Chemistry A Molecular Approach 2 nd Ed Nivaldo


























- Slides: 112

Chemistry: A Molecular Approach, 2 nd Ed. Nivaldo Tro Chapter 19 Radioactivity and Nuclear Chemistry Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA Copyright 2011 Pearson Education, Inc.

Nuclear Medicine • Changes in the structure of the nucleus are • used in many ways in medicine Nuclear radiation can be used to visualize or test structures in your body to see if they are operating properly ü e. g. labeling atoms so their intake and output can be monitored • Nuclear radiation can also be used to treat diseases because the radiation is ionizing, allowing it to attack unhealthy tissue Tro: Chemistry: A Molecular Approach 2 Copyright 2011 Pearson Education, Inc.

The Discovery of Radioactivity • Antoine-Henri Becquerel designed an experiment to determine if phosphorescent minerals also gave off X-rays ü phosphorescence is the long-lived emission of light by atoms or molecules that sometimes occurs after they absorb light ü X-rays are detected by their ability to penetrate matter and expose a photographic plate Tro: Chemistry: A Molecular Approach 3 Copyright 2011 Pearson Education, Inc.

The Discovery of Radioactivity • Becquerel discovered that certain minerals were • constantly producing energy rays that could penetrate matter Becquerel determined that 1. all the minerals that produced these rays contained uranium 2. the rays were produced even though the mineral was not exposed to outside energy • He called them uranic rays because they were emitted from minerals that contained uranium ü like X-rays ü but not related to phosphorescence • Energy apparently being produced without energy input? ? Tro: Chemistry: A Molecular Approach 4 Copyright 2011 Pearson Education, Inc.

The Curies • Marie Curie broke down these • • minerals and used an electroscope to detect where the uranic rays were coming from She discovered the rays were emitted from specific elements She also discovered new elements by detecting their rays ü radium named for its green phosphorescence ü polonium named for her homeland • Because these rays were no longer just a property of uranium, she renamed it radioactivity Tro: Chemistry: A Molecular Approach 5 Copyright 2011 Pearson Education, Inc.

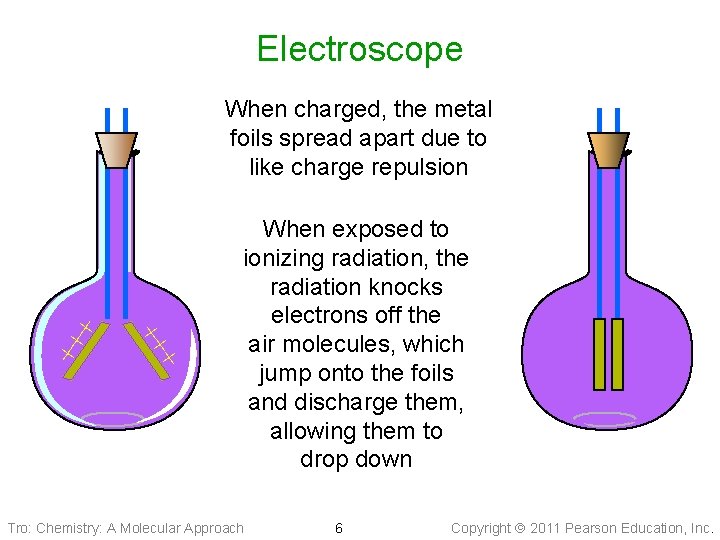
Electroscope ++ + When charged, the metal foils spread apart due to like charge repulsion When exposed to ionizing radiation, the radiation knocks electrons off the air molecules, which jump onto the foils and discharge them, allowing them to drop down Tro: Chemistry: A Molecular Approach 6 Copyright 2011 Pearson Education, Inc.

Other Properties of Radioactivity • Radioactive rays can ionize matter ü cause uncharged matter to become charged ü basis of Geiger Counter and electroscope • Radioactive rays have high energy • Radioactive rays can penetrate matter • Radioactive rays cause phosphorescent chemicals to glow ü basis of scintillation counter Tro: Chemistry: A Molecular Approach 7 Copyright 2011 Pearson Education, Inc.

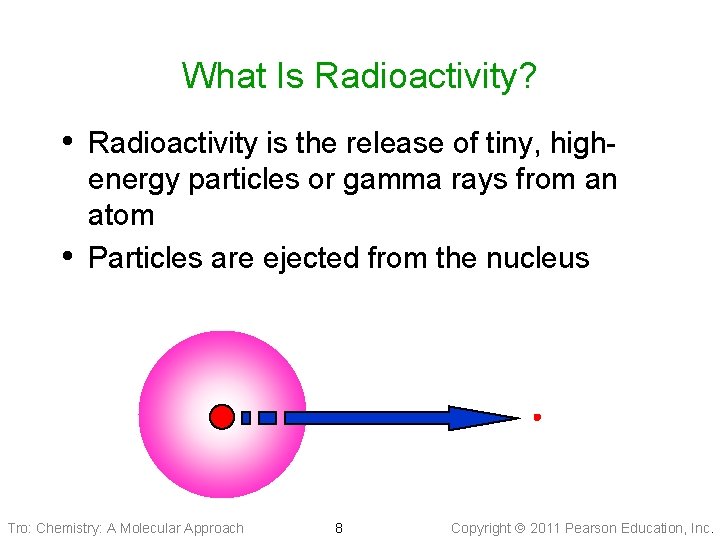
What Is Radioactivity? • Radioactivity is the release of tiny, high • energy particles or gamma rays from an atom Particles are ejected from the nucleus Tro: Chemistry: A Molecular Approach 8 Copyright 2011 Pearson Education, Inc.

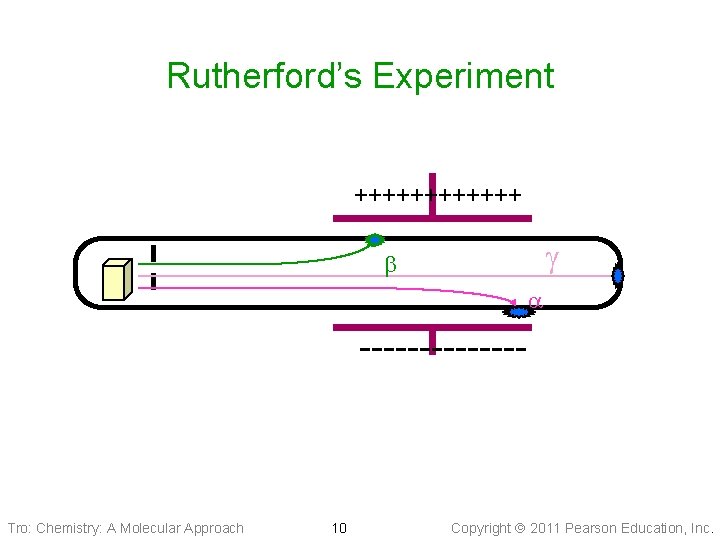
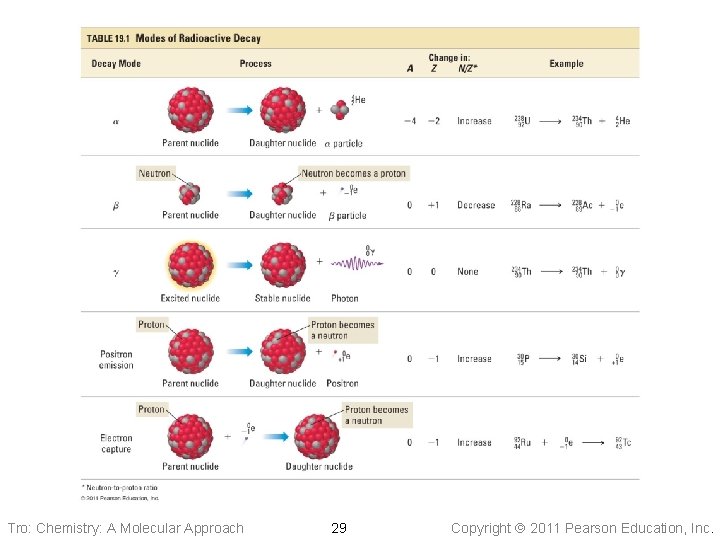
Types of Radioactive Decay • Rutherford discovered three types of rays ü alpha ( ) rays Øhave a charge of +2 c. u. and a mass of 4 amu Øwhat we now know to be helium nucleus ü beta ( ) rays Øhave a charge of − 1 c. u. and negligible mass Øelectron-like ü gamma (g) rays Øform of light energy (not a particle like and ) • In addition, some unstable nuclei emit positrons ü like a positively charged electron • Some unstable nuclei will undergo electron capture ü a low energy electron is pulled into the nucleus Tro: Chemistry: A Molecular Approach 9 Copyright 2011 Pearson Education, Inc.

Rutherford’s Experiment ++++++ g ------- Tro: Chemistry: A Molecular Approach 10 Copyright 2011 Pearson Education, Inc.

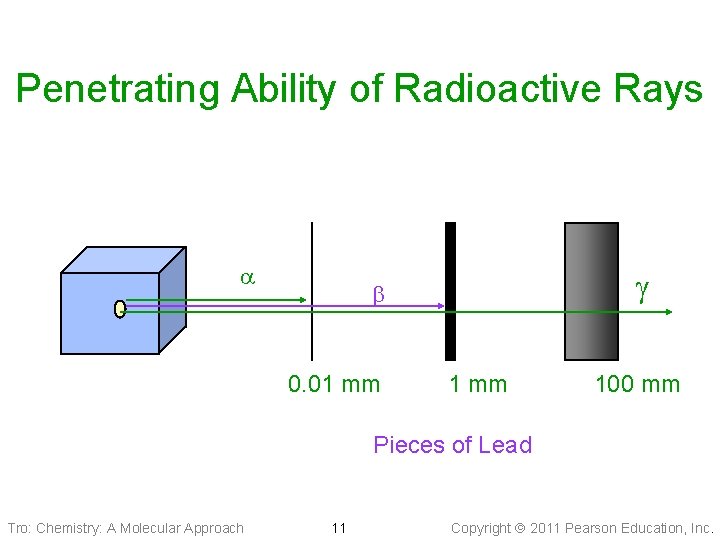
Penetrating Ability of Radioactive Rays g 0. 01 mm 100 mm Pieces of Lead Tro: Chemistry: A Molecular Approach 11 Copyright 2011 Pearson Education, Inc.

Facts About the Nucleus • Very small volume compared to volume of • • • the atom Essentially entire mass of atom Very dense Composed of protons and neutrons that are tightly held together ü the particles that make up the nucleus are called nucleons Tro: Chemistry: A Molecular Approach 12 Copyright 2011 Pearson Education, Inc.

