Chemistry A Molecular Approach 1 st Ed Nivaldo









- Slides: 62

Chemistry: A Molecular Approach, 1 st Ed. Nivaldo Tro Chapter 8 Periodic Properties of the Elements Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA 2007, Prentice Hall

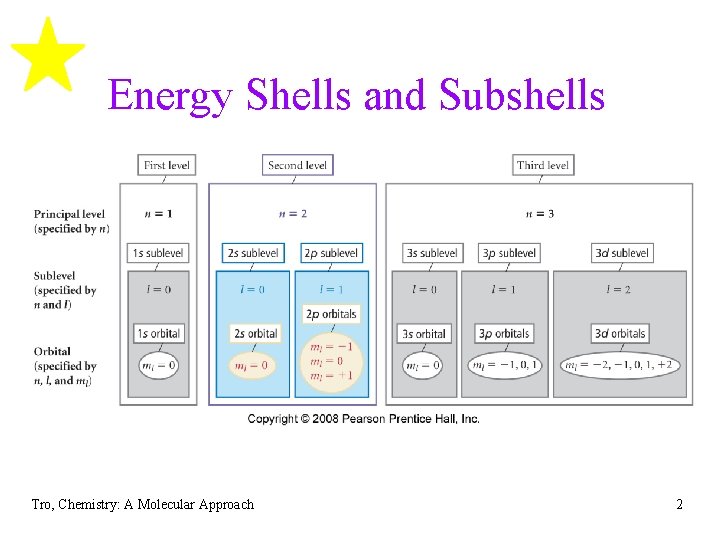
Energy Shells and Subshells Tro, Chemistry: A Molecular Approach 2

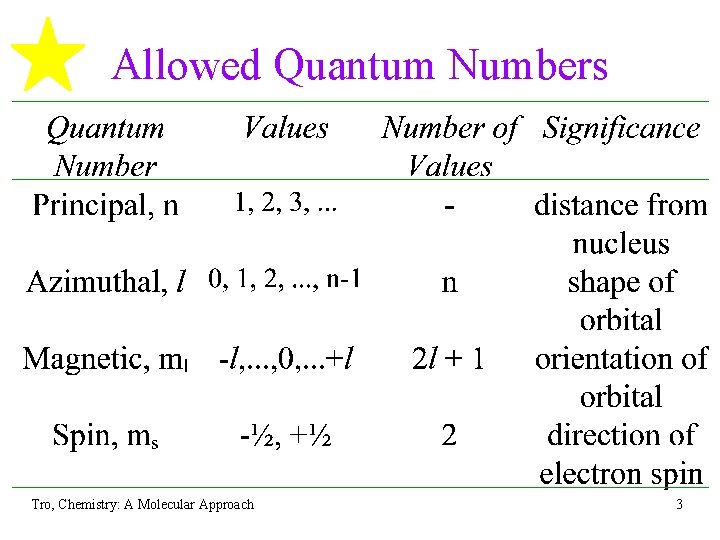
Allowed Quantum Numbers Tro, Chemistry: A Molecular Approach 3

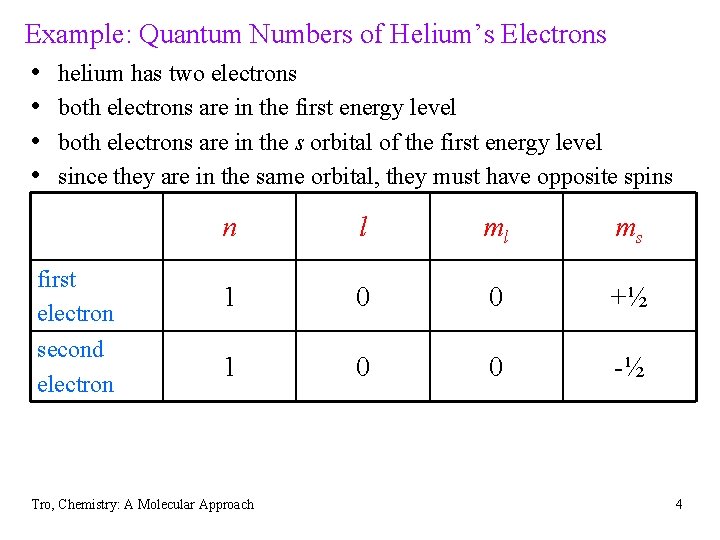
Example: Quantum Numbers of Helium’s Electrons • helium has two electrons • both electrons are in the first energy level • both electrons are in the s orbital of the first energy level • since they are in the same orbital, they must have opposite spins n l ml ms first electron 1 0 0 +½ second electron 1 0 0 -½ Tro, Chemistry: A Molecular Approach 4

Pauli Exclusion Principle • no two electrons in an atom may have the same set of 4 • • quantum numbers therefore no orbital may have more than 2 electrons, and they must have with opposite spins knowing the number orbitals in a sublevel allows us to determine the maximum number of electrons in the sublevel ü s sublevel has 1 orbital, therefore it can hold 2 electrons ü p sublevel has 3 orbitals, therefore it can hold 6 electrons ü d sublevel has 5 orbitals, therefore it can hold 10 electrons ü f sublevel has 7 orbitals, therefore it can hold 14 electrons Tro, Chemistry: A Molecular Approach 5

Electron Configurations • the ground state of the electron is the lowest • • • energy orbital it can occupy the distribution of electrons into the various orbitals in an atom in its ground state is called its electron configuration the number designates the principal energy level the letter designates the sublevel and type of orbital the superscript designates the number of electrons in that sublevel He = 1 s 2 Tro, Chemistry: A Molecular Approach 6

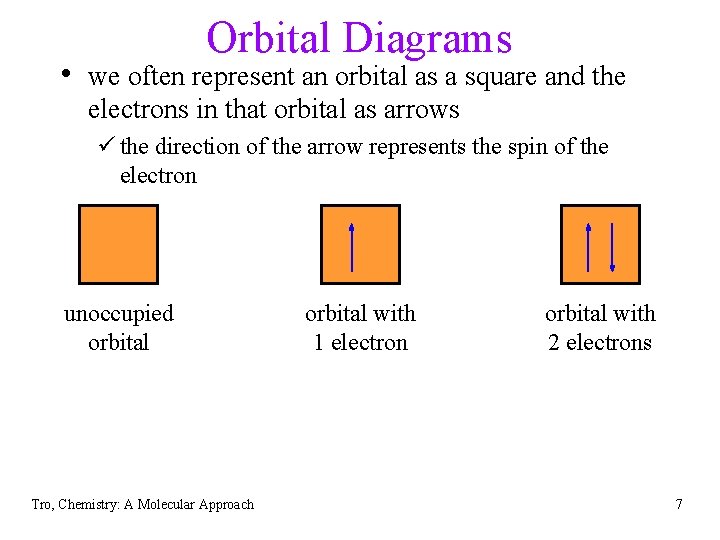
Orbital Diagrams • we often represent an orbital as a square and the electrons in that orbital as arrows ü the direction of the arrow represents the spin of the electron unoccupied orbital Tro, Chemistry: A Molecular Approach orbital with 1 electron orbital with 2 electrons 7

Sublevel Splitting in Multielectron Atoms • the sublevels in each principal energy level of Hydrogen all have the same energy – we call orbitals with the same energy degenerate ü or other single electron systems • for multielectron atoms, the energies of the sublevels are split ü caused by electron-electron repulsion • the lower the value of the l quantum number, the less energy the sublevel has ü s (l = 0) < p (l = 1) < d (l = 2) < f (l = 3) Tro, Chemistry: A Molecular Approach 8

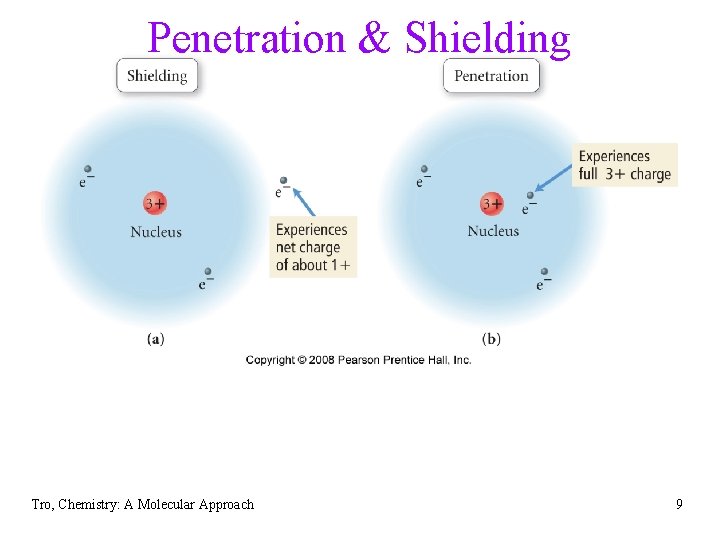
Penetration & Shielding Tro, Chemistry: A Molecular Approach 9

