VSEPR Theory l Types of e Pairs Bonding
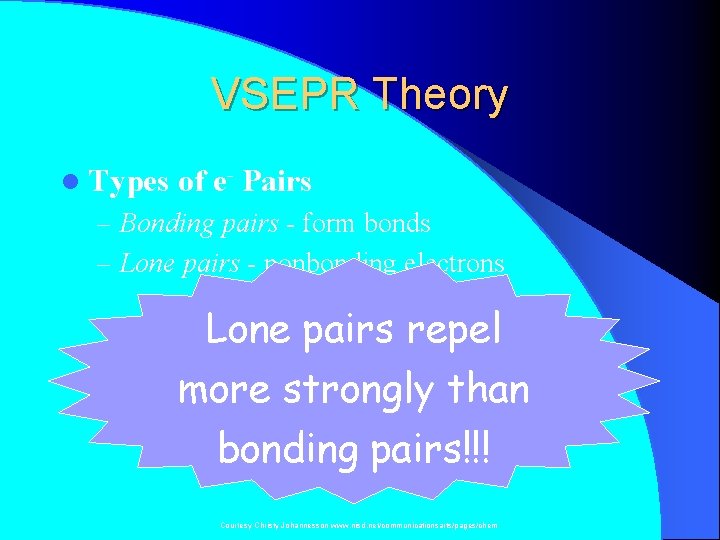
VSEPR Theory l Types of e- Pairs – Bonding pairs - form bonds – Lone pairs - nonbonding electrons Lone pairs repel more strongly than bonding pairs!!! Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
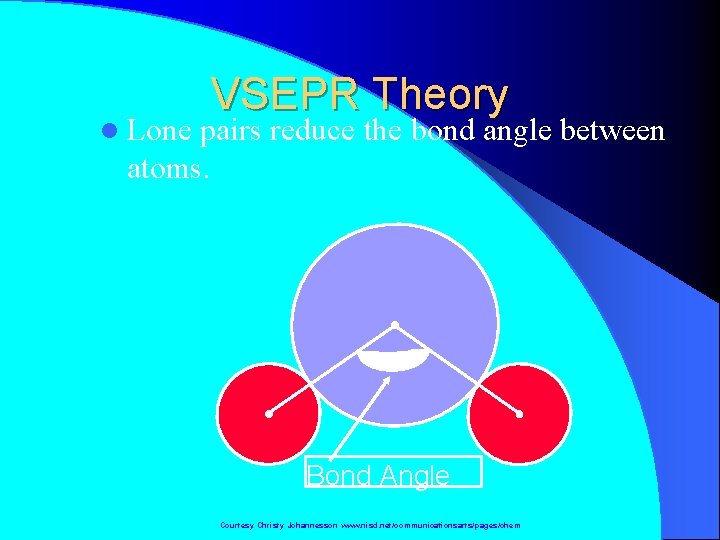
l Lone VSEPR Theory pairs reduce the bond angle between atoms. Bond Angle Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
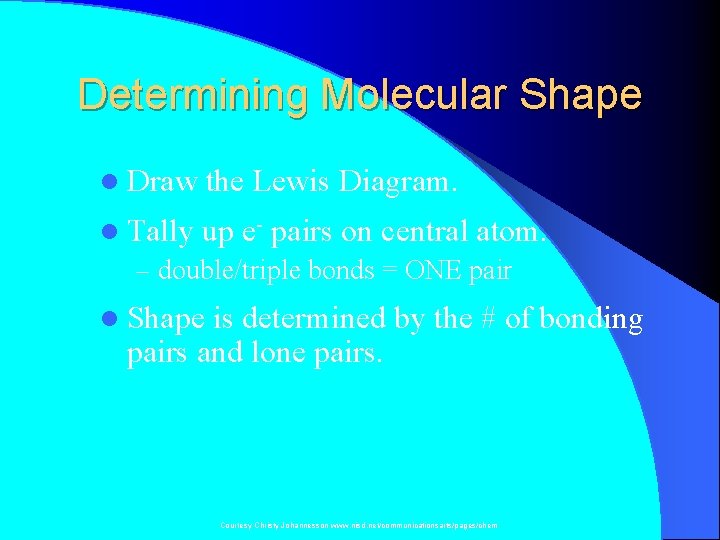
Determining Molecular Shape l Draw the Lewis Diagram. l Tally up e- pairs on central atom. – double/triple bonds = ONE pair l Shape is determined by the # of bonding pairs and lone pairs. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
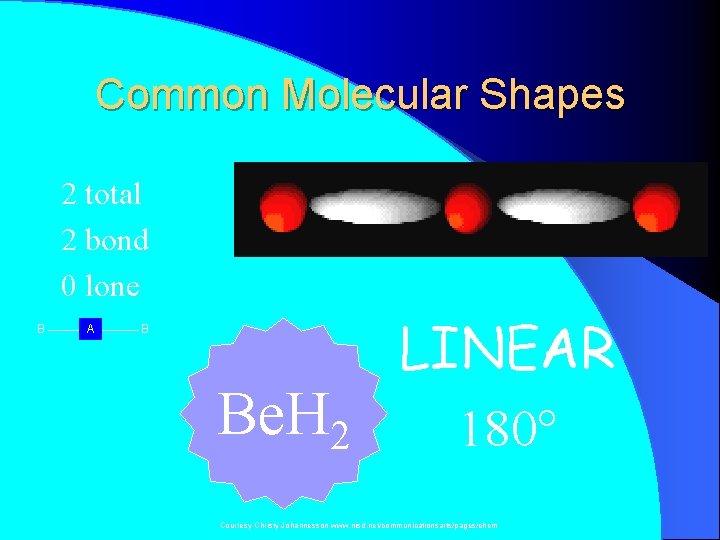
Common Molecular Shapes 2 total 2 bond 0 lone B A B Be. H 2 LINEAR 180° Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
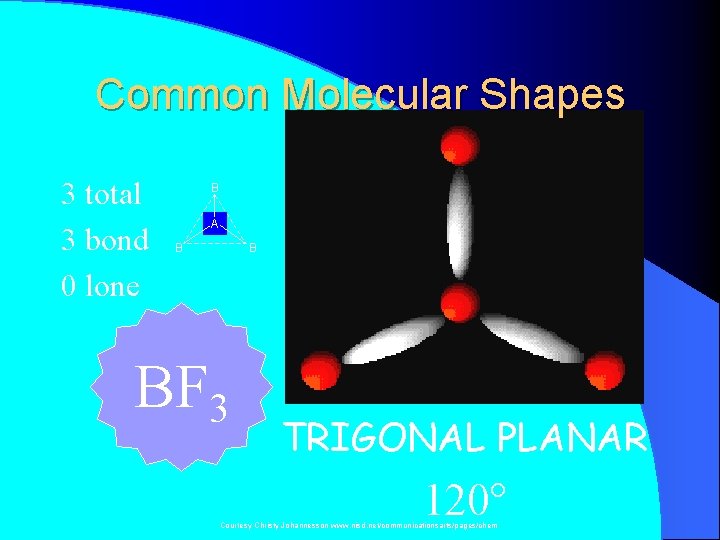
Common Molecular Shapes 3 total 3 bond 0 lone B A B B BF 3 TRIGONAL PLANAR 120° Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