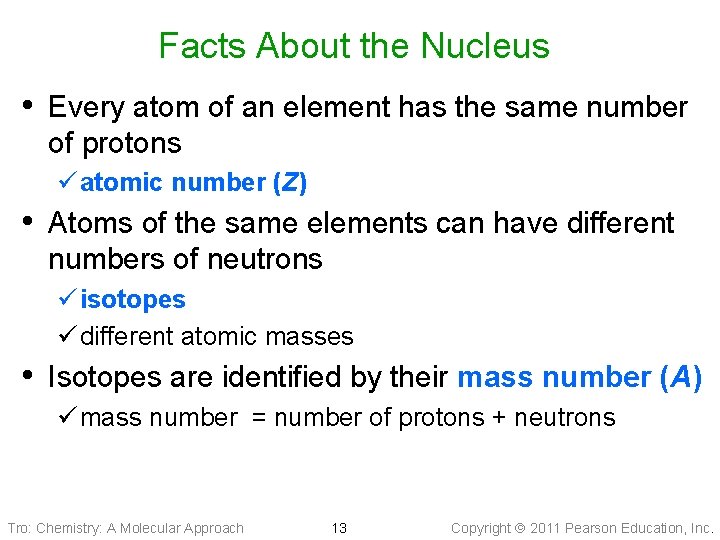
Facts About the Nucleus • Every atom of an element has the same number of protons ü atomic number (Z) • Atoms of the same elements can have different numbers of neutrons ü isotopes ü different atomic masses • Isotopes are identified by their mass number (A) ü mass number = number of protons + neutrons Tro: Chemistry: A Molecular Approach 13 Copyright 2011 Pearson Education, Inc.

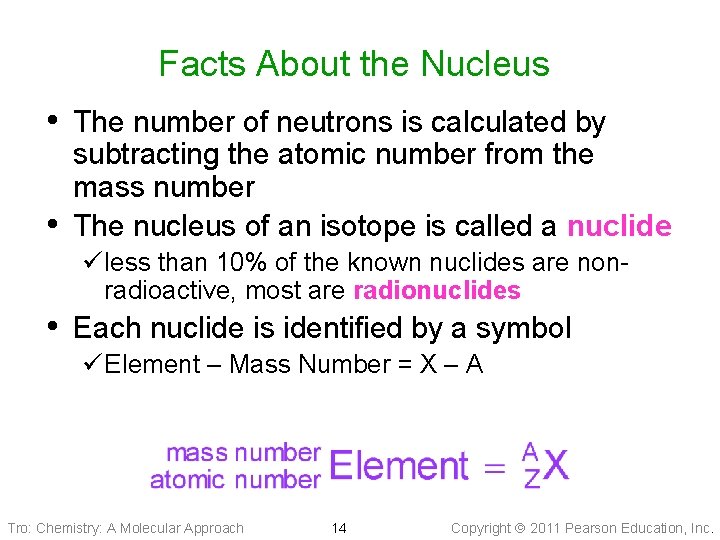
Facts About the Nucleus • The number of neutrons is calculated by • subtracting the atomic number from the mass number The nucleus of an isotope is called a nuclide ü less than 10% of the known nuclides are nonradioactive, most are radionuclides • Each nuclide is identified by a symbol ü Element – Mass Number = X – A Tro: Chemistry: A Molecular Approach 14 Copyright 2011 Pearson Education, Inc.

Radioactivity • Radioactive nuclei spontaneously decompose into smaller nuclei ü radioactive decay ü we say that radioactive nuclei are unstable ü decomposing involves the nuclide emitting a particle and/or energy • The parent nuclide is the nucleus that is • • undergoing radioactive decay The daughter nuclide is the new nucleus that is made All nuclides with 84 or more protons are radioactive Tro: Chemistry: A Molecular Approach 15 Copyright 2011 Pearson Education, Inc.

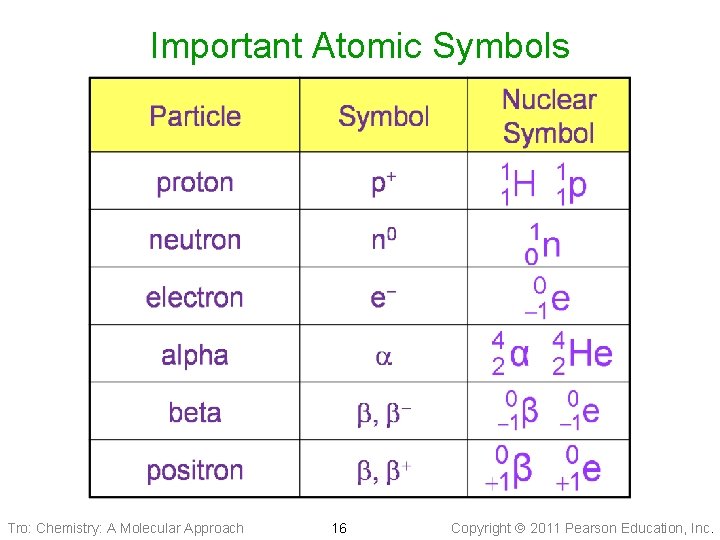
Important Atomic Symbols Tro: Chemistry: A Molecular Approach 16 Copyright 2011 Pearson Education, Inc.

Transmutation • Rutherford discovered that during the radioactive process, atoms of one element are changed into atoms of a different element – transmutation ü showing that statement 3 of Dalton’s Atomic Theory is not valid all the time, only for chemical reactions • For one element to change into another, the number of protons in the nucleus must change! Tro: Chemistry: A Molecular Approach 17 Copyright 2011 Pearson Education, Inc.

Chemical Processes vs. Nuclear Processes • Chemical reactions involve changes in the electronic structure of the atom ü atoms gain, lose, or share electrons ü no change in the nuclei occurs • Nuclear reactions involve changes in the structure of the nucleus ü when the number of protons in the nucleus changes, the atom becomes a different element Tro: Chemistry: A Molecular Approach 18 Copyright 2011 Pearson Education, Inc.

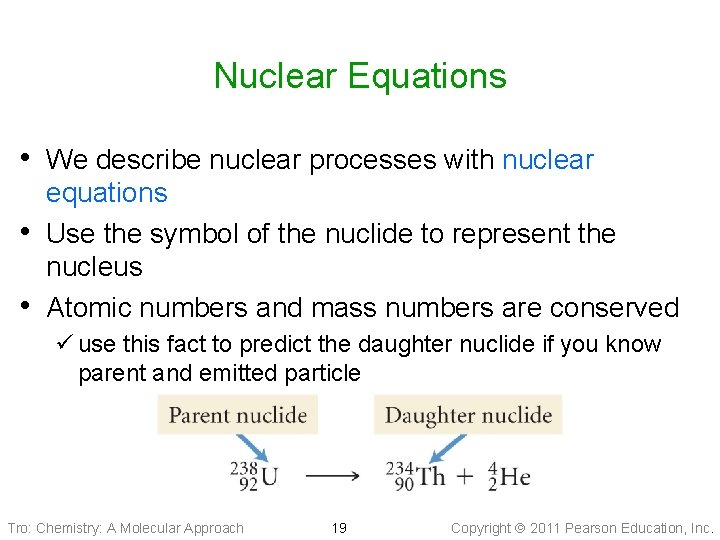
Nuclear Equations • We describe nuclear processes with nuclear • • equations Use the symbol of the nuclide to represent the nucleus Atomic numbers and mass numbers are conserved ü use this fact to predict the daughter nuclide if you know parent and emitted particle Tro: Chemistry: A Molecular Approach 19 Copyright 2011 Pearson Education, Inc.

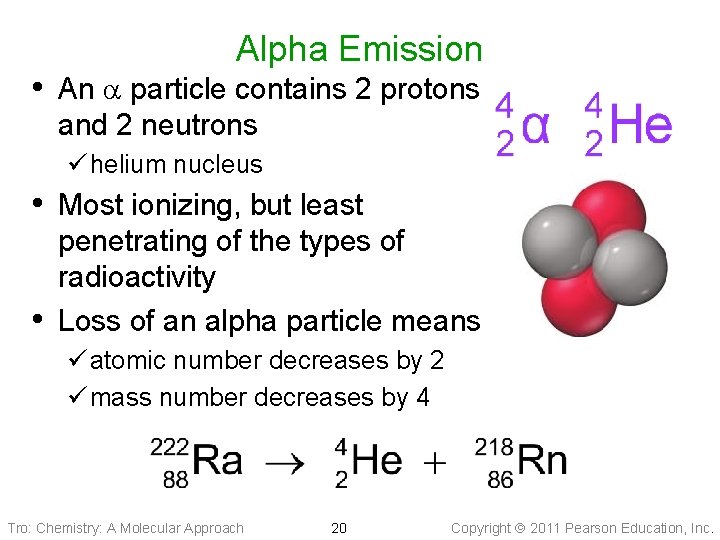
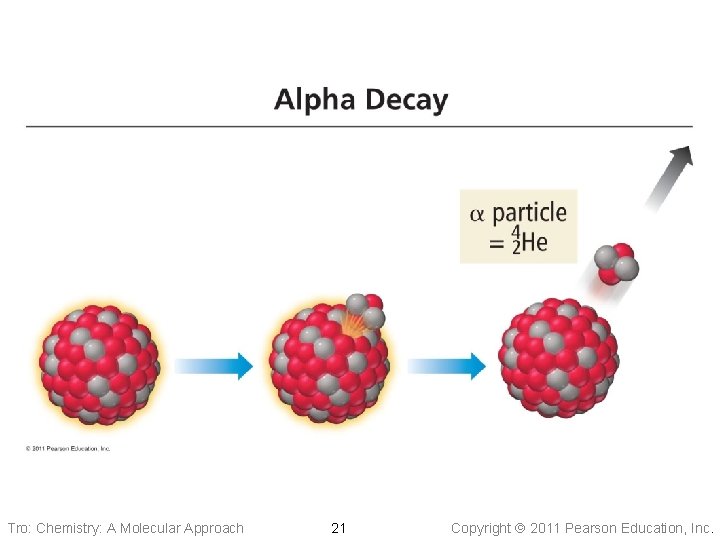
Alpha Emission • An particle contains 2 protons and 2 neutrons ü helium nucleus • Most ionizing, but least • penetrating of the types of radioactivity Loss of an alpha particle means ü atomic number decreases by 2 ü mass number decreases by 4 Tro: Chemistry: A Molecular Approach 20 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 21 Copyright 2011 Pearson Education, Inc.