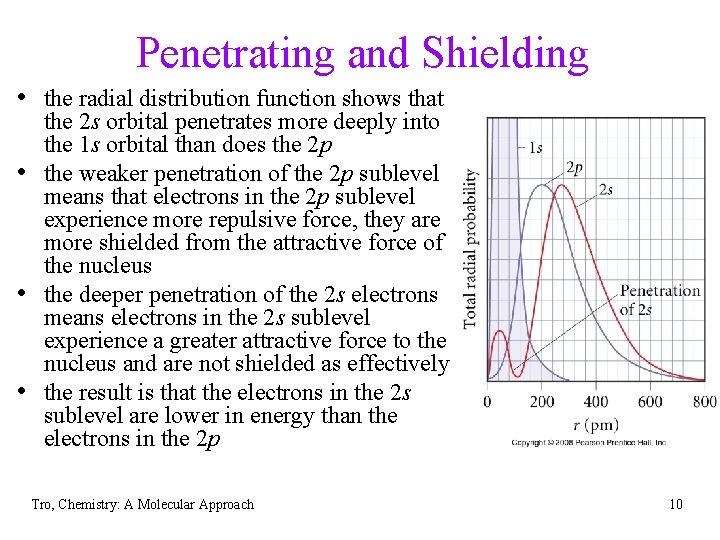
Penetrating and Shielding • the radial distribution function shows that • • • the 2 s orbital penetrates more deeply into the 1 s orbital than does the 2 p the weaker penetration of the 2 p sublevel means that electrons in the 2 p sublevel experience more repulsive force, they are more shielded from the attractive force of the nucleus the deeper penetration of the 2 s electrons means electrons in the 2 s sublevel experience a greater attractive force to the nucleus and are not shielded as effectively the result is that the electrons in the 2 s sublevel are lower in energy than the electrons in the 2 p Tro, Chemistry: A Molecular Approach 10

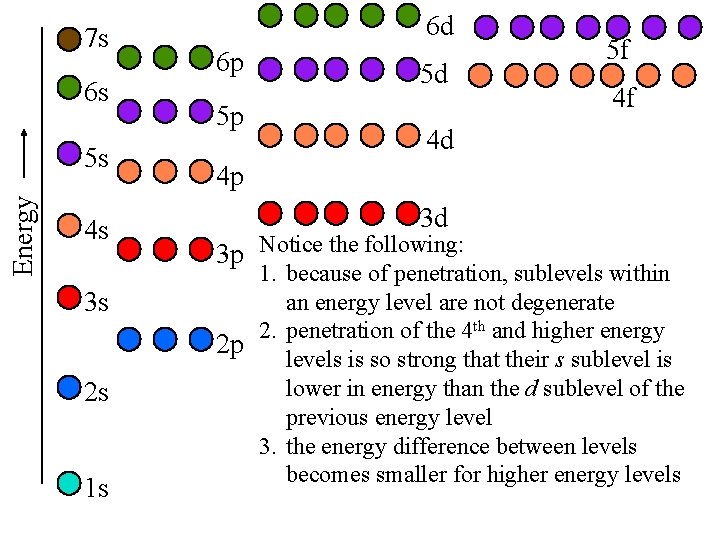
7 s 6 s Energy 5 s 4 s 3 s 2 s 1 s 6 d 6 p 5 p 5 d 5 f 4 f 4 d 4 p 3 d 3 p Notice the following: 1. because of penetration, sublevels within an energy level are not degenerate 2. penetration of the 4 th and higher energy 2 p levels is so strong that their s sublevel is lower in energy than the d sublevel of the previous energy level 3. the energy difference between levels becomes smaller for higher energy levels

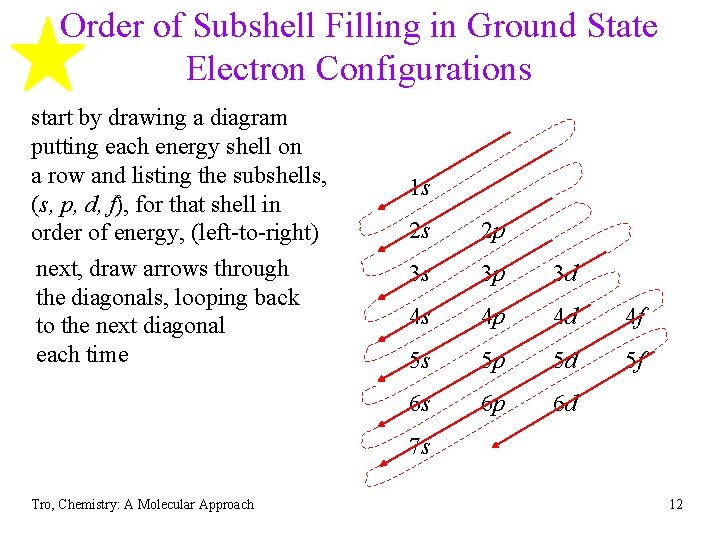
Order of Subshell Filling in Ground State Electron Configurations start by drawing a diagram putting each energy shell on a row and listing the subshells, (s, p, d, f), for that shell in order of energy, (left-to-right) next, draw arrows through the diagonals, looping back to the next diagonal each time 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 7 s Tro, Chemistry: A Molecular Approach 12

Filling the Orbitals with Electrons • energy shells fill from lowest energy to high • subshells fill from lowest energy to high üs → p → d → f ü Aufbau Principle • orbitals that are in the same subshell have the same • energy no more than 2 electrons per orbital ü Pauli Exclusion Principle • when filling orbitals that have the same energy, place one electron in each before completing pairs ü Hund’s Rule Tro, Chemistry: A Molecular Approach 13

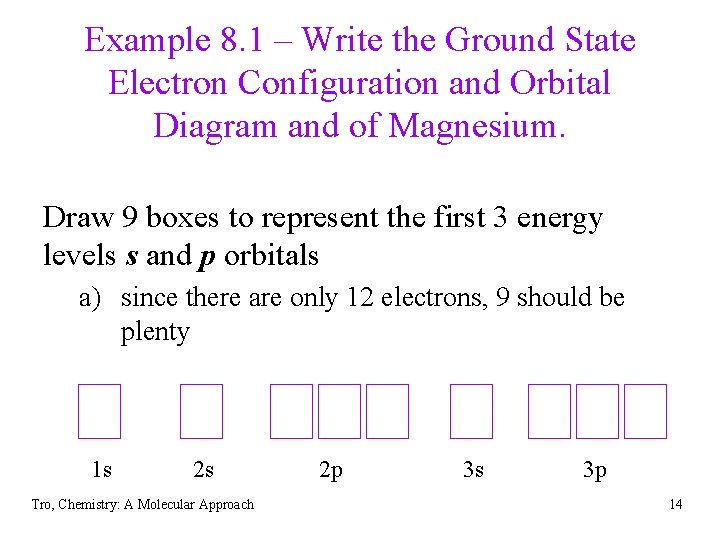
Example 8. 1 – Write the Ground State Electron Configuration and Orbital Diagram and of Magnesium. Draw 9 boxes to represent the first 3 energy levels s and p orbitals a) since there are only 12 electrons, 9 should be plenty 1 s 2 s Tro, Chemistry: A Molecular Approach 2 p 3 s 3 p 14

Valence Electrons • the electrons in all the subshells with the • • highest principal energy shell are called the valence electrons in lower energy shells are called core electrons chemists have observed that one of the most important factors in the way an atom behaves, both chemically and physically, is the number of valence electrons Tro, Chemistry: A Molecular Approach 15

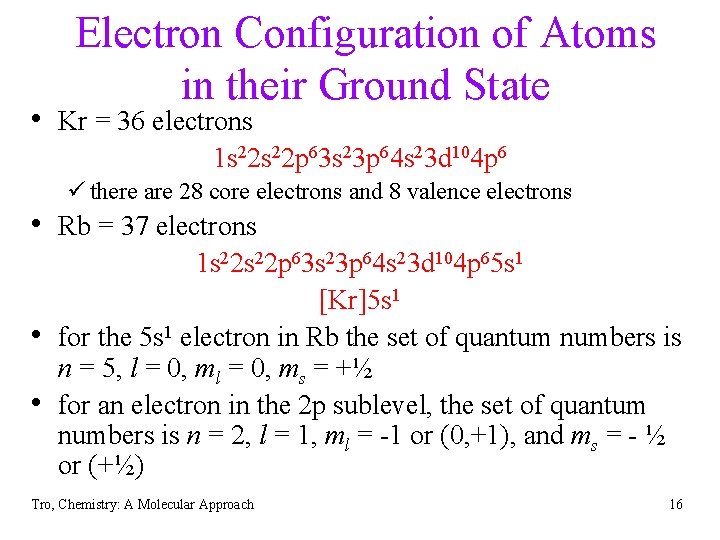
Electron Configuration of Atoms in their Ground State • Kr = 36 electrons 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 ü there are 28 core electrons and 8 valence electrons • Rb = 37 electrons • • 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 [Kr]5 s 1 for the 5 s 1 electron in Rb the set of quantum numbers is n = 5, l = 0, ms = +½ for an electron in the 2 p sublevel, the set of quantum numbers is n = 2, l = 1, ml = -1 or (0, +1), and ms = - ½ or (+½) Tro, Chemistry: A Molecular Approach 16