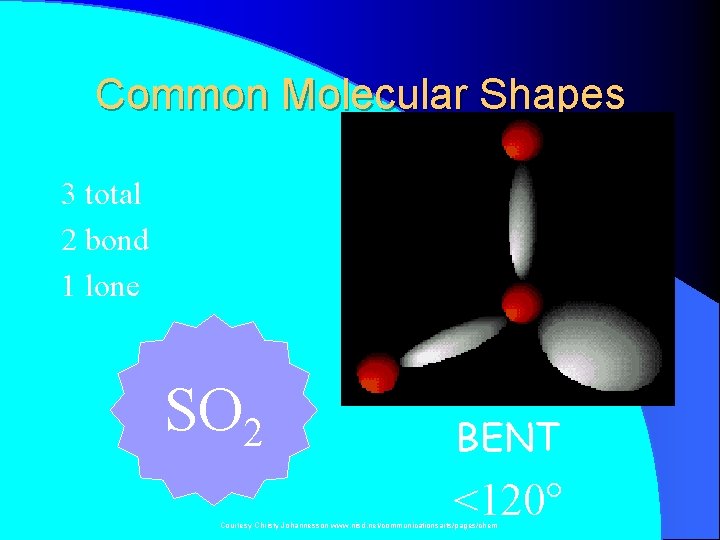
Common Molecular Shapes 3 total 2 bond 1 lone SO 2 BENT <120° Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Common Molecular Shapes B 4 total 4 bond 0 lone A B B B CH 4 TETRAHEDRAL 109. 5° Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
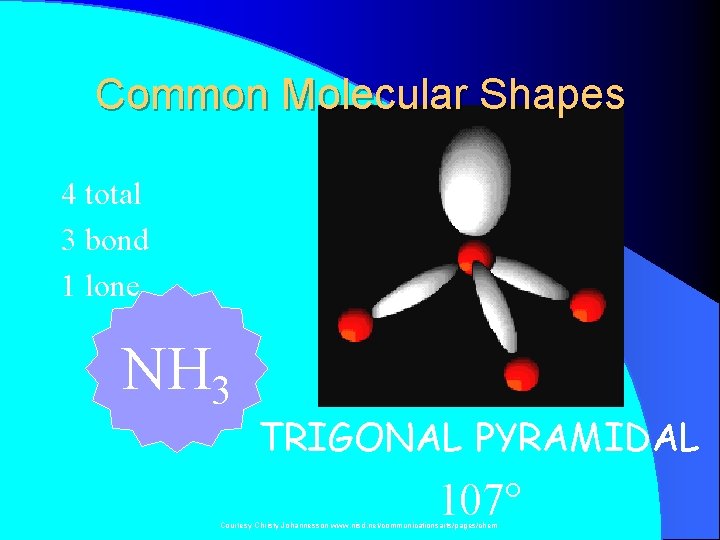
Common Molecular Shapes 4 total 3 bond 1 lone NH 3 TRIGONAL PYRAMIDAL 107° Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
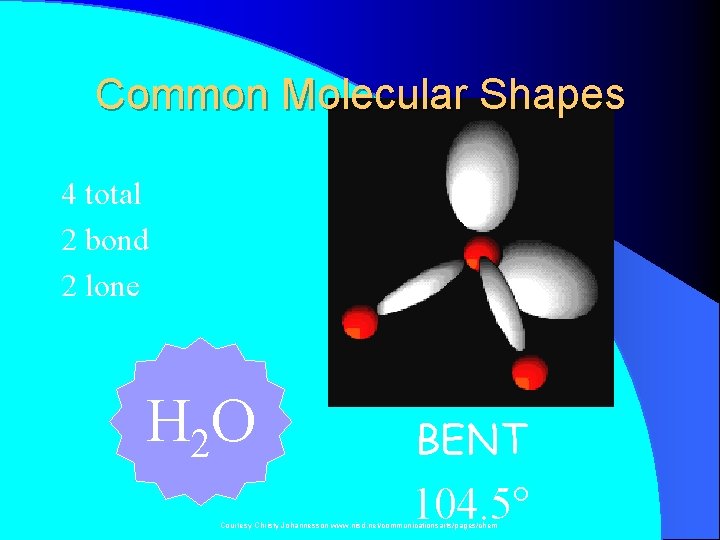
Common Molecular Shapes 4 total 2 bond 2 lone H 2 O BENT 104. 5° Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
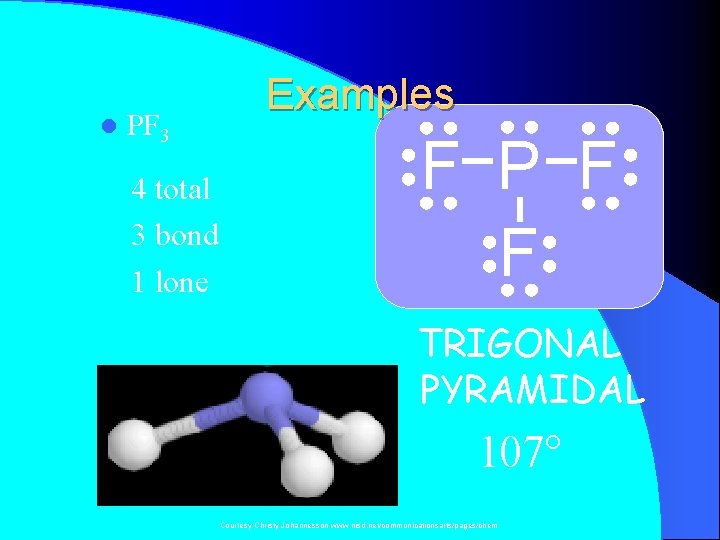
l PF 3 4 total 3 bond 1 lone Examples F P F F TRIGONAL PYRAMIDAL 107° Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
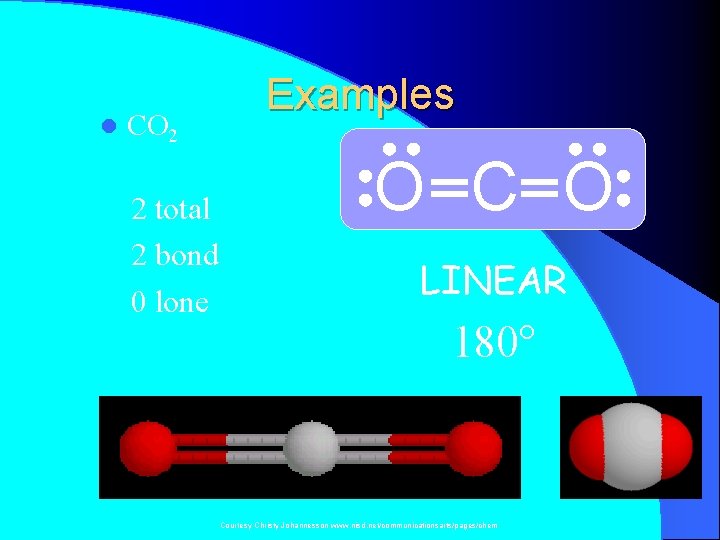
l CO 2 2 total 2 bond 0 lone Examples O C O LINEAR 180° Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
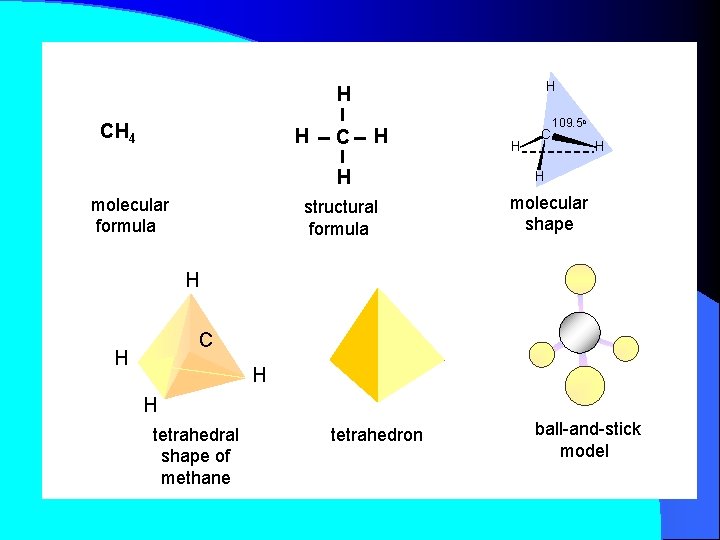