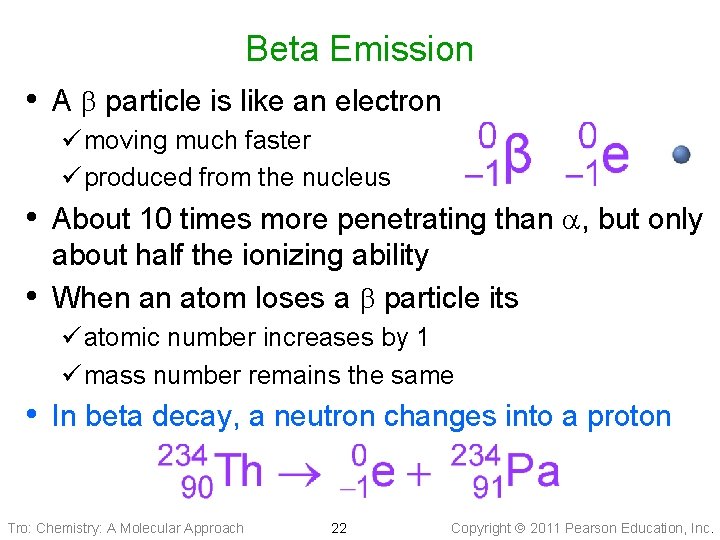
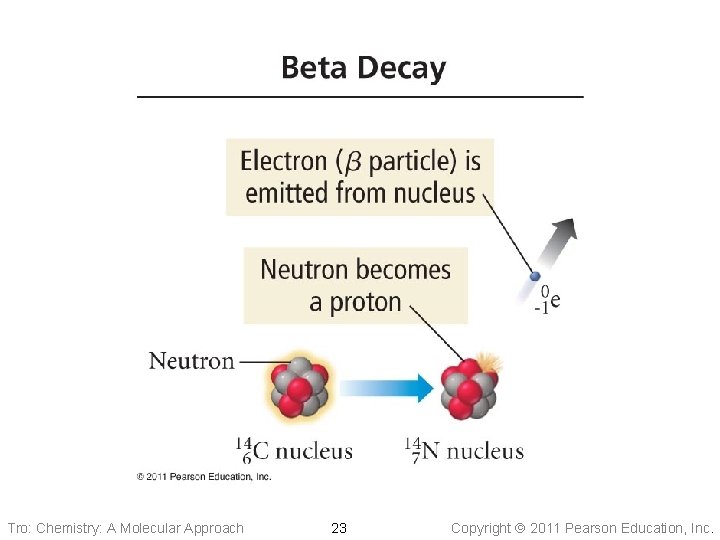
Beta Emission • A particle is like an electron ü moving much faster ü produced from the nucleus • About 10 times more penetrating than , but only • about half the ionizing ability When an atom loses a particle its ü atomic number increases by 1 ü mass number remains the same • In beta decay, a neutron changes into a proton Tro: Chemistry: A Molecular Approach 22 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 23 Copyright 2011 Pearson Education, Inc.

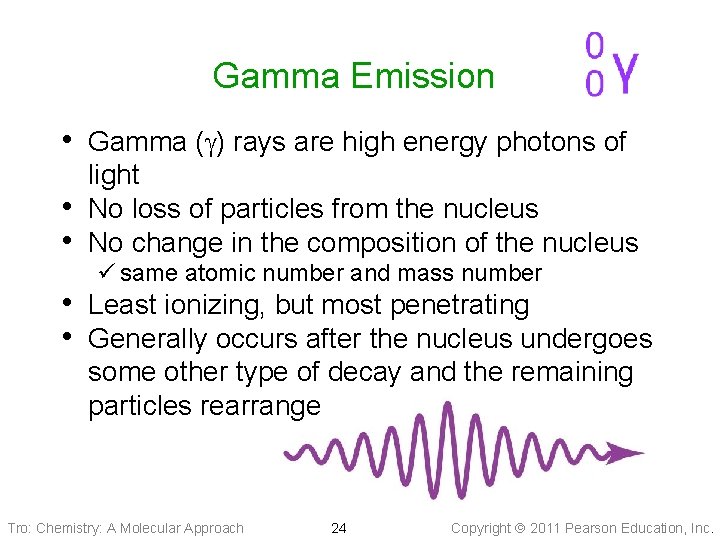
Gamma Emission • Gamma (g) rays are high energy photons of • • light No loss of particles from the nucleus No change in the composition of the nucleus ü same atomic number and mass number • Least ionizing, but most penetrating • Generally occurs after the nucleus undergoes some other type of decay and the remaining particles rearrange Tro: Chemistry: A Molecular Approach 24 Copyright 2011 Pearson Education, Inc.

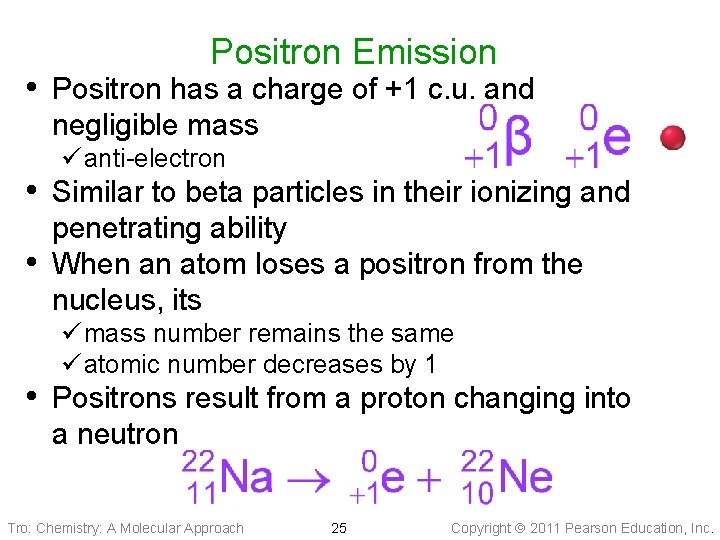
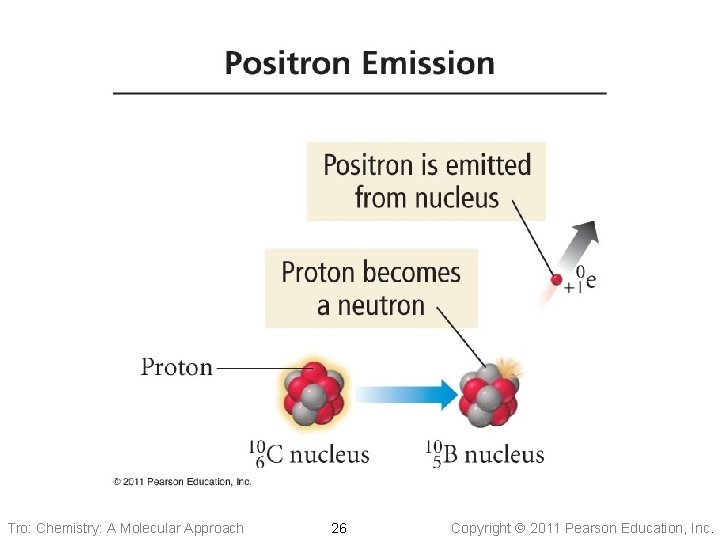
Positron Emission • Positron has a charge of +1 c. u. and negligible mass ü anti-electron • Similar to beta particles in their ionizing and • penetrating ability When an atom loses a positron from the nucleus, its ü mass number remains the same ü atomic number decreases by 1 • Positrons result from a proton changing into a neutron Tro: Chemistry: A Molecular Approach 25 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 26 Copyright 2011 Pearson Education, Inc.

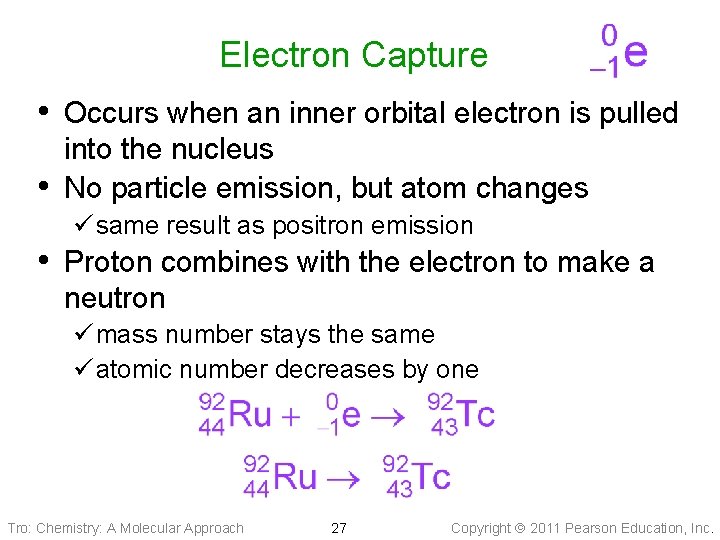
Electron Capture • Occurs when an inner orbital electron is pulled • into the nucleus No particle emission, but atom changes ü same result as positron emission • Proton combines with the electron to make a neutron ü mass number stays the same ü atomic number decreases by one Tro: Chemistry: A Molecular Approach 27 Copyright 2011 Pearson Education, Inc.

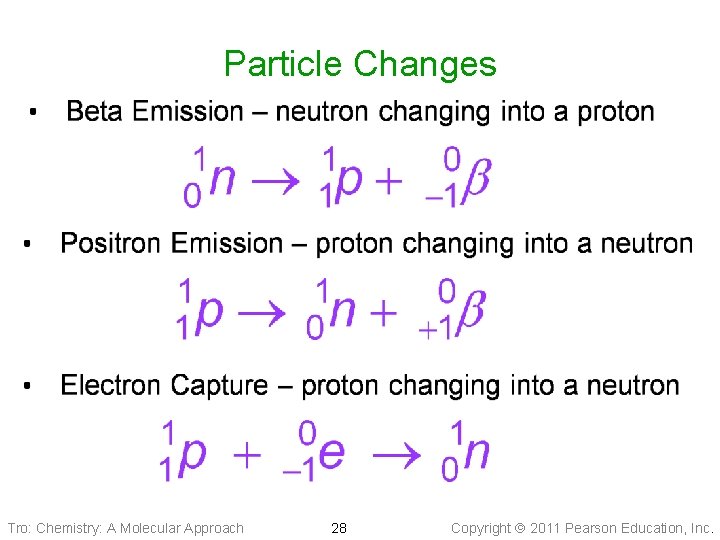
Particle Changes Tro: Chemistry: A Molecular Approach 28 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 29 Copyright 2011 Pearson Education, Inc.