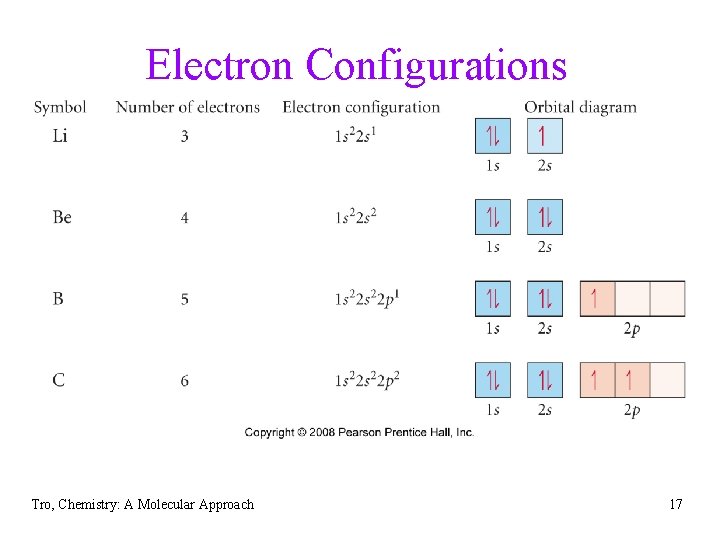
Electron Configurations Tro, Chemistry: A Molecular Approach 17

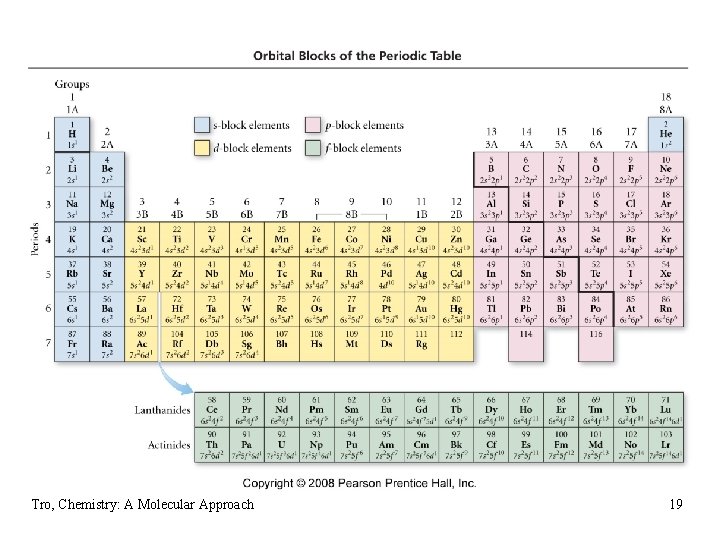
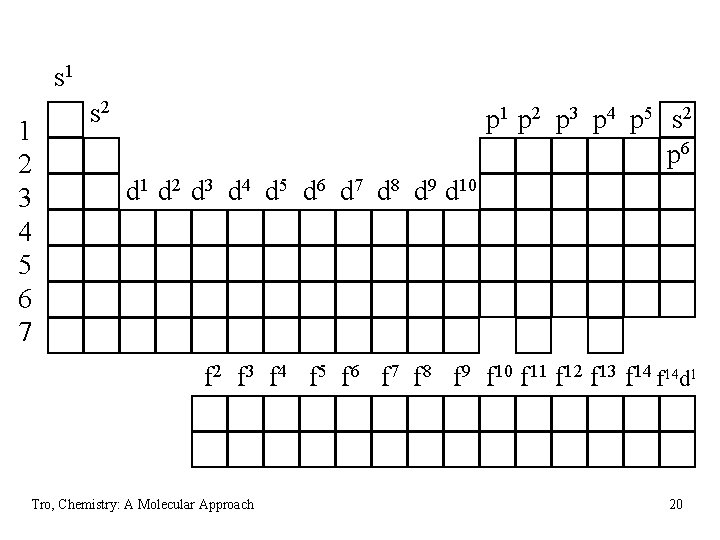
Electron Configuration & the Periodic Table • the Group number corresponds to the number of • • valence electrons the length of each “block” is the maximum number of electrons the sublevel can hold the Period number corresponds to the principal energy level of the valence electrons Tro, Chemistry: A Molecular Approach 18

Tro, Chemistry: A Molecular Approach 19

s 1 1 2 3 4 5 6 7 s 2 p 1 p 2 p 3 p 4 p 5 s 2 p 6 d 1 d 2 d 3 d 4 d 5 d 6 d 7 d 8 d 9 d 10 f 2 f 3 f 4 f 5 f 6 f 7 f 8 f 9 f 10 f 11 f 12 f 13 f 14 d 1 Tro, Chemistry: A Molecular Approach 20

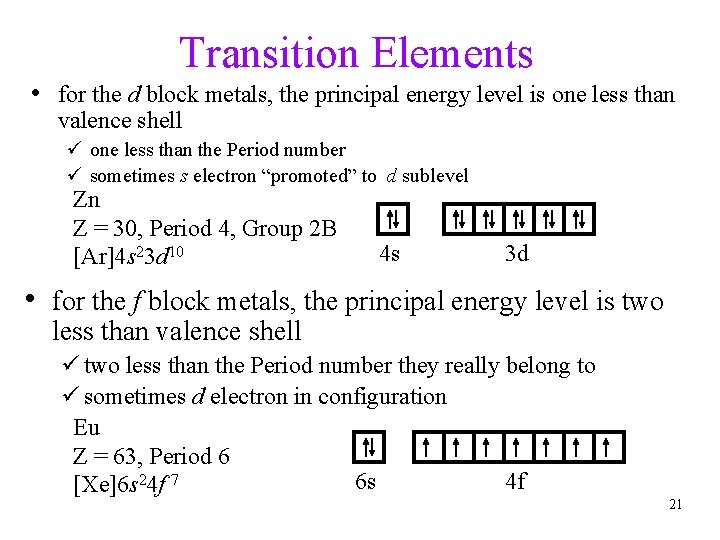
Transition Elements • for the d block metals, the principal energy level is one less than valence shell ü one less than the Period number ü sometimes s electron “promoted” to d sublevel Zn Z = 30, Period 4, Group 2 B [Ar]4 s 23 d 10 4 s 3 d • for the f block metals, the principal energy level is two less than valence shell ü two less than the Period number they really belong to ü sometimes d electron in configuration Eu Z = 63, Period 6 6 s 4 f [Xe]6 s 24 f 7 21

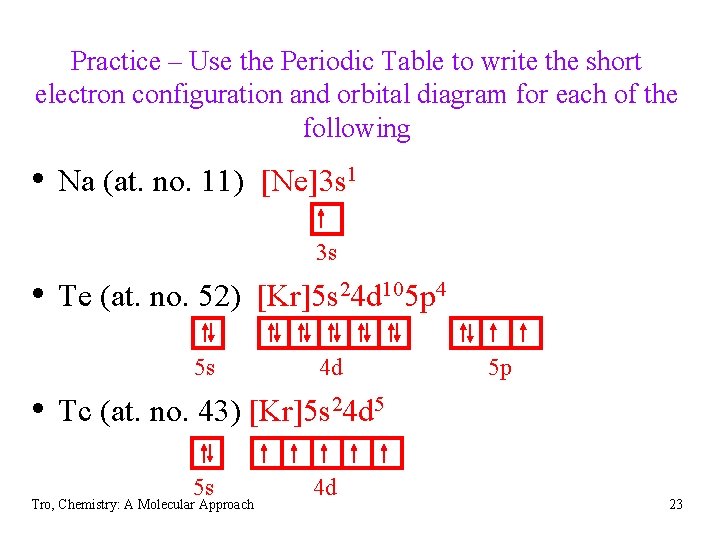
Practice – Use the Periodic Table to write the short electron configuration and orbital diagram for each of the following • Na (at. no. 11) • Te (at. no. 52) • Tc (at. no. 43) Tro, Chemistry: A Molecular Approach 22

Practice – Use the Periodic Table to write the short electron configuration and orbital diagram for each of the following • Na (at. no. 11) [Ne]3 s 1 3 s • Te (at. no. 52) [Kr]5 s 24 d 105 p 4 5 s 4 d 5 p • Tc (at. no. 43) [Kr]5 s 24 d 5 5 s Tro, Chemistry: A Molecular Approach 4 d 23

Properties & Electron Configuration • elements in the same column have similar chemical and physical properties because they have the same number of valence electrons in the same kinds of orbitals Tro, Chemistry: A Molecular Approach 24

Electron Configuration & Element Properties • the number of valence electrons largely determines the behavior of an element ü chemical and some physical • since the number of valence electrons follows a Periodic pattern, • • the properties of the elements should also be periodic quantum mechanical calculations show that 8 valence electrons should result in a very unreactive atom, an atom that is very stable – and the noble gases, that have 8 valence electrons are all very stable and unreactive conversely, elements that have either one more or one less electron should be very reactive – and the halogens are the most reactive nonmetals and alkali metals the most reactive metals ü as a group Tro, Chemistry: A Molecular Approach 25