H H CH 4 H C H H molecular formula structural formula H C 109. 5 o H H molecular shape H C H H H tetrahedral shape of methane tetrahedron ball-and-stick model
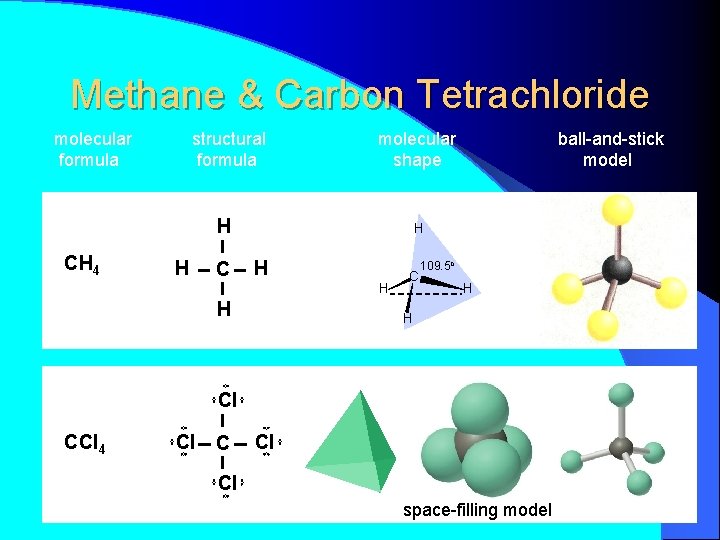
Methane & Carbon Tetrachloride molecular formula structural formula molecular shape H CH 4 H C ball-and-stick model H H C 109. 5 o H H Cl CCl 4 Cl Cl space-filling model
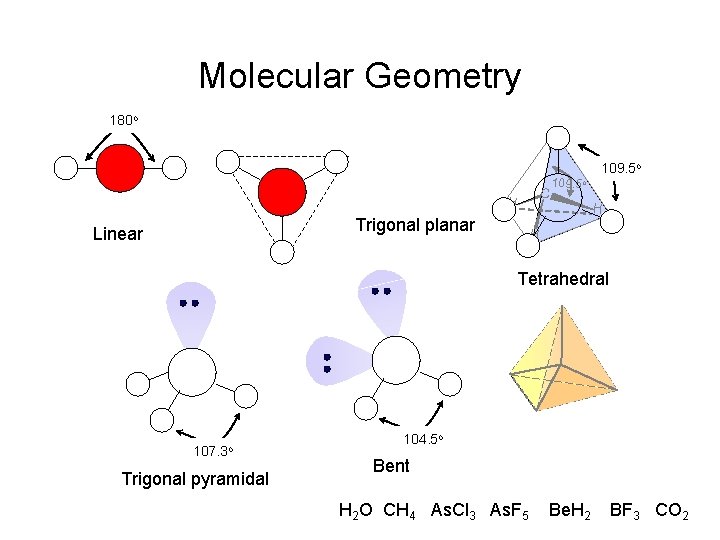
Molecular Geometry 180 o H H Trigonal planar Linear C 109. 5 o H H Tetrahedral 107. 3 o Trigonal pyramidal 104. 5 o Bent H 2 O CH 4 As. Cl 3 As. F 5 Be. H 2 BF 3 CO 2
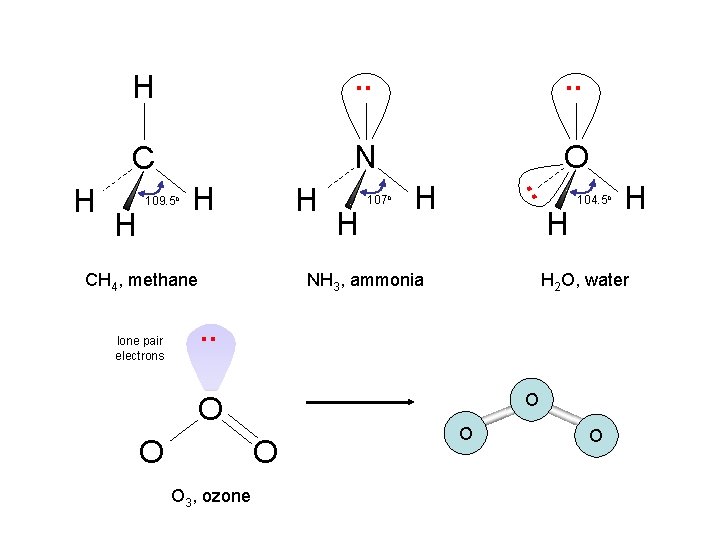
. . C N O H 109. 5 o H H CH 4, methane lone pair electrons H 107 o H . . H H H NH 3, ammonia 104. 5 o H 2 O, water . . O O O 3, ozone H O O

Molecular Shapes Three atoms (AB 2) Four atoms (AB 3) • Linear (180 o) • Bent B A linear B B • Trigonal planar (120 o) • Trigonal pyramidal • T-shaped B A B trigonal planar B Five atoms (AB 4) • Tetrahedral (109. 47 o) • Square planar • Seesaw tetrahedral A B Bailar, Moeller, Kleinberg, Guss, Castellion, Metz, Chemistry, 1984, page 313.