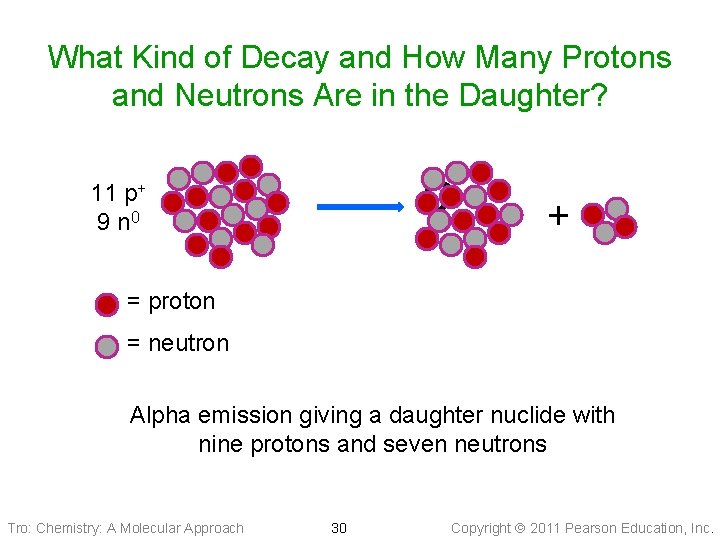
What Kind of Decay and How Many Protons and Neutrons Are in the Daughter? ? 11 p+ 9 n 0 + = proton = neutron Alpha emission giving a daughter nuclide with nine protons and seven neutrons Tro: Chemistry: A Molecular Approach 30 Copyright 2011 Pearson Education, Inc.

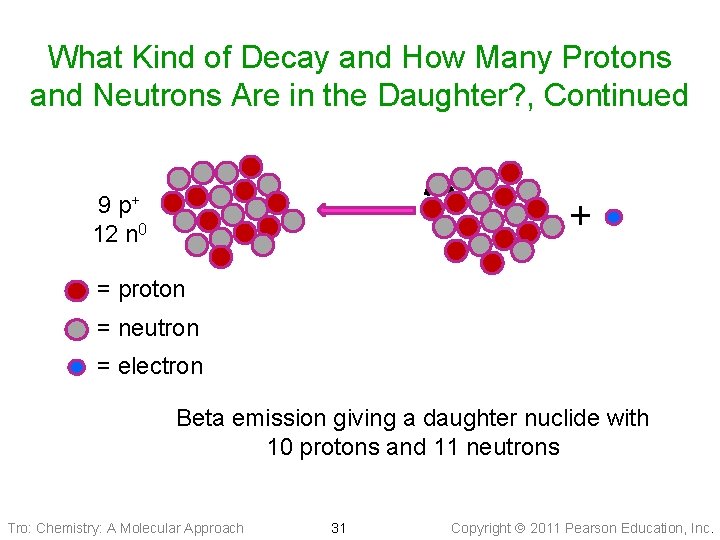
What Kind of Decay and How Many Protons and Neutrons Are in the Daughter? , Continued ? 9 p+ 12 n 0 + = proton = neutron = electron Beta emission giving a daughter nuclide with 10 protons and 11 neutrons Tro: Chemistry: A Molecular Approach 31 Copyright 2011 Pearson Education, Inc.

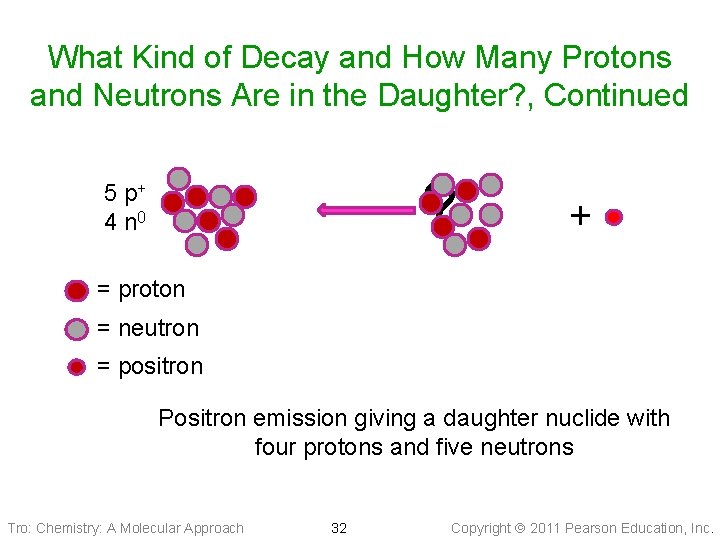
What Kind of Decay and How Many Protons and Neutrons Are in the Daughter? , Continued ? 5 p+ 4 n 0 + = proton = neutron = positron Positron emission giving a daughter nuclide with four protons and five neutrons Tro: Chemistry: A Molecular Approach 32 Copyright 2011 Pearson Education, Inc.

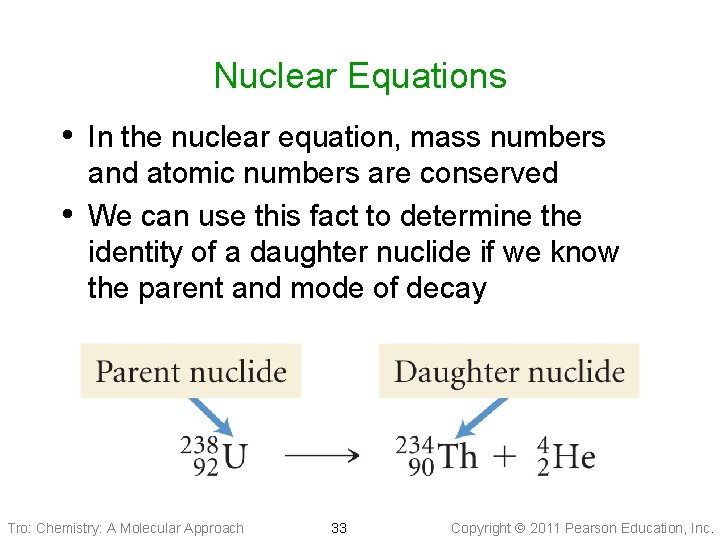
Nuclear Equations • In the nuclear equation, mass numbers • and atomic numbers are conserved We can use this fact to determine the identity of a daughter nuclide if we know the parent and mode of decay Tro: Chemistry: A Molecular Approach 33 Copyright 2011 Pearson Education, Inc.

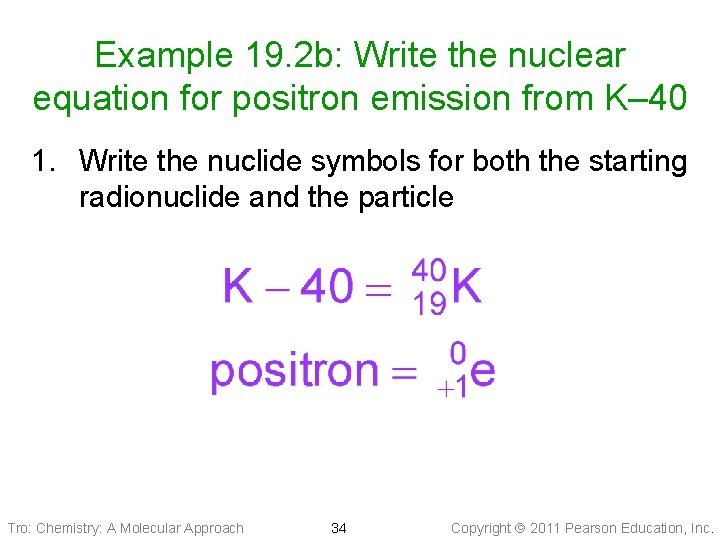
Example 19. 2 b: Write the nuclear equation for positron emission from K– 40 1. Write the nuclide symbols for both the starting radionuclide and the particle Tro: Chemistry: A Molecular Approach 34 Copyright 2011 Pearson Education, Inc.

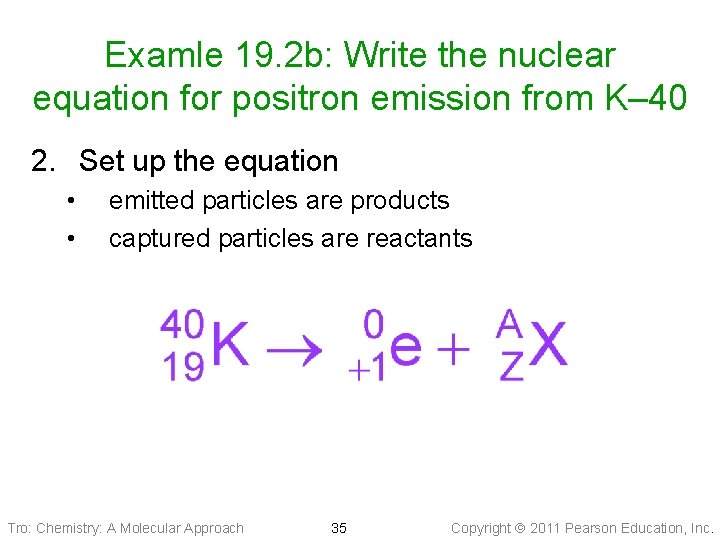
Examle 19. 2 b: Write the nuclear equation for positron emission from K– 40 2. Set up the equation • • emitted particles are products captured particles are reactants Tro: Chemistry: A Molecular Approach 35 Copyright 2011 Pearson Education, Inc.

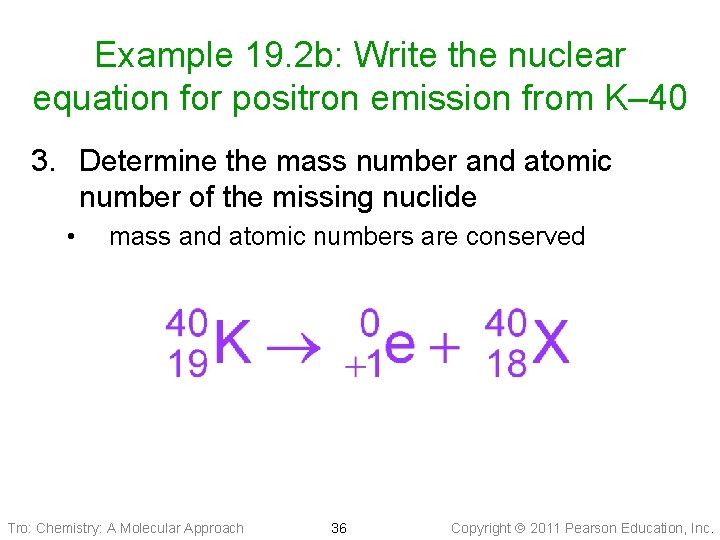
Example 19. 2 b: Write the nuclear equation for positron emission from K– 40 3. Determine the mass number and atomic number of the missing nuclide • mass and atomic numbers are conserved Tro: Chemistry: A Molecular Approach 36 Copyright 2011 Pearson Education, Inc.