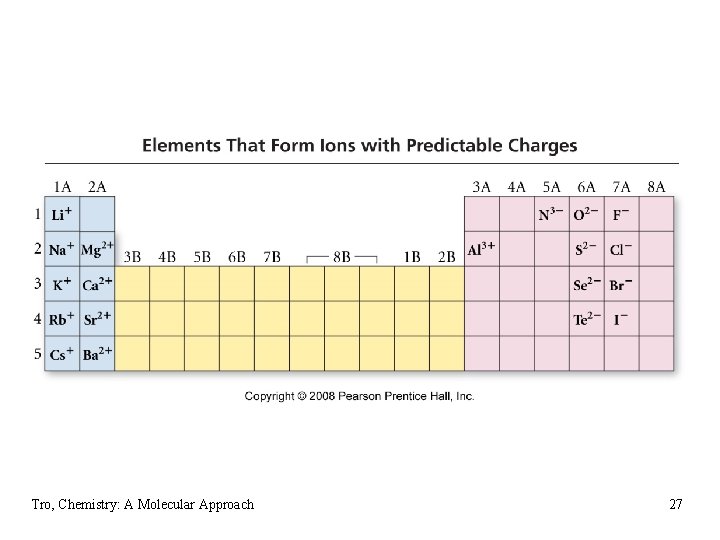
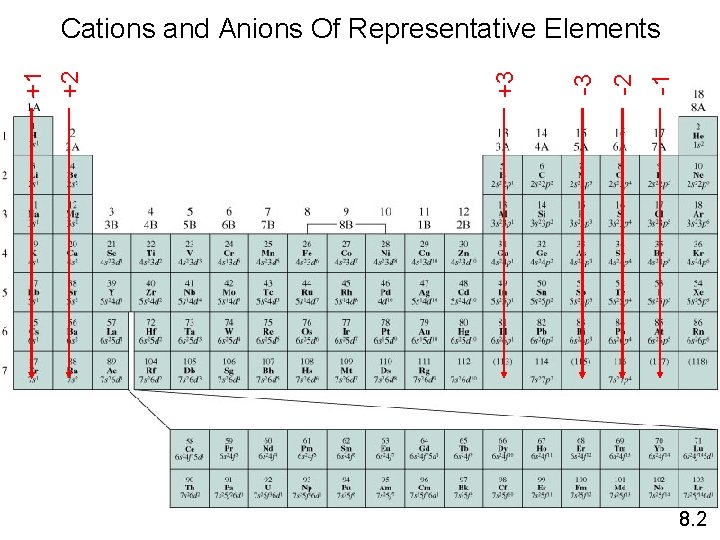
Electron Configuration & Ion Charge • we have seen that many metals and nonmetals form one ion, and that the charge on that ion is predictable based on its position on the Periodic Table üGroup 1 A = +1, Group 2 A = +2, Group 7 A = -1, Group 6 A = -2, etc. • these atoms form ions that will result in an electron configuration that is the same as the nearest noble gas Tro, Chemistry: A Molecular Approach 26

Tro, Chemistry: A Molecular Approach 27

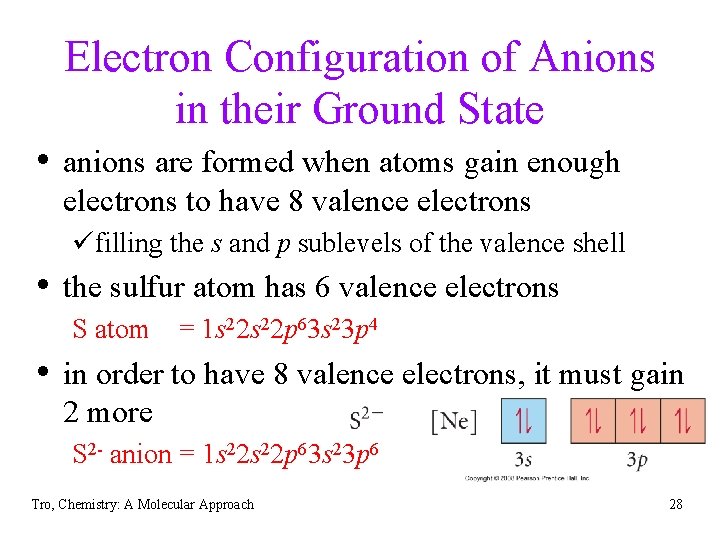
Electron Configuration of Anions in their Ground State • anions are formed when atoms gain enough electrons to have 8 valence electrons üfilling the s and p sublevels of the valence shell • the sulfur atom has 6 valence electrons S atom = 1 s 22 p 63 s 23 p 4 • in order to have 8 valence electrons, it must gain 2 more S 2 - anion = 1 s 22 p 63 s 23 p 6 Tro, Chemistry: A Molecular Approach 28

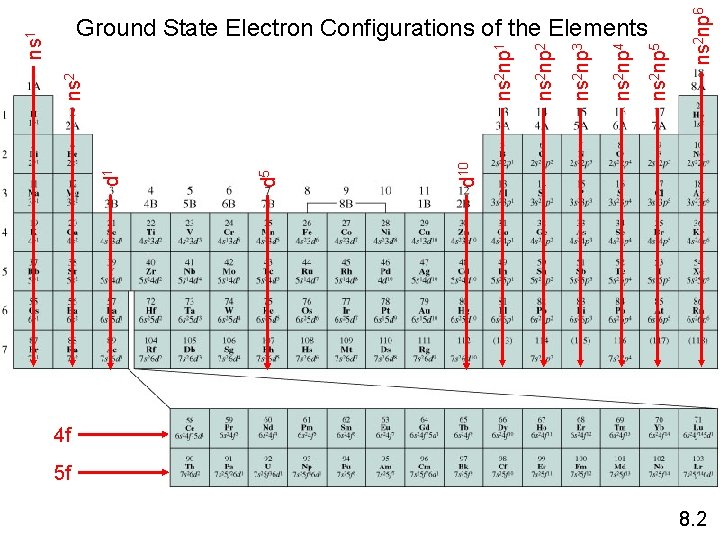
ns 2 np 6 ns 2 np 5 ns 2 np 4 ns 2 np 3 ns 2 np 2 ns 2 np 1 d 10 d 5 d 1 ns 2 ns 1 Ground State Electron Configurations of the Elements 4 f 5 f 8. 2

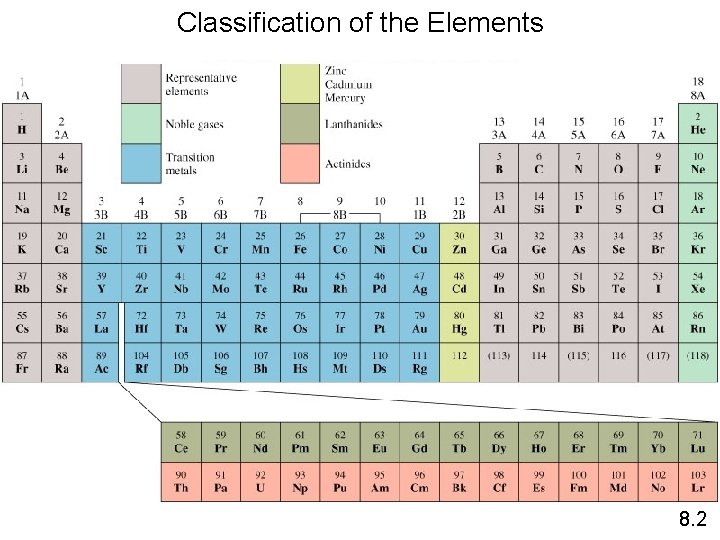
Classification of the Elements 8. 2

-1 -2 -3 +3 +2 +1 Cations and Anions Of Representative Elements 8. 2

To Understand Periodic Relationships Among the Elements: 1. 2. 3. Be able to define the property or characteristic that is being compared Keep in mind how the Periodic Table is built up Specify the direction (top to bottom or left to right) along which the property or characteristic changes 4. Use the words increases or decreases to describe the trend in a given direction Ex. “Atomic size decreases from left to right” 5. Use the phrases greater than or less than when comparing the characteristics of 2 elements Ex. “The electron affinity of F is greater then Cl” 5. When comparing how two characteristics change relative to each other, use the phrases: “Increases with increasing/decreasing ______” or “Decreases with increasing/decreasing ______” Ex. “Ionization Energy increases with increasing nuclear charge and decreasing ionic radius” The properties that will be studied are as follows: Atomic and Ionic Size, Effective Nuclear charge, Ionization Energy, Electron Affinity, Electronegativity, Reactivity, Acidity

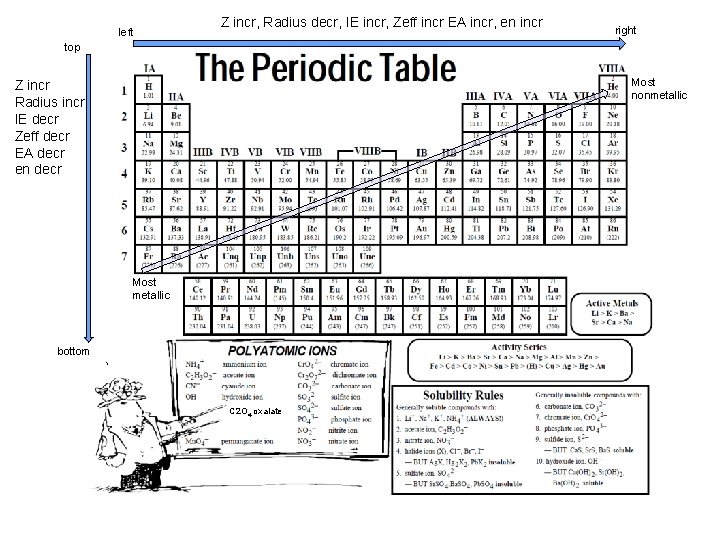
Z incr Radius incr IE decr Zeff decr EA decr en decr bottom Radius incr, IE decr, Z incr, Zeff decr EA decr, en decr top left Z incr, Radius decr, IE incr, Zeff incr EA incr, en incr right Most nonmetallic Most metallic C 2 O 4 oxalate