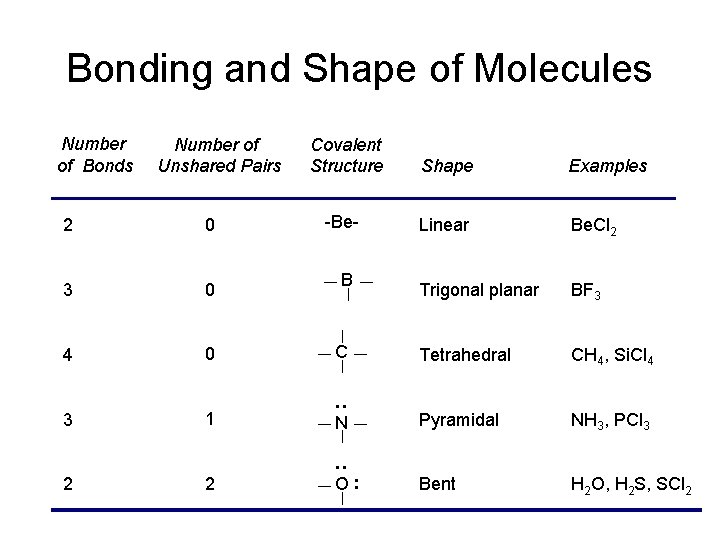
Bonding and Shape of Molecules Number of Unshared Pairs Covalent Structure Shape Examples -Be- Linear Be. Cl 2 Trigonal planar BF 3 2 0 3 0 4 0 C Tetrahedral CH 4, Si. Cl 4 3 1 : Number of Bonds Pyramidal NH 3, PCl 3 2 2 Bent H 2 O, H 2 S, SCl 2 B : N O:
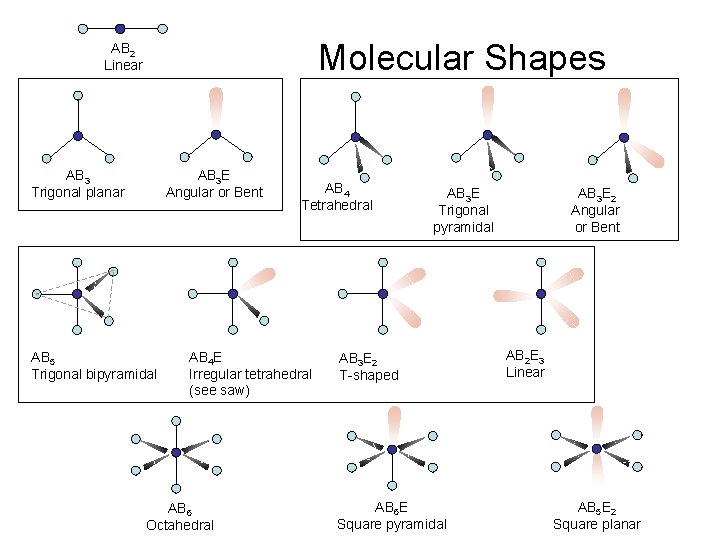
Molecular Shapes AB 2 Linear AB 3 Trigonal planar AB 3 E Angular or Bent AB 5 Trigonal bipyramidal AB 4 Tetrahedral AB 4 E Irregular tetrahedral (see saw) AB 6 Octahedral AB 3 E Trigonal pyramidal AB 3 E 2 T-shaped AB 6 E Square pyramidal AB 3 E 2 Angular or Bent AB 2 E 3 Linear AB 5 E 2 Square planar
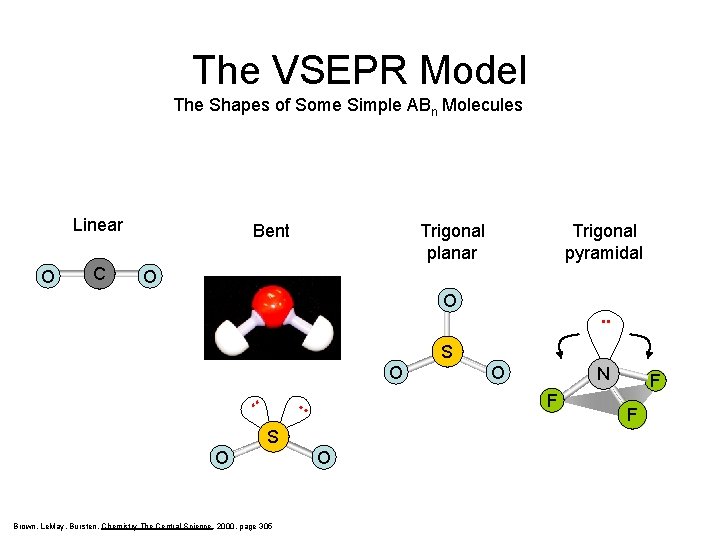
The VSEPR Model The Shapes of Some Simple ABn Molecules Linear O C Bent Trigonal planar Trigonal pyramidal O O SF 6 . . S O . . SO 2 S O Brown, Le. May, Bursten, Chemistry The Central Science, 2000, page 305 O O N F F F
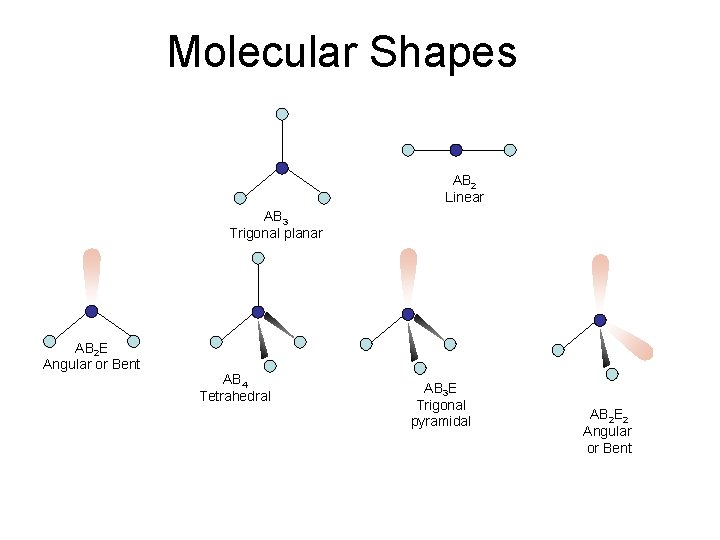
Molecular Shapes AB 2 Linear AB 3 Trigonal planar AB 2 E Angular or Bent AB 4 Tetrahedral AB 3 E Trigonal pyramidal AB 2 E 2 Angular or Bent