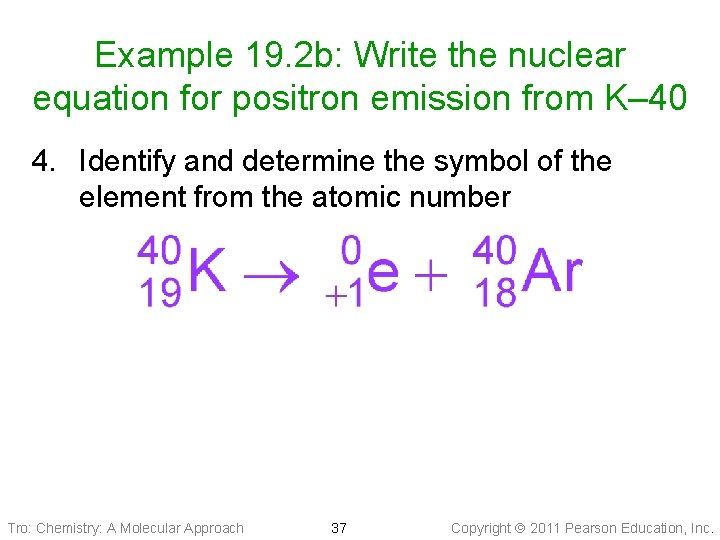
Example 19. 2 b: Write the nuclear equation for positron emission from K– 40 4. Identify and determine the symbol of the element from the atomic number Tro: Chemistry: A Molecular Approach 37 Copyright 2011 Pearson Education, Inc.

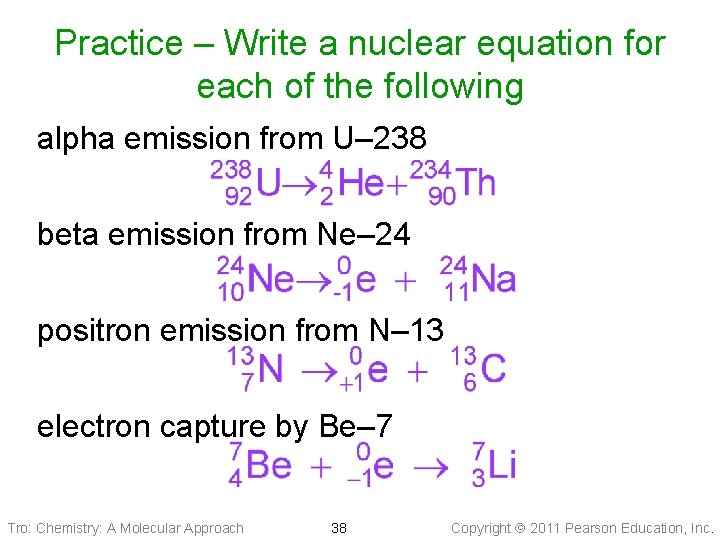
Practice – Write a nuclear equation for each of the following alpha emission from U– 238 beta emission from Ne– 24 positron emission from N– 13 electron capture by Be– 7 Tro: Chemistry: A Molecular Approach 38 Copyright 2011 Pearson Education, Inc.

What Causes Nuclei to Decompose? • The particles in the nucleus are held together by a very strong attractive force only found in the nucleus called the strong force ü acts only over very short distances • The neutrons play an important role in stabilizing the nucleus, as they add to the strong force, but don’t repel each other like the protons do Tro: Chemistry: A Molecular Approach 39 Copyright 2011 Pearson Education, Inc.

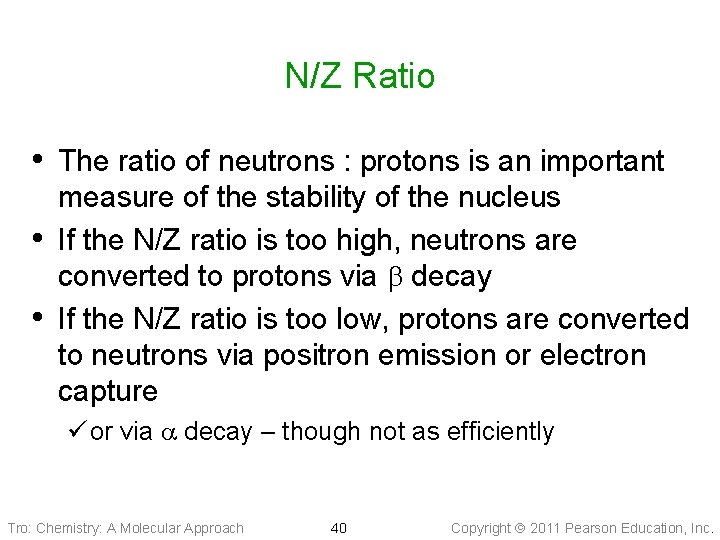
N/Z Ratio • The ratio of neutrons : protons is an important • • measure of the stability of the nucleus If the N/Z ratio is too high, neutrons are converted to protons via decay If the N/Z ratio is too low, protons are converted to neutrons via positron emission or electron capture ü or via decay – though not as efficiently Tro: Chemistry: A Molecular Approach 40 Copyright 2011 Pearson Education, Inc.

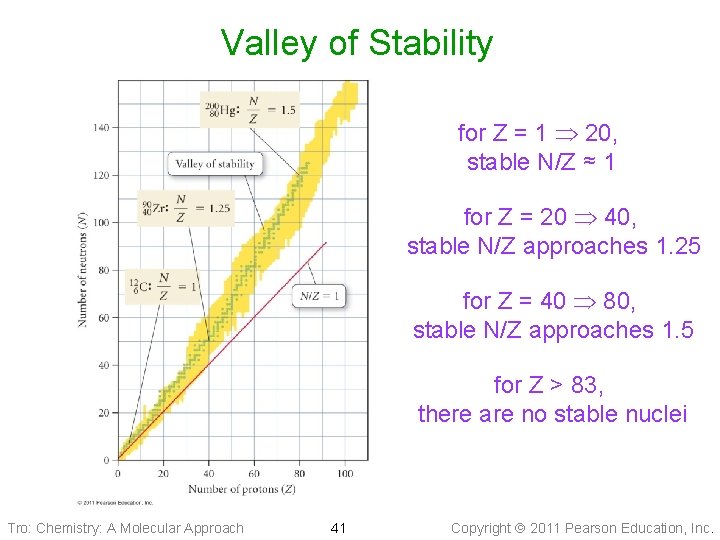
Valley of Stability for Z = 1 20, stable N/Z ≈ 1 for Z = 20 40, stable N/Z approaches 1. 25 for Z = 40 80, stable N/Z approaches 1. 5 for Z > 83, there are no stable nuclei Tro: Chemistry: A Molecular Approach 41 Copyright 2011 Pearson Education, Inc.

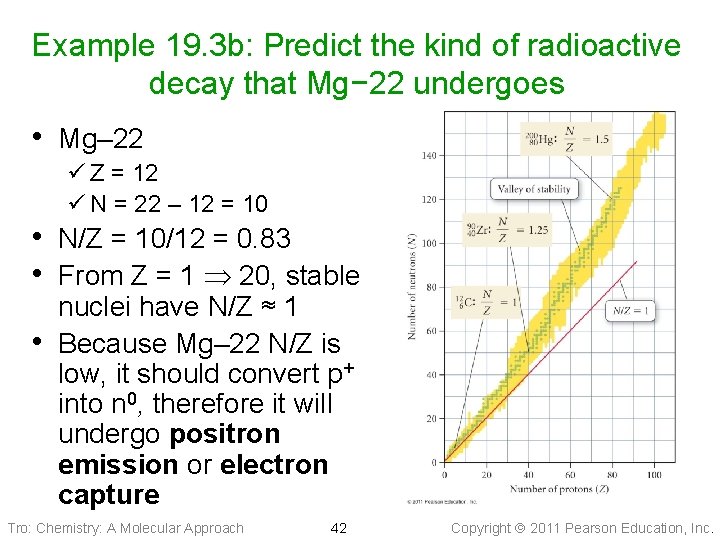
Example 19. 3 b: Predict the kind of radioactive decay that Mg− 22 undergoes • Mg– 22 ü Z = 12 ü N = 22 – 12 = 10 • N/Z = 10/12 = 0. 83 • From Z = 1 20, stable • nuclei have N/Z ≈ 1 Because Mg– 22 N/Z is low, it should convert p+ into n 0, therefore it will undergo positron emission or electron capture Tro: Chemistry: A Molecular Approach 42 Copyright 2011 Pearson Education, Inc.

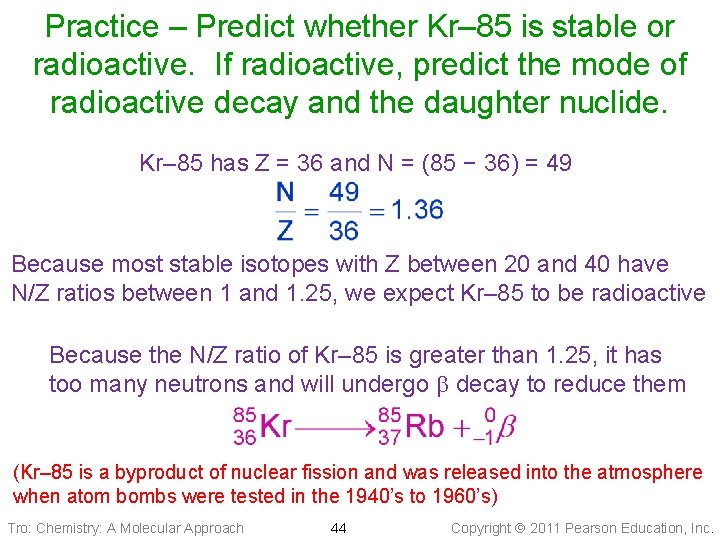
Practice – Predict whether Kr– 85 is stable or radioactive. If radioactive, predict the mode of radioactive decay and the daughter nuclide. Tro: Chemistry: A Molecular Approach 43 Copyright 2011 Pearson Education, Inc.

Practice – Predict whether Kr– 85 is stable or radioactive. If radioactive, predict the mode of radioactive decay and the daughter nuclide. Kr– 85 has Z = 36 and N = (85 − 36) = 49 Because most stable isotopes with Z between 20 and 40 have N/Z ratios between 1 and 1. 25, we expect Kr– 85 to be radioactive Because the N/Z ratio of Kr– 85 is greater than 1. 25, it has too many neutrons and will undergo decay to reduce them (Kr– 85 is a byproduct of nuclear fission and was released into the atmosphere when atom bombs were tested in the 1940’s to 1960’s) Tro: Chemistry: A Molecular Approach 44 Copyright 2011 Pearson Education, Inc.