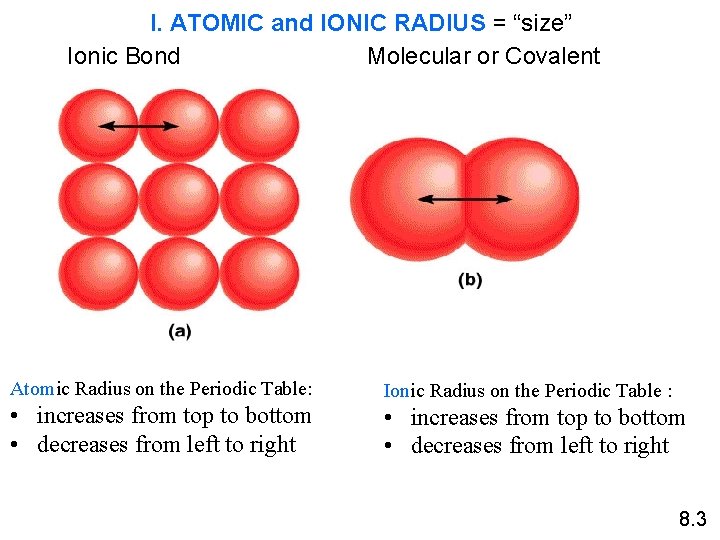
I. ATOMIC and IONIC RADIUS = “size” Ionic Bond Molecular or Covalent Atomic Radius on the Periodic Table: • increases from top to bottom • decreases from left to right Ionic Radius on the Periodic Table : • increases from top to bottom • decreases from left to right 8. 3

Trend in Atomic Radius – Main Group • Different methods for measuring the radius of an atom, and they give slightly different trends ü van der Waals radius = nonbonding ü covalent radius = bonding radius ü atomic radius is an average radius of an atom based on measuring large numbers of elements and compounds • Atomic Radius Increases down group ü valence shell farther from nucleus ü effective nuclear charge fairly close • Atomic Radius Decreases across period (left to right) ü adding electrons to same valence shell ü effective nuclear charge increases ü valence shell held closer Tro, Chemistry: A Molecular Approach 35

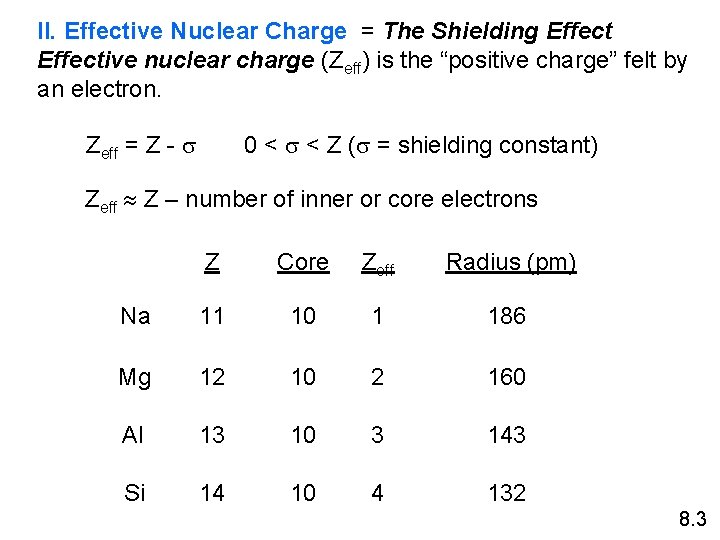
II. Effective Nuclear Charge = The Shielding Effective nuclear charge (Zeff) is the “positive charge” felt by an electron. Zeff = Z - s 0 < s < Z (s = shielding constant) Zeff Z – number of inner or core electrons Z Core Zeff Radius (pm) Na 11 10 1 186 Mg 12 10 2 160 Al 13 10 3 143 Si 14 10 4 132 8. 3

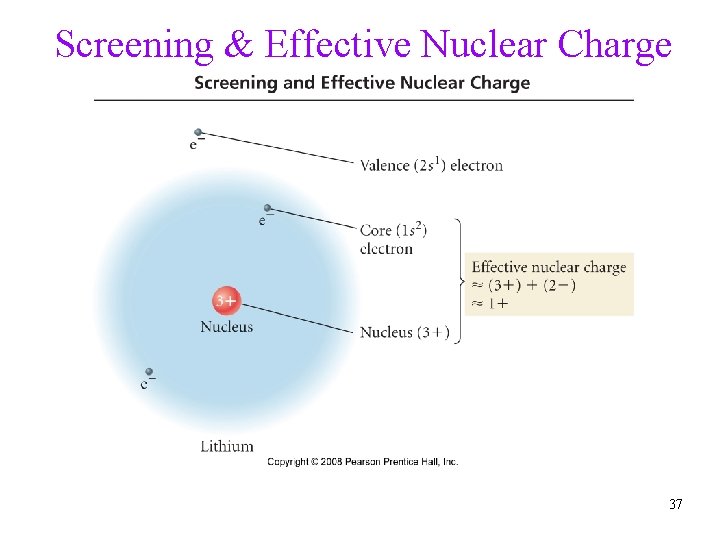
Screening & Effective Nuclear Charge 37

• • Effective Nuclear Charge in a multi-electron system, electrons are simultaneously attracted to the nucleus and repelled by each other outer electrons are shielded from full strength of nucleus üscreening effective nuclear charge is net positive charge that is attracting a particular electron Z is nuclear charge, S is electrons in lower energy levels ü electrons in same energy level contribute to screening, but very little ü effective nuclear charge on sublevels trend, s > p > d > f Zeffective = Z - S Tro, Chemistry: A Molecular Approach 38

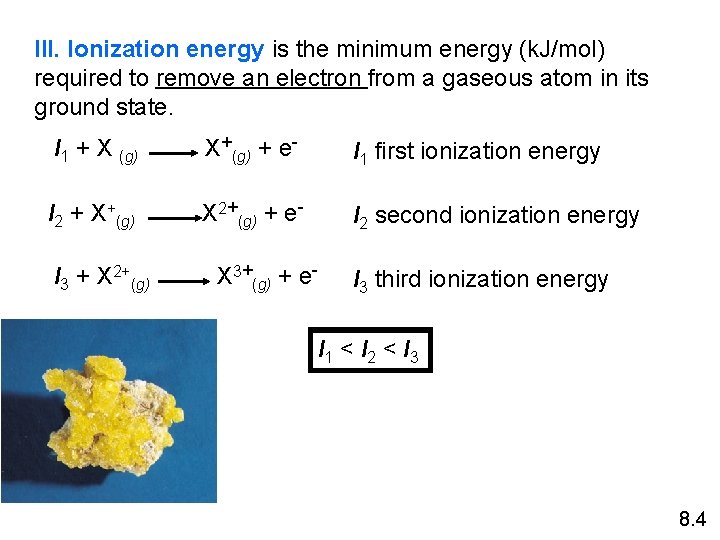
III. Ionization energy is the minimum energy (k. J/mol) required to remove an electron from a gaseous atom in its ground state. I 1 + X (g) X+(g) + e- I 1 first ionization energy I 2 + X+(g) X 2+(g) + e- I 2 second ionization energy I 3 + X 2+(g) X 3+(g) + e- I 3 third ionization energy I 1 < I 2 < I 3 8. 4

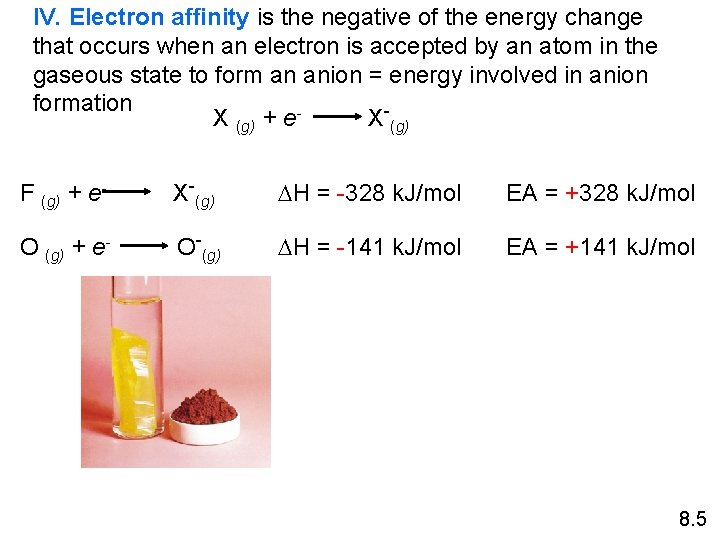
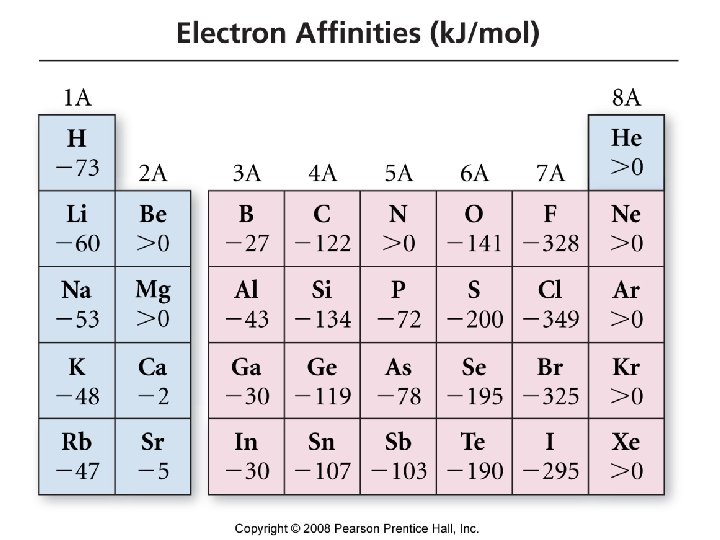
IV. Electron affinity is the negative of the energy change that occurs when an electron is accepted by an atom in the gaseous state to form an anion = energy involved in anion formation X (g) + e. X-(g) F (g) + e- X-(g) DH = -328 k. J/mol EA = +328 k. J/mol O (g) + e- O-(g) DH = -141 k. J/mol EA = +141 k. J/mol 8. 5