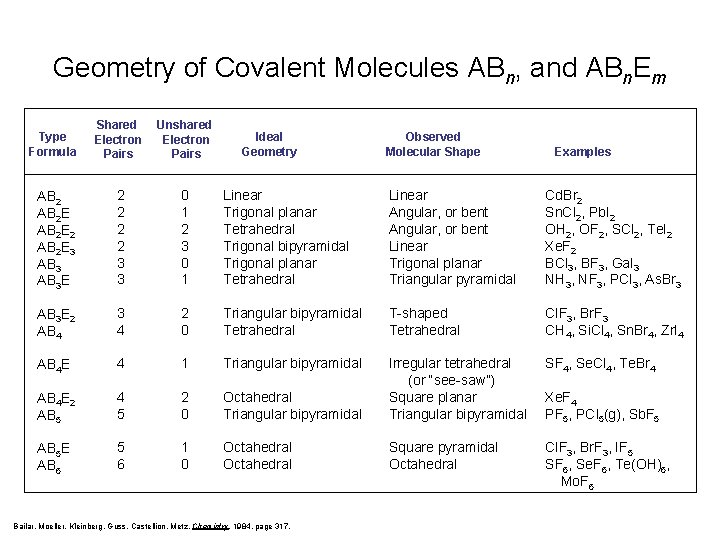
Geometry of Covalent Molecules ABn, and ABn. Em Type Formula Shared Electron Pairs Unshared Electron Pairs AB 2 E 2 AB 2 E 3 AB 3 E 2 2 3 3 0 1 2 3 0 1 Linear Trigonal planar Tetrahedral Trigonal bipyramidal Trigonal planar Tetrahedral Linear Angular, or bent Linear Trigonal planar Triangular pyramidal Cd. Br 2 Sn. Cl 2, Pb. I 2 OH 2, OF 2, SCl 2, Te. I 2 Xe. F 2 BCl 3, BF 3, Ga. I 3 NH 3, NF 3, PCl 3, As. Br 3 AB 3 E 2 AB 4 3 4 2 0 Triangular bipyramidal Tetrahedral T-shaped Tetrahedral Cl. F 3, Br. F 3 CH 4, Si. Cl 4, Sn. Br 4, Zr. I 4 AB 4 E 4 1 Triangular bipyramidal SF 4, Se. Cl 4, Te. Br 4 AB 4 E 2 AB 5 4 5 2 0 Octahedral Triangular bipyramidal Irregular tetrahedral (or “see-saw”) Square planar Triangular bipyramidal AB 5 E AB 6 5 6 1 0 Octahedral Square pyramidal Octahedral Cl. F 3, Br. F 3, IF 5 SF 6, Se. F 6, Te(OH)6, Mo. F 6 Ideal Geometry Bailar, Moeller, Kleinberg, Guss, Castellion, Metz, Chemistry, 1984, page 317. Observed Molecular Shape Examples Xe. F 4 PF 5, PCl 5(g), Sb. F 5