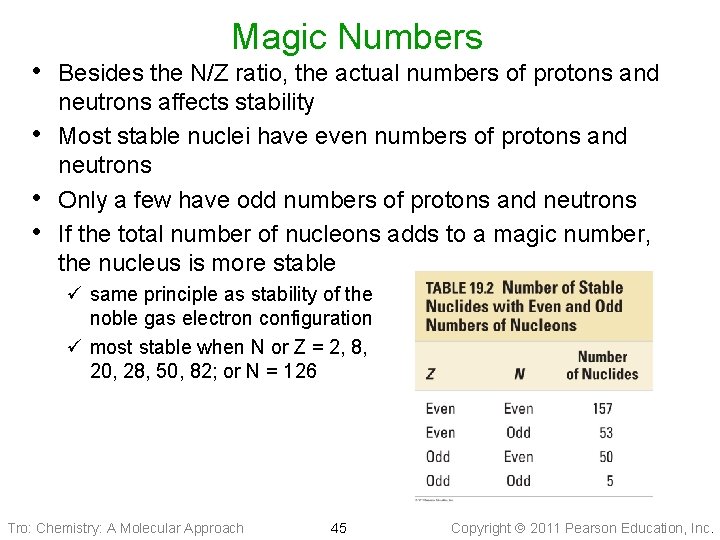
Magic Numbers • Besides the N/Z ratio, the actual numbers of protons and • • • neutrons affects stability Most stable nuclei have even numbers of protons and neutrons Only a few have odd numbers of protons and neutrons If the total number of nucleons adds to a magic number, the nucleus is more stable ü same principle as stability of the noble gas electron configuration ü most stable when N or Z = 2, 8, 20, 28, 50, 82; or N = 126 Tro: Chemistry: A Molecular Approach 45 Copyright 2011 Pearson Education, Inc.

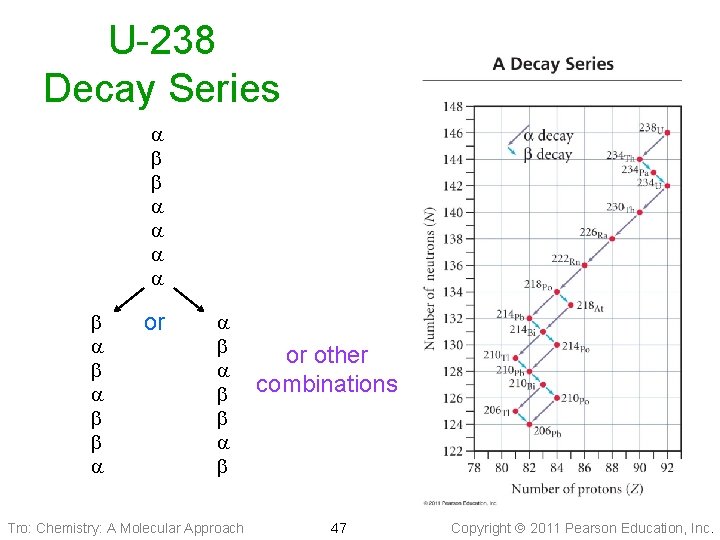
Decay Series • In nature, often one radioactive nuclide changes into another radioactive nuclide ü i. e. the daughter nuclide is also radioactive • All of the radioactive nuclides that are • produced one after the other until a stable nuclide is made is called a decay series To determine the stable nuclide at the end of the series without writing it all out 1. count the number of and decays 2. from the mass no. subtract 4 for each decay 3. from the atomic no. subtract 2 for each decay and add 1 for each Tro: Chemistry: A Molecular Approach 46 Copyright 2011 Pearson Education, Inc.

U-238 Decay Series or Tro: Chemistry: A Molecular Approach or other combinations 47 Copyright 2011 Pearson Education, Inc.

Detecting Radioactivity To detect something, you need to identify what it does • Radioactive rays can expose light-protected photographic film • We may use photographic film to detect the presence of radioactive rays – film badge dosimeters Tro: Chemistry: A Molecular Approach 48 Copyright 2011 Pearson Education, Inc.

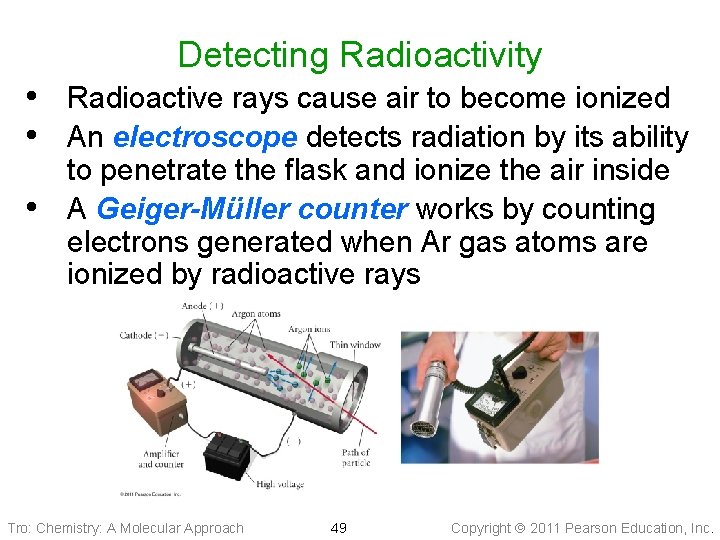
Detecting Radioactivity • Radioactive rays cause air to become ionized • An electroscope detects radiation by its ability • to penetrate the flask and ionize the air inside A Geiger-Müller counter works by counting electrons generated when Ar gas atoms are ionized by radioactive rays Tro: Chemistry: A Molecular Approach 49 Copyright 2011 Pearson Education, Inc.

Detecting Radioactivity • Radioactive rays cause certain chemicals to • give off a flash of light when they strike the chemical A scintillation counter is able to count the number of flashes per minute Tro: Chemistry: A Molecular Approach 50 Copyright 2011 Pearson Education, Inc.

Natural Radioactivity • There are small amounts of radioactive • • minerals in the air, ground, and water Even in the food you eat! The radiation you are exposed to from natural sources is called background radiation Tro: Chemistry: A Molecular Approach 51 Copyright 2011 Pearson Education, Inc.

Rate of Radioactive Decay • It was discovered that the rate of change in the amount of radioactivity was constant, and different for each radioactive “isotope” ü change in radioactivity measured with Geiger counter Øcounts per minute • Each radionuclide had a particular length of time it required to lose half its radioactivity ü a constant half-life ü we know that processes with a constant half-life follow first order kinetic rate laws • The rate of radioactive change was not affected by temperature ü meaning radioactivity is not a chemical reaction! Tro: Chemistry: A Molecular Approach 52 Copyright 2011 Pearson Education, Inc.

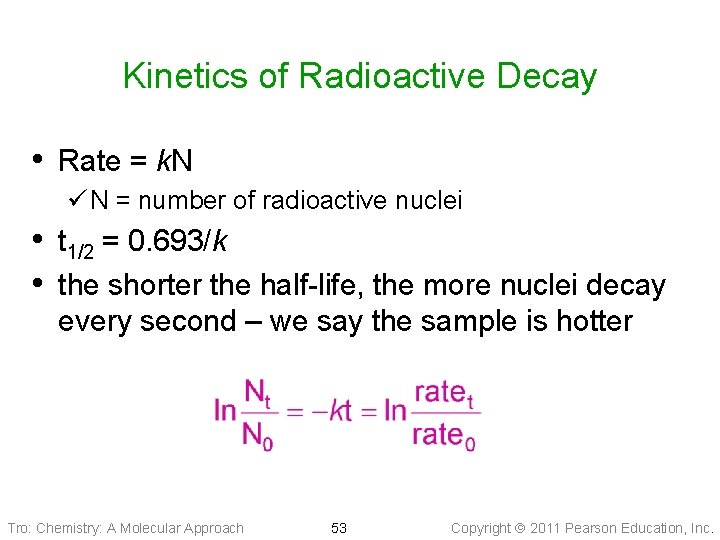
Kinetics of Radioactive Decay • Rate = k. N ü N = number of radioactive nuclei • t 1/2 = 0. 693/k • the shorter the half-life, the more nuclei decay every second – we say the sample is hotter Tro: Chemistry: A Molecular Approach 53 Copyright 2011 Pearson Education, Inc.

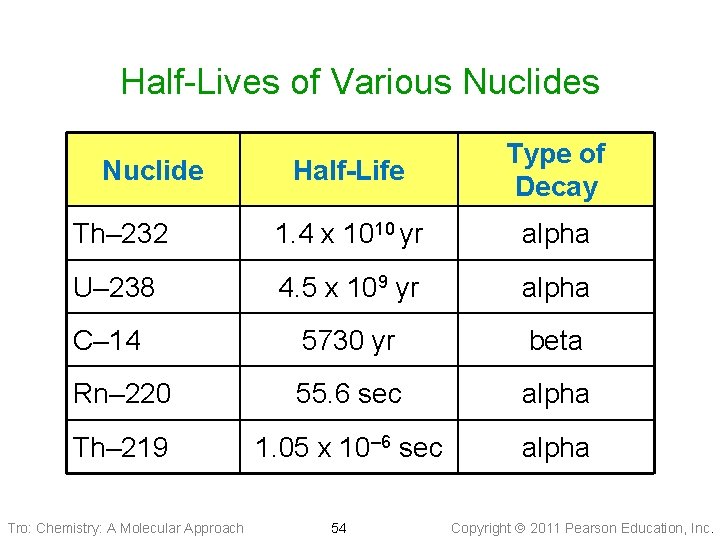
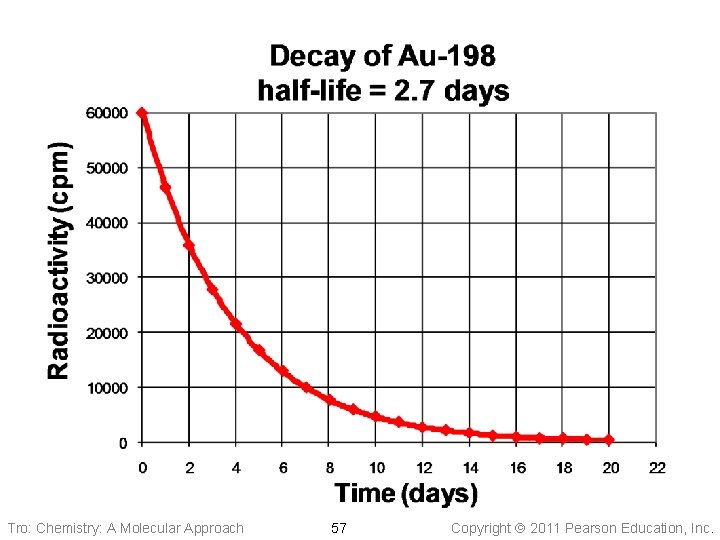
Half-Lives of Various Nuclides Half-Life Type of Decay Th– 232 1. 4 x 1010 yr alpha U– 238 4. 5 x 109 yr alpha C– 14 5730 yr beta Rn– 220 55. 6 sec alpha Th– 219 1. 05 x 10– 6 sec alpha Nuclide Tro: Chemistry: A Molecular Approach 54 Copyright 2011 Pearson Education, Inc.