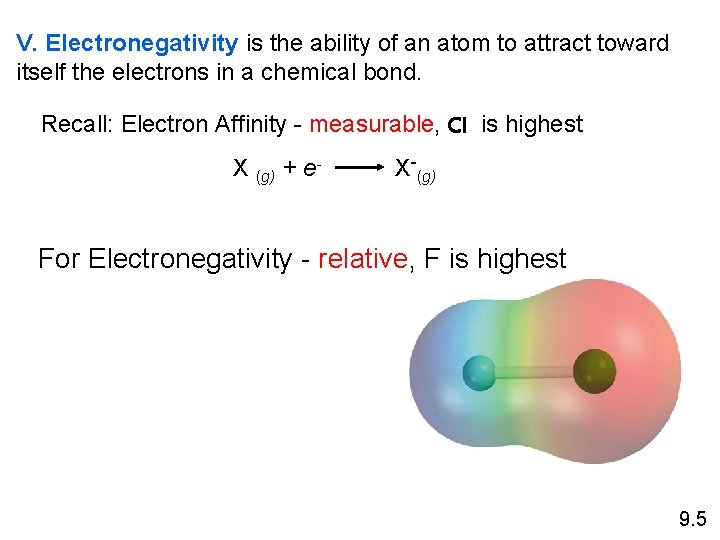
V. Electronegativity is the ability of an atom to attract toward itself the electrons in a chemical bond. Recall: Electron Affinity - measurable, Cl is highest X (g) + e- X-(g) For Electronegativity - relative, F is highest 9. 5

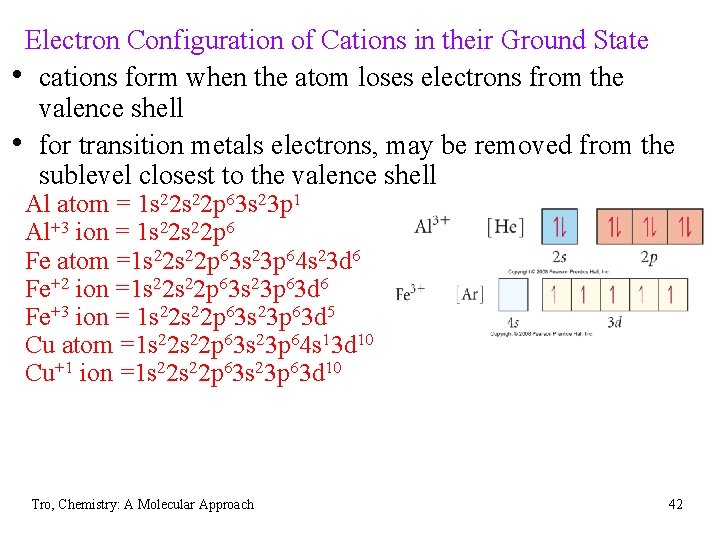
Electron Configuration of Cations in their Ground State • cations form when the atom loses electrons from the valence shell • for transition metals electrons, may be removed from the sublevel closest to the valence shell Al atom = 1 s 22 p 63 s 23 p 1 Al+3 ion = 1 s 22 p 6 Fe atom =1 s 22 p 63 s 23 p 64 s 23 d 6 Fe+2 ion =1 s 22 p 63 s 23 p 63 d 6 Fe+3 ion = 1 s 22 p 63 s 23 p 63 d 5 Cu atom =1 s 22 p 63 s 23 p 64 s 13 d 10 Cu+1 ion =1 s 22 p 63 s 23 p 63 d 10 Tro, Chemistry: A Molecular Approach 42

Magnetic Properties of Transition Metal Atoms & Ions • electron configurations that result in unpaired electrons mean that the atom or ion will have a net magnetic field – this is called paramagnetism ü will be attracted to a magnetic field • electron configurations that result in all paired electrons mean that the atom or ion will have no magnetic field – this is called diamagnetism ü slightly repelled by a magnetic field • both Zn atoms and Zn 2+ ions are diamagnetic, showing that the two 4 s electrons are lost before the 3 d ü Zn atoms [Ar]4 s 23 d 10 ü Zn 2+ ions [Ar]4 s 03 d 10 Tro, Chemistry: A Molecular Approach 43

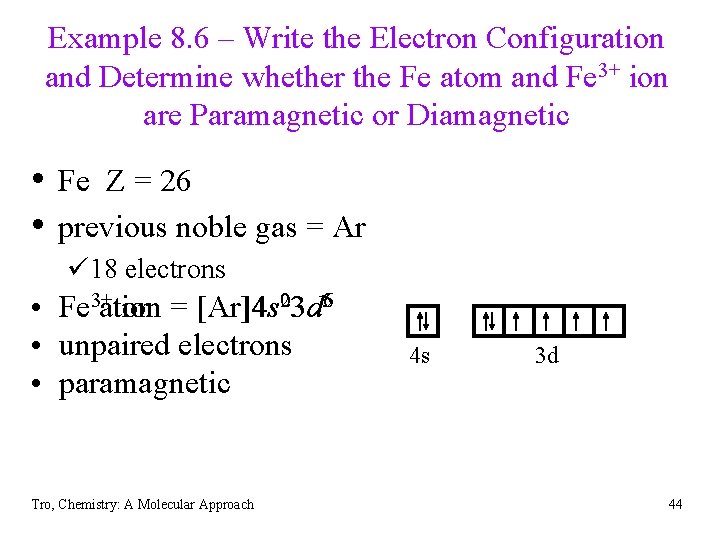
Example 8. 6 – Write the Electron Configuration and Determine whether the Fe atom and Fe 3+ ion are Paramagnetic or Diamagnetic • Fe Z = 26 • previous noble gas = Ar ü 18 electrons • Fe 3+atom ion = [Ar]4 s 023 d 56 • unpaired electrons • paramagnetic Tro, Chemistry: A Molecular Approach 4 s 3 d 44

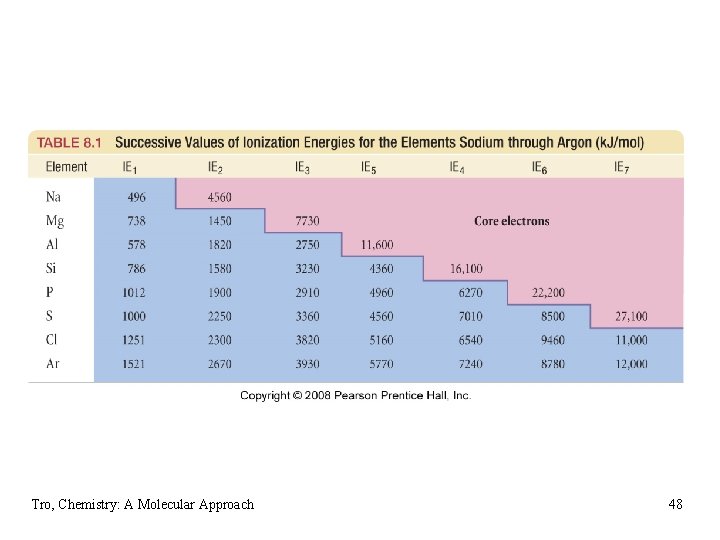
Ionization Energy • minimum energy needed to remove an electron from an atom ügas state üendothermic process üvalence electron easiest to remove üM(g) + IE 1 M 1+(g) + 1 eüM+1(g) + IE 2 M 2+(g) + 1 eØfirst ionization energy = energy to remove electron from neutral atom; 2 nd IE = energy to remove from +1 ion; etc. Tro, Chemistry: A Molecular Approach 45

General Trends in 1 st Ionization Energy • larger the effective nuclear charge on the • • electron, the more energy it takes to remove it the farther the most probable distance the electron is from the nucleus, the less energy it takes to remove it 1 st IE decreases down the group üvalence electron farther from nucleus • 1 st IE generally increases across the period üeffective nuclear charge increases Tro, Chemistry: A Molecular Approach 46