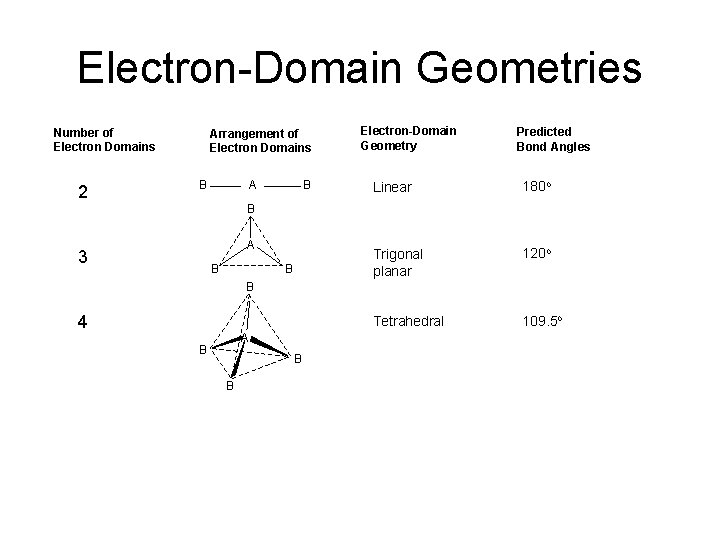
Electron-Domain Geometries Number of Electron Domains 2 Arrangement of Electron Domains B A B Electron-Domain Geometry Predicted Bond Angles Linear 180 o Trigonal planar 120 o Tetrahedral 109. 5 o B A 3 B B B 4 A B B B
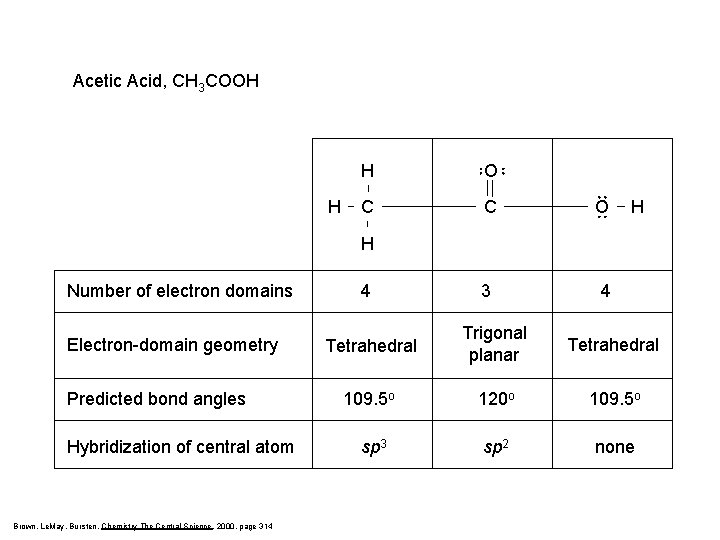
Acetic Acid, CH 3 COOH H H O C C O 3 4 H H Number of electron domains Electron-domain geometry Predicted bond angles Hybridization of central atom Brown, Le. May, Bursten, Chemistry The Central Science, 2000, page 314 4 Tetrahedral Trigonal planar Tetrahedral 109. 5 o 120 o 109. 5 o sp 3 sp 2 none
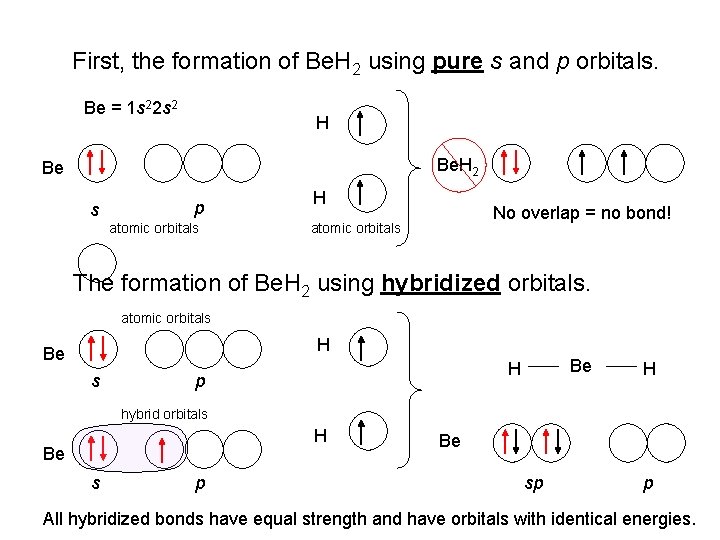
First, the formation of Be. H 2 using pure s and p orbitals. Be = 1 s 22 s 2 H Be. H 2 Be s p atomic orbitals H No overlap = no bond! atomic orbitals The formation of Be. H 2 using hybridized orbitals. atomic orbitals H Be s Be H p H hybrid orbitals H Be s p Be. H 2 Be sp p All hybridized bonds have equal strength and have orbitals with identical energies.