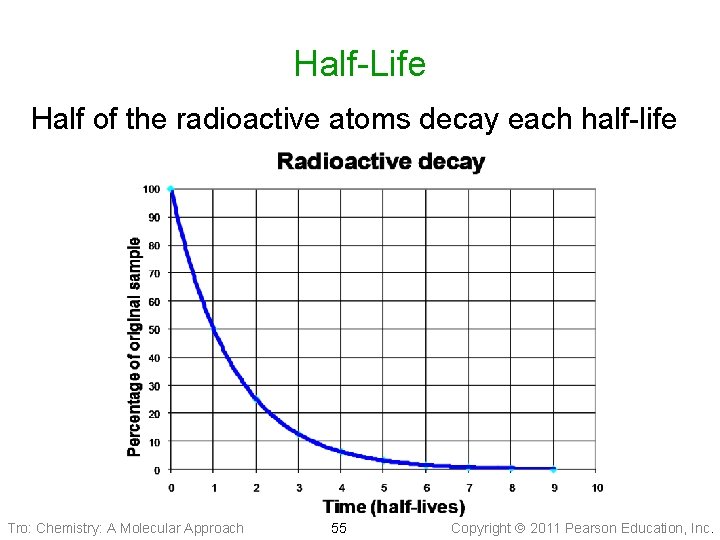
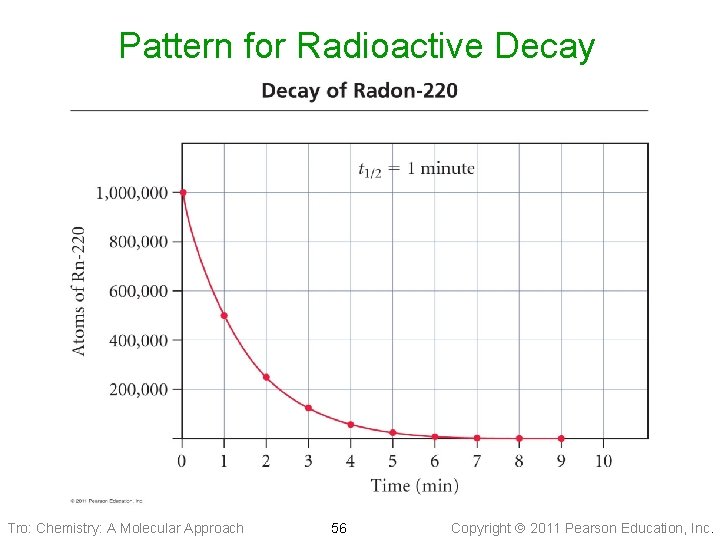
Half-Life Half of the radioactive atoms decay each half-life Tro: Chemistry: A Molecular Approach 55 Copyright 2011 Pearson Education, Inc.

Pattern for Radioactive Decay Tro: Chemistry: A Molecular Approach 56 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 57 Copyright 2011 Pearson Education, Inc.

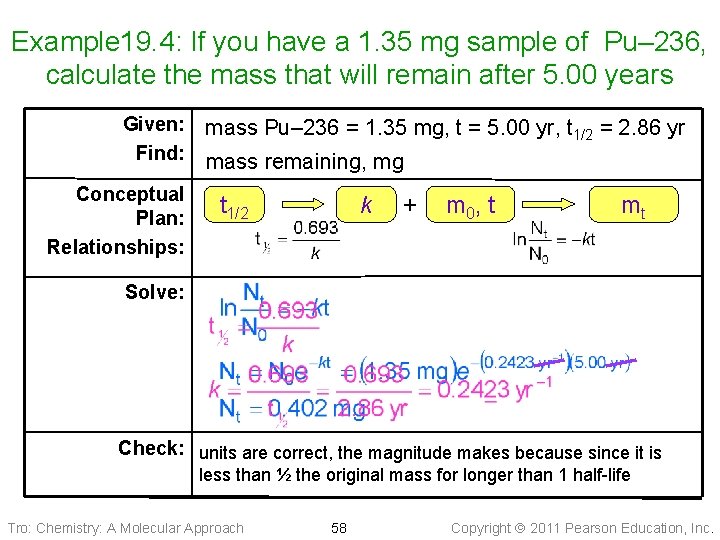
Example 19. 4: If you have a 1. 35 mg sample of Pu– 236, calculate the mass that will remain after 5. 00 years Given: Find: Conceptual Plan: Relationships: mass Pu– 236 = 1. 35 mg, t = 5. 00 yr, t 1/2 = 2. 86 yr mass remaining, mg t 1/2 k + m 0 , t mt Solve: Check: units are correct, the magnitude makes because since it is less than ½ the original mass for longer than 1 half-life Tro: Chemistry: A Molecular Approach 58 Copyright 2011 Pearson Education, Inc.

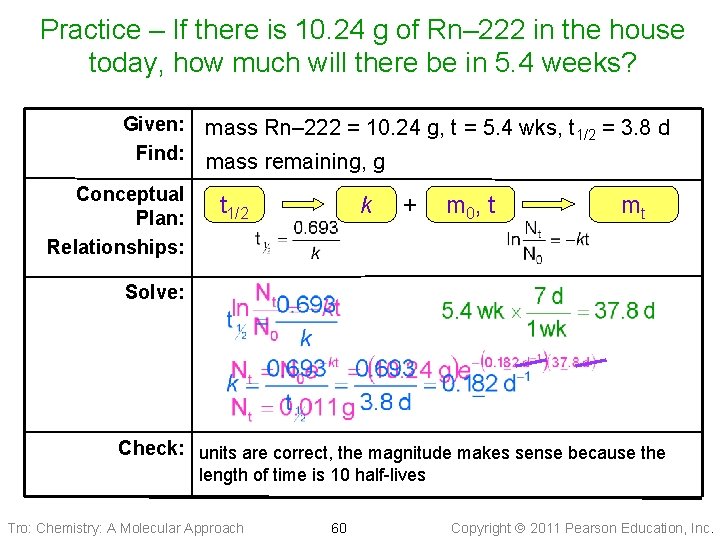
Practice—Radon– 222 is a gas that is suspected of causing lung cancer as it leaks into houses. It is produced by uranium decay. Assuming no loss or gain from leakage, if there is 10. 24 g of Rn– 222 in the house today, how much will there be in 5. 4 weeks? ( Rn– 222 half-life is 3. 8 Days) Tro: Chemistry: A Molecular Approach 59 Copyright 2011 Pearson Education, Inc.

Practice – If there is 10. 24 g of Rn– 222 in the house today, how much will there be in 5. 4 weeks? Given: Find: Conceptual Plan: Relationships: mass Rn– 222 = 10. 24 g, t = 5. 4 wks, t 1/2 = 3. 8 d mass remaining, g t 1/2 k + m 0 , t mt Solve: Check: units are correct, the magnitude makes sense because the length of time is 10 half-lives Tro: Chemistry: A Molecular Approach 60 Copyright 2011 Pearson Education, Inc.

Radiometric Dating • The change in the amount of radioactivity of a • particular radionuclide is predictable and not affected by environmental factors By measuring and comparing the amount of a parent radioactive isotope and its stable daughter we can determine the age of the object ü by using the half-life and the previous equations • Mineral (geological) dating ü compare the amount of U-238 to Pb-206 in volcanic rocks and meteorites Ødates the Earth to between 4. 0 and 4. 5 billion yrs. old ü compare amount of K-40 to Ar-40 Tro: Chemistry: A Molecular Approach 61 Copyright 2011 Pearson Education, Inc.

Radiocarbon Dating • All things that are alive or were once alive • contain carbon Three isotopes of carbon exist in nature, one of which, C– 14, is radioactive ü C– 14 radioactive with half-life = 5730 yrs • We would normally expect a radioisotope with this relatively short half-life to have disappeared long ago, but atmospheric chemistry keeps producing C– 14 at nearly the same rate it decays Tro: Chemistry: A Molecular Approach 62 Copyright 2011 Pearson Education, Inc.

Radiocarbon Dating • While still living, C– 14/C– 12 is constant because the organism replenishes its supply of carbon ü CO 2 in air ultimate source of all C in organism • Once the organism dies the C– 14/C– 12 ratio • • decreases By measuring the C– 14/C– 12 ratio in a once living artifact and comparing it to the C– 14/C– 12 ratio in a living organism, we can tell how long ago the organism was alive The limit for this technique is 50, 000 years old ü about 9 half-lives, after which radioactivity from C– 14 will be below the background radiation Tro: Chemistry: A Molecular Approach 63 Copyright 2011 Pearson Education, Inc.

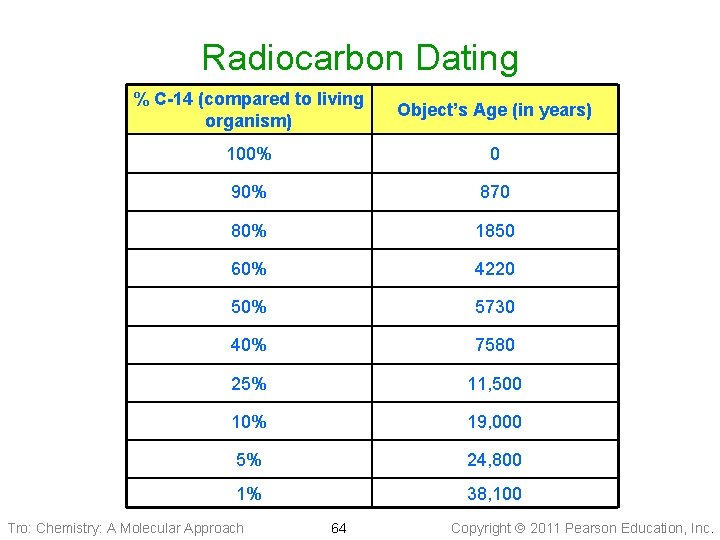
Radiocarbon Dating % C-14 (compared to living organism) Object’s Age (in years) 100% 0 90% 870 80% 1850 60% 4220 50% 5730 40% 7580 25% 11, 500 10% 19, 000 5% 24, 800 1% 38, 100 Tro: Chemistry: A Molecular Approach 64 Copyright 2011 Pearson Education, Inc.

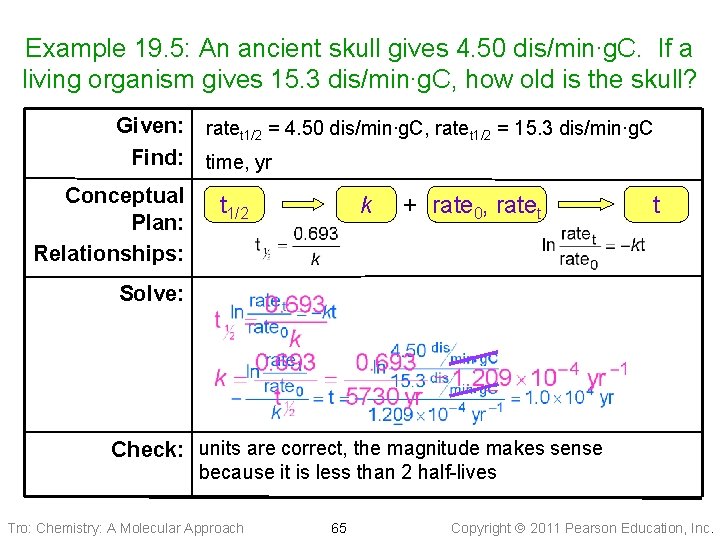
Example 19. 5: An ancient skull gives 4. 50 dis/min∙g. C. If a living organism gives 15. 3 dis/min∙g. C, how old is the skull? Given: ratet 1/2 = 4. 50 dis/min∙g. C, ratet 1/2 = 15. 3 dis/min∙g. C Find: time, yr Conceptual Plan: Relationships: t 1/2 k + rate 0, ratet t Solve: Check: units are correct, the magnitude makes sense because it is less than 2 half-lives Tro: Chemistry: A Molecular Approach 65 Copyright 2011 Pearson Education, Inc.

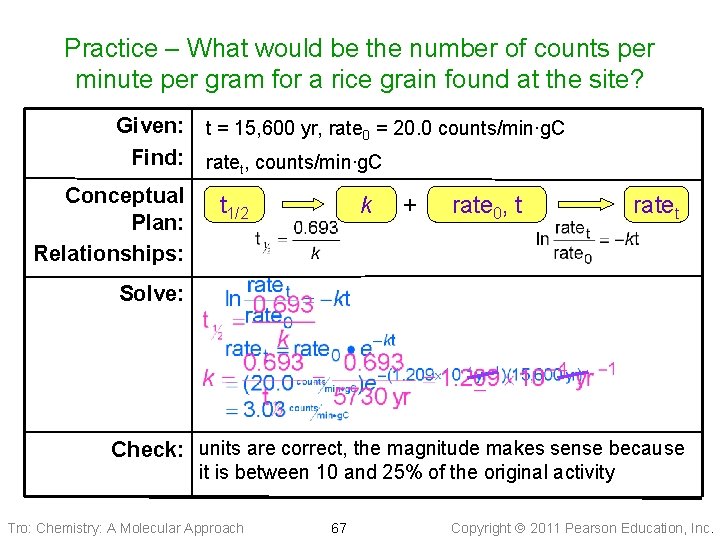
Practice – Archeologists have dated a civilization to 15, 600 yrs ago. If a living sample gives 20. 0 counts per minute per gram C, what would be the number of counts per minute per gram C for a rice grain found at the site? Tro: Chemistry: A Molecular Approach 66 Copyright 2011 Pearson Education, Inc.

Practice – What would be the number of counts per minute per gram for a rice grain found at the site? Given: t = 15, 600 yr, rate 0 = 20. 0 counts/min∙g. C Find: ratet, counts/min∙g. C Conceptual Plan: Relationships: t 1/2 k + rate 0, t ratet Solve: Check: units are correct, the magnitude makes sense because it is between 10 and 25% of the original activity Tro: Chemistry: A Molecular Approach 67 Copyright 2011 Pearson Education, Inc.

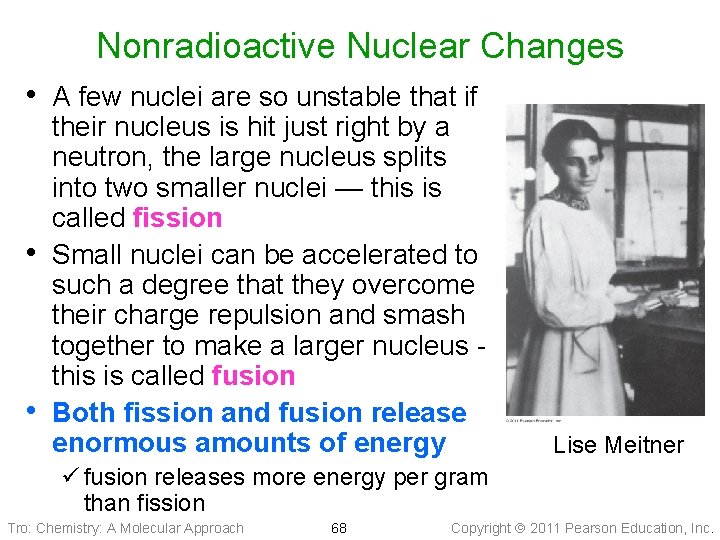
Nonradioactive Nuclear Changes • A few nuclei are so unstable that if • • their nucleus is hit just right by a neutron, the large nucleus splits into two smaller nuclei — this is called fission Small nuclei can be accelerated to such a degree that they overcome their charge repulsion and smash together to make a larger nucleus this is called fusion Both fission and fusion release enormous amounts of energy Lise Meitner ü fusion releases more energy per gram than fission Tro: Chemistry: A Molecular Approach 68 Copyright 2011 Pearson Education, Inc.

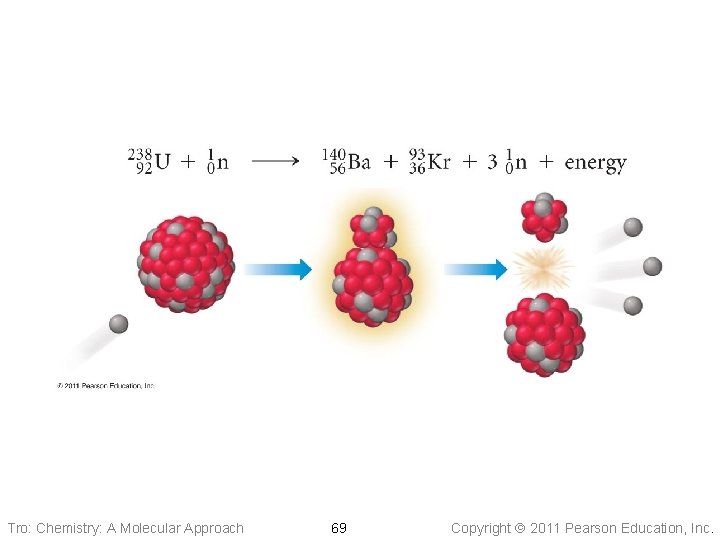
Tro: Chemistry: A Molecular Approach 69 Copyright 2011 Pearson Education, Inc.

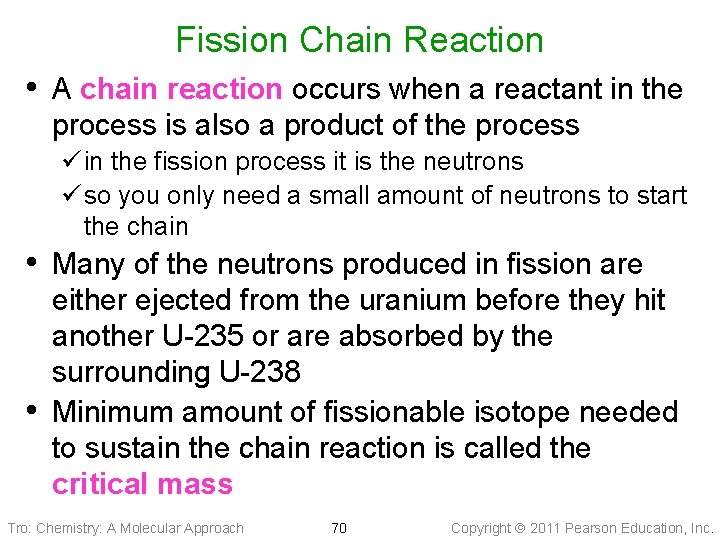
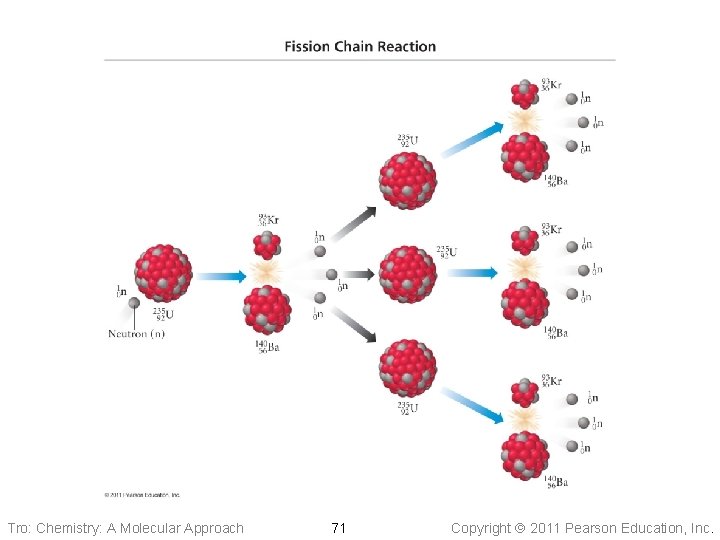
Fission Chain Reaction • A chain reaction occurs when a reactant in the process is also a product of the process ü in the fission process it is the neutrons ü so you only need a small amount of neutrons to start the chain • Many of the neutrons produced in fission are • either ejected from the uranium before they hit another U-235 or are absorbed by the surrounding U-238 Minimum amount of fissionable isotope needed to sustain the chain reaction is called the critical mass Tro: Chemistry: A Molecular Approach 70 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 71 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 72 Copyright 2011 Pearson Education, Inc.

Fissionable Material • Fissionable isotopes include U– 235, Pu– 239, • and Pu– 240 Natural uranium is less than 1% U– 235 ü rest mostly U– 238 ü not enough U– 235 to sustain chain reaction • To produce fissionable uranium, the natural uranium must be enriched in U– 235 ü to about 7% for “weapons grade” ü to about 3% for reactor grade Tro: Chemistry: A Molecular Approach 73 Copyright 2011 Pearson Education, Inc.

Nuclear Power • Nuclear reactors use fission to generate electricity ü about 20% of U. S. electricity ü uses the fission of U– 235 to produce heat • The heat boils water