Example 8. 8 – Choose the Atom in Each Pair with the Higher First Ionization Energy 1) 2) 3) 4) Al or S S, Al is further left As or Sb Sb, Sb is further down N or Si, Si Si is further down & left O or Cl? opposing trends Tro, Chemistry: A Molecular Approach 47

Tro, Chemistry: A Molecular Approach 48

Trends in Electron Affinity • energy released when an neutral atom gains an electron ü gas state ü M(g) + 1 e- M-1(g) + EA • defined as exothermic (-), but may actually be endothermic (+) ü alkali earth metals & noble gases endothermic, WHY? • more energy released (more -); the larger the EA • generally increases across period ü becomes more negative from left to right ü not absolute ü lowest EA in period = alkali earth metal or noble gas ü highest EA in period = halogen Tro, Chemistry: A Molecular Approach 49

Tro, Chemistry: A Molecular Approach 50

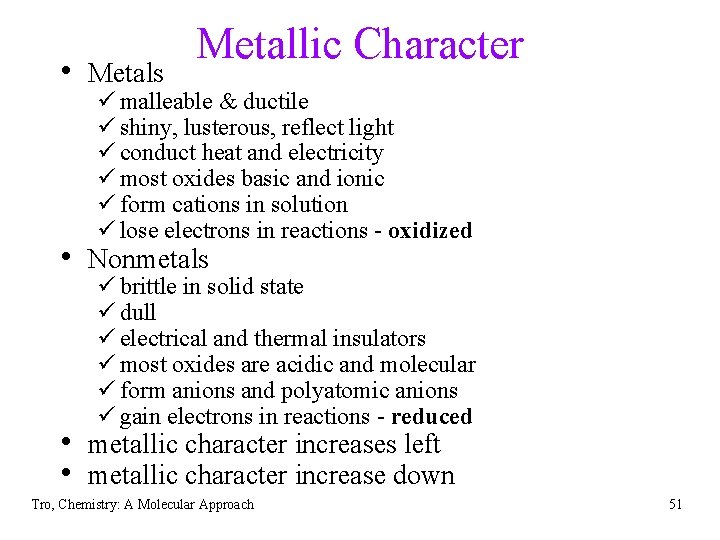
• Metals Metallic Character ü malleable & ductile ü shiny, lusterous, reflect light ü conduct heat and electricity ü most oxides basic and ionic ü form cations in solution ü lose electrons in reactions - oxidized • Nonmetals ü brittle in solid state ü dull ü electrical and thermal insulators ü most oxides are acidic and molecular ü form anions and polyatomic anions ü gain electrons in reactions - reduced • metallic character increases left • metallic character increase down Tro, Chemistry: A Molecular Approach 51

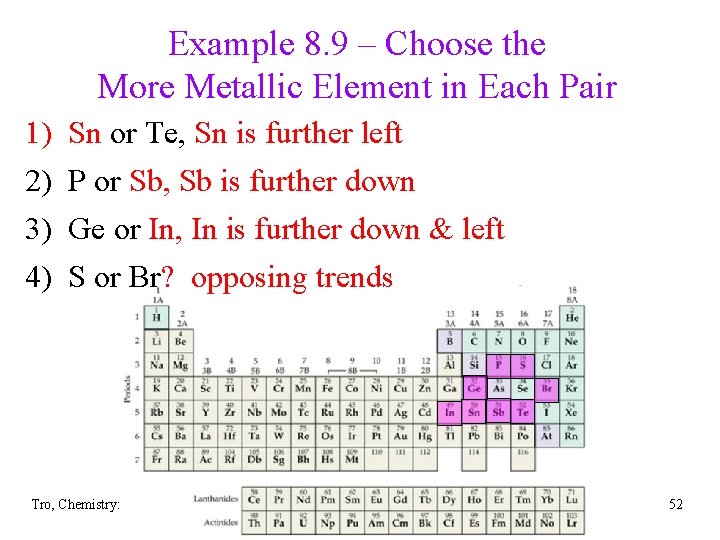
Example 8. 9 – Choose the More Metallic Element in Each Pair 1) 2) 3) 4) Sn or Te, Te Sn is further left P or Sb, Sb Sb is further down Ge or In In, In is further down & left S or Br? opposing trends Tro, Chemistry: A Molecular Approach 52

Tro, Chemistry: A Molecular Approach 53

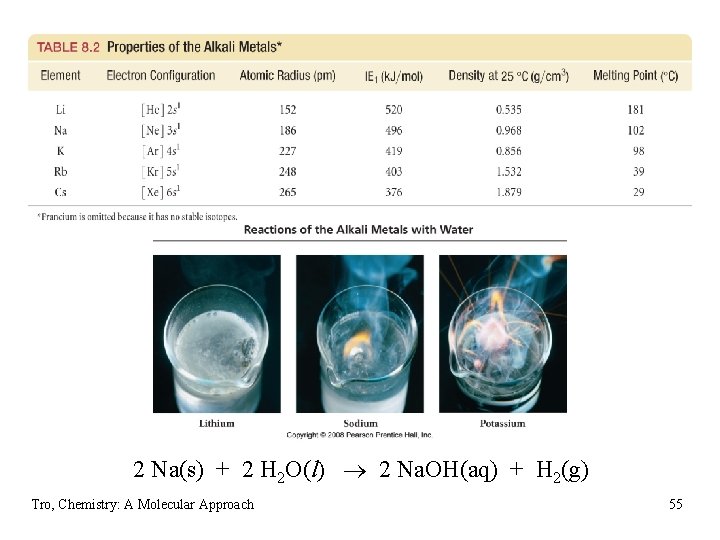
Trends in Alkali Metals • atomic radius increases down the column • ionization energy decreases down the column • very low ionization energies ü good reducing agents, easy to oxidize ü very reactive, not found uncombined in nature ü react with nonmetals to form salts ü compounds generally soluble in water found in seawater • electron affinity decreases down the column • melting point decreases down the column ü all very low MP for metals • density increases down the column ü except K ü in general, the increase in mass is greater than the increase in volume Tro, Chemistry: A Molecular Approach 54

2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g) Tro, Chemistry: A Molecular Approach 55

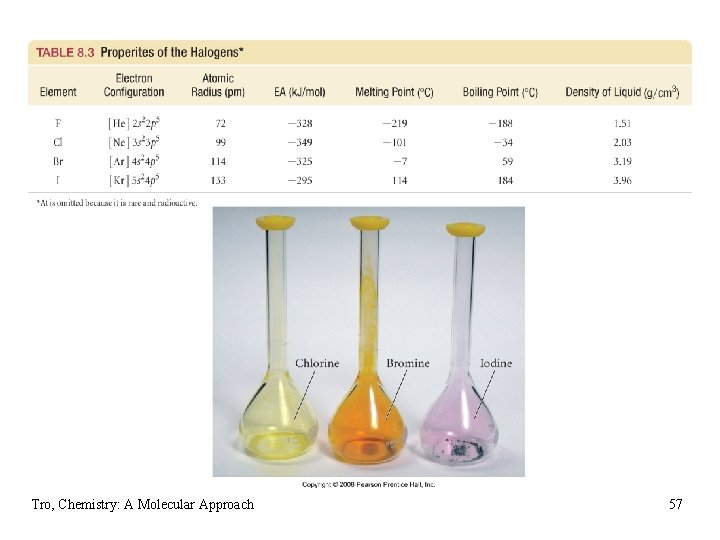
Trends in the Halogens • atomic radius increases down the column • ionization energy decreases down the column • very high electron affinities ü good oxidizing agents, easy to reduce ü very reactive, not found uncombined in nature ü react with metals to form salts ü compounds generally soluble in water found in seawater • reactivity increases down the column • react with hydrogen to form HX, acids • melting point and boiling point increases down the • column density increases down the column ü in general, the increase in mass is greater than the increase in volume Tro, Chemistry: A Molecular Approach 56

Tro, Chemistry: A Molecular Approach 57

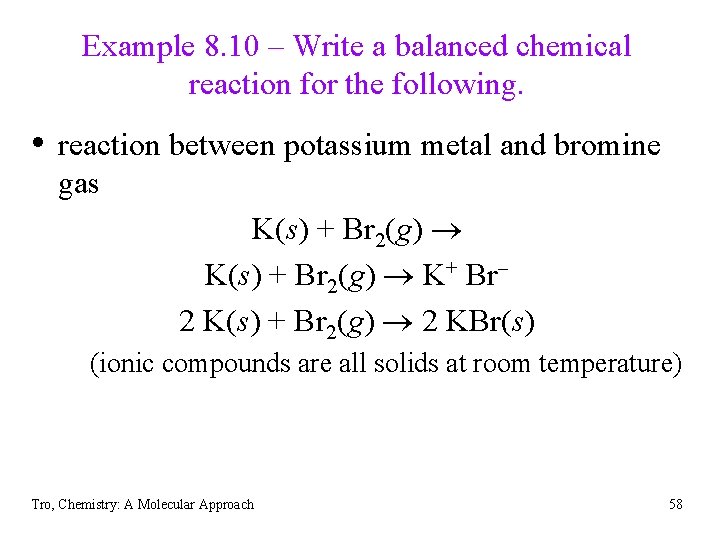
Example 8. 10 – Write a balanced chemical reaction for the following. • reaction between potassium metal and bromine gas K(s) + Br 2(g) K+ Br 2 K(s) + Br 2(g) 2 KBr(s) (ionic compounds are all solids at room temperature) Tro, Chemistry: A Molecular Approach 58

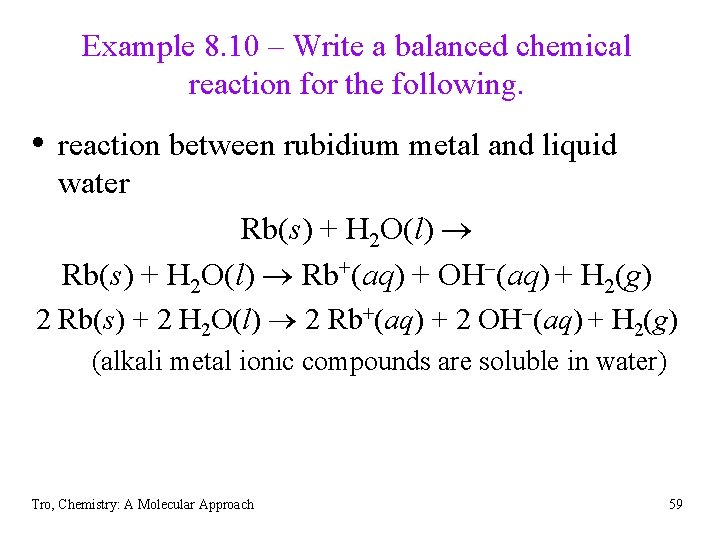
Example 8. 10 – Write a balanced chemical reaction for the following. • reaction between rubidium metal and liquid water Rb(s) + H 2 O(l) Rb+(aq) + OH (aq) + H 2(g) 2 Rb(s) + 2 H 2 O(l) 2 Rb+(aq) + 2 OH (aq) + H 2(g) (alkali metal ionic compounds are soluble in water) Tro, Chemistry: A Molecular Approach 59

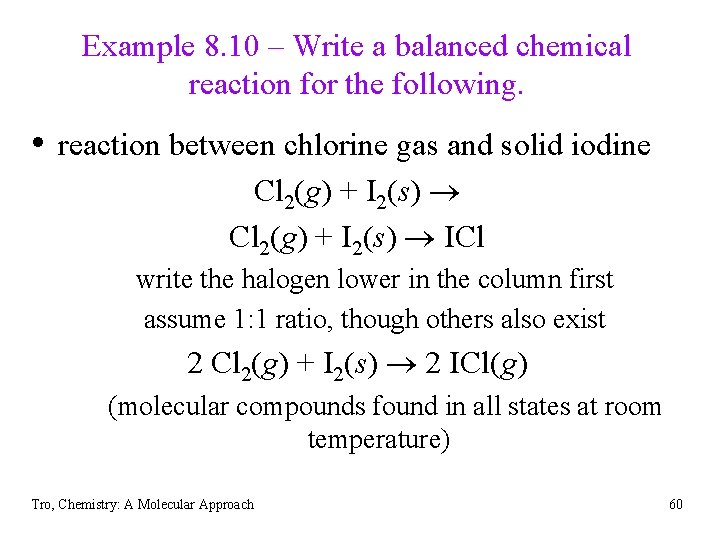
Example 8. 10 – Write a balanced chemical reaction for the following. • reaction between chlorine gas and solid iodine Cl 2(g) + I 2(s) ICl write the halogen lower in the column first assume 1: 1 ratio, though others also exist 2 Cl 2(g) + I 2(s) 2 ICl(g) (molecular compounds found in all states at room temperature) Tro, Chemistry: A Molecular Approach 60

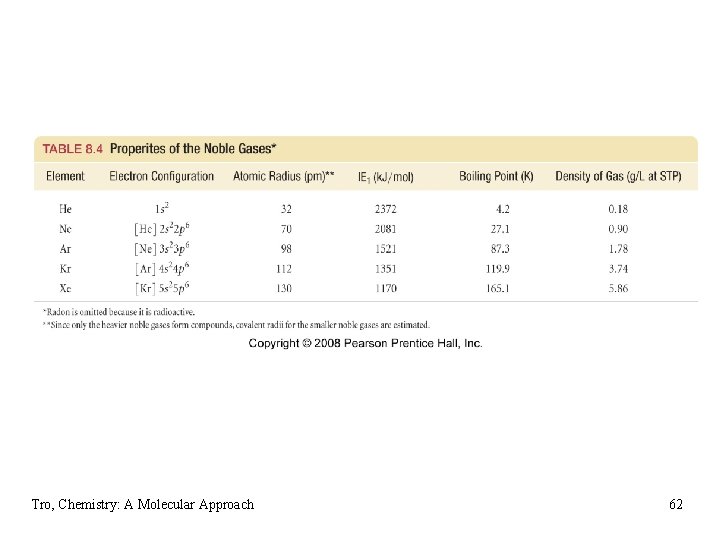
Trends in the Noble Gases • atomic radius increases down the column • ionization energy decreases down the column ü very high IE • very unreactive ü only found uncombined in nature ü used as “inert” atmosphere when reactions with other gases would be undersirable • melting point and boiling point increases down the column ü all gases at room temperature ü very low boiling points • density increases down the column ü in general, the increase in mass is greater than the increase in volume Tro, Chemistry: A Molecular Approach 61

Tro, Chemistry: A Molecular Approach 62
 Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach What is a covalent bond simple definition
What is a covalent bond simple definition Ionic covalent metallic
Ionic covalent metallic Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Ap chemistry molecular geometry
Ap chemistry molecular geometry Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Approach chemistry chalk chapter
Approach chemistry chalk chapter A switched wan is normally implemented as a network
A switched wan is normally implemented as a network Theoretical models of counseling
Theoretical models of counseling Waterfall and shower approach
Waterfall and shower approach Multiple approach-avoidance
Multiple approach-avoidance Bandura's reciprocal determinism
Bandura's reciprocal determinism Research approach means
Research approach means Traditional approach to system development
Traditional approach to system development Tony wagner's seven survival skills
Tony wagner's seven survival skills Vsepr theory assignment
Vsepr theory assignment Shapes of molecules in chemistry
Shapes of molecules in chemistry Molecular geometry of pf3
Molecular geometry of pf3 Crash course molecular biology
Crash course molecular biology Molecular level vs cellular level
Molecular level vs cellular level Aniline uv spectrum
Aniline uv spectrum Sf5cl lewis structure
Sf5cl lewis structure Kenitic molecular theory
Kenitic molecular theory Properties of network covalent solids
Properties of network covalent solids Kinetic molecular theory of solids
Kinetic molecular theory of solids The chemical formula of a compound indicates
The chemical formula of a compound indicates Criterios de amsterdam cancer de colon
Criterios de amsterdam cancer de colon Unit chemical bonding molecular geometry
Unit chemical bonding molecular geometry Palindromic sequence
Palindromic sequence Molecular rebar
Molecular rebar Covalent molecular substances
Covalent molecular substances Empirical formula vs molecular formula
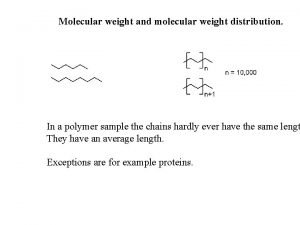
Empirical formula vs molecular formula Number-average molecular weight
Number-average molecular weight Molecular clock theory
Molecular clock theory Percent composition of magnesium nitrate
Percent composition of magnesium nitrate Molecular mass of kmno4
Molecular mass of kmno4 Empirical and molecular formula
Empirical and molecular formula How to find empirical formula from percent
How to find empirical formula from percent Molecular modelling laboratory
Molecular modelling laboratory Molecular replacement method
Molecular replacement method Condensed structural formula of alkenes
Condensed structural formula of alkenes Naming compounds and writing formulas
Naming compounds and writing formulas Binary molecular formula
Binary molecular formula N srinivasan iisc passed away
N srinivasan iisc passed away Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Number-average molecular weight
Number-average molecular weight Cl4 lewis structure
Cl4 lewis structure Kahoot shapes
Kahoot shapes Nh3 shape and polarity
Nh3 shape and polarity Patterson function
Patterson function Patterson
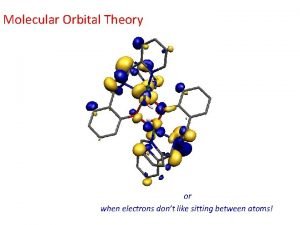
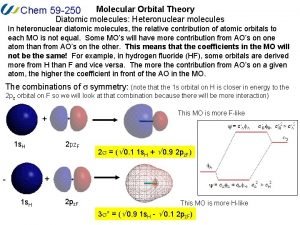
Patterson Molecular orbital theory
Molecular orbital theory Heteronuclear diatomic molecules molecular orbital diagram
Heteronuclear diatomic molecules molecular orbital diagram Li2 molecular orbital diagram
Li2 molecular orbital diagram Cml treatment milestones
Cml treatment milestones H - c
H - c Define microbiology
Define microbiology Vsepr shapes
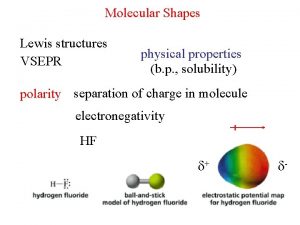
Vsepr shapes Polar and nonpolar
Polar and nonpolar