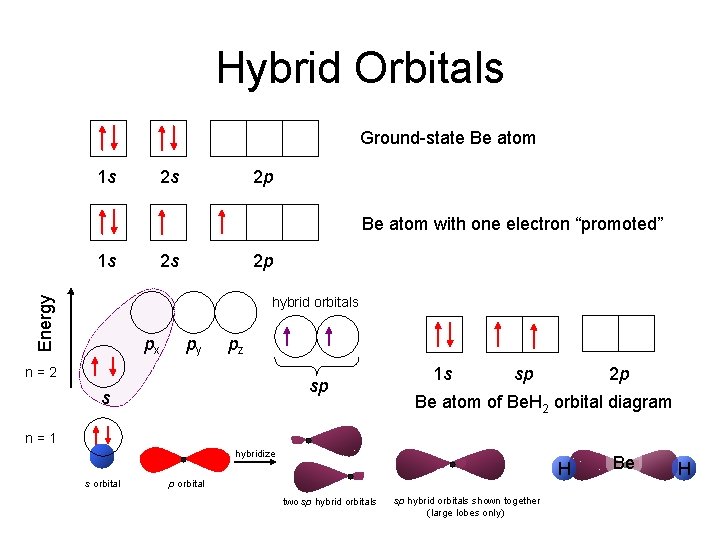
Hybrid Orbitals Ground-state Be atom 1 s 2 s 2 p Be atom with one electron “promoted” 1 s 2 s 2 p Energy hybrid orbitals px py pz n=2 sp s 1 s sp 2 p Be atom of Be. H 2 orbital diagram n=1 hybridize s orbital H p orbital two sp hybrid orbitals shown together (large lobes only) Be H
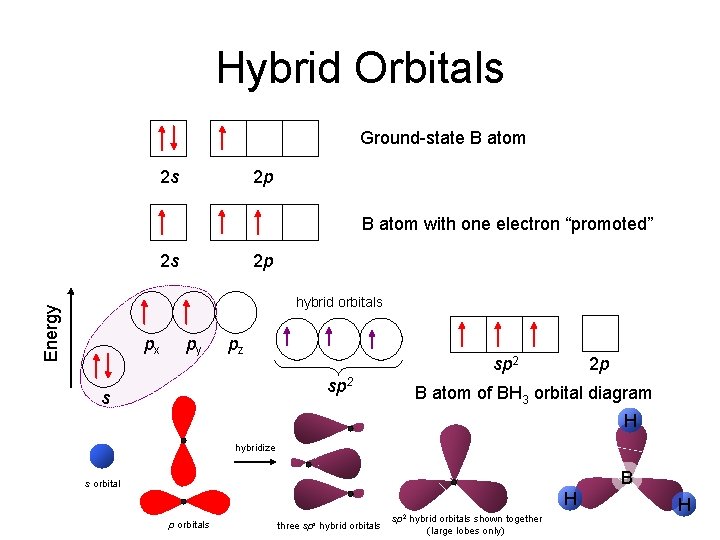
Hybrid Orbitals Ground-state B atom 2 s 2 p B atom with one electron “promoted” 2 s 2 p Energy hybrid orbitals px py pz sp 2 s 2 p B atom of BH 3 orbital diagram H hybridize B s orbital H p orbitals three sps hybrid orbitals sp 2 hybrid orbitals shown together (large lobes only) H
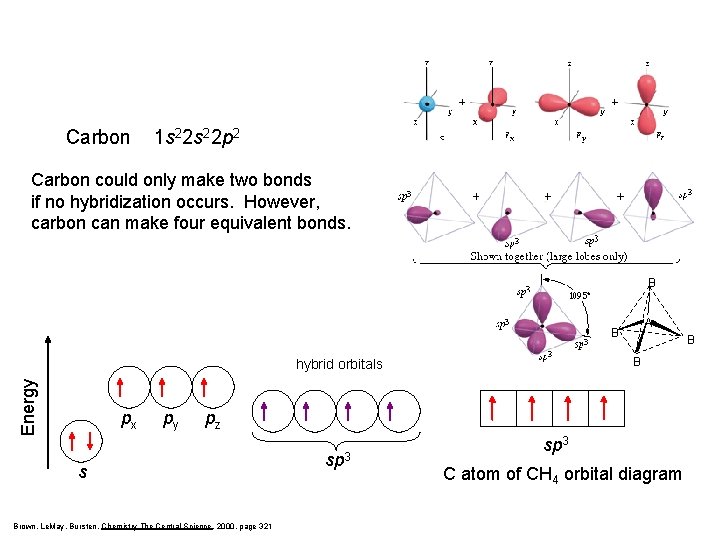
Carbon 1 s 22 p 2 Carbon could only make two bonds if no hybridization occurs. However, carbon can make four equivalent bonds. B A B B Energy hybrid orbitals px py B pz s Brown, Le. May, Bursten, Chemistry The Central Science, 2000, page 321 sp 3 C atom of CH 4 orbital diagram
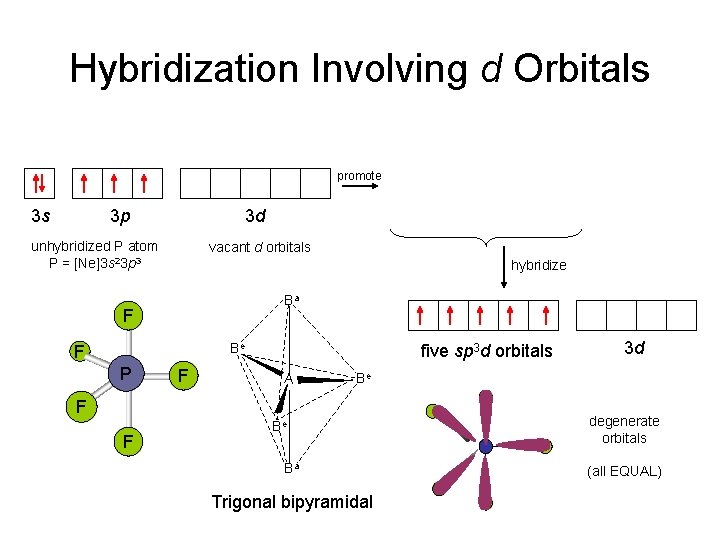
Hybridization Involving d Orbitals promote 3 s 3 p 3 d unhybridized P atom P = [Ne]3 s 23 p 3 3 s hybridize Ba Be F F five sp 3 d orbitals A 3 d Be F F 3 d vacant d orbitals F P 3 p Be Ba Trigonal bipyramidal degenerate orbitals (all EQUAL)
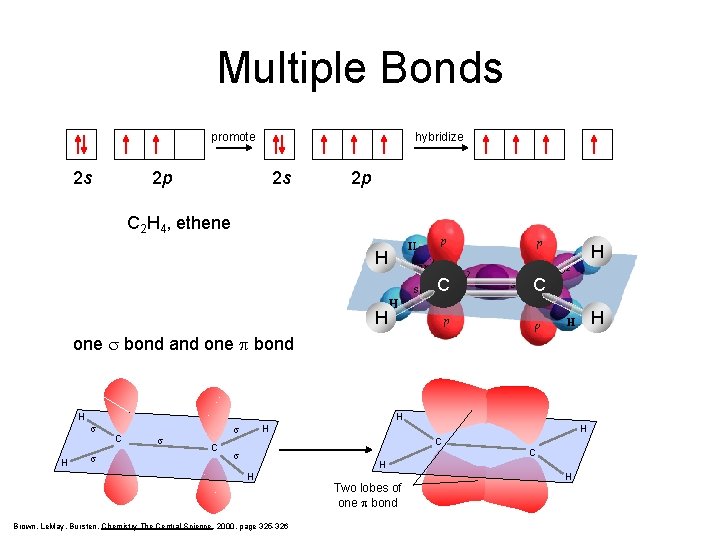
Multiple Bonds hybridize promote 2 s 2 p sp 2 2 p C 2 H 4, ethene H H C C H H one s bond and one p bond H H s C s H s C H C s C H H Brown, Le. May, Bursten, Chemistry The Central Science, 2000, page 325 -326 Two lobes of one p bond H
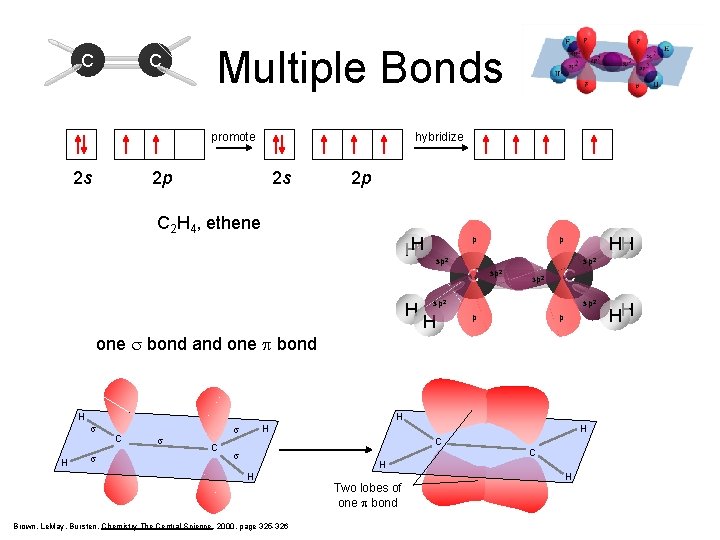
C C Multiple Bonds hybridize promote 2 s 2 p sp 2 C 2 H 4, ethene HH H p sp 2 C 2 p HH p sp 2 C sp 2 H sp 2 p p one s bond and one p bond H H s C s H s C H C s C H H Brown, Le. May, Bursten, Chemistry The Central Science, 2000, page 325 -326 Two lobes of one p bond H HH
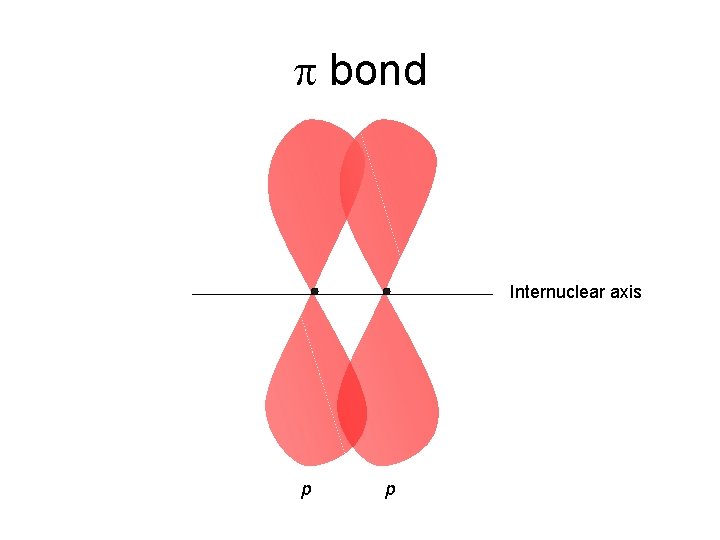
p bond Internuclear axis p p
- Slides: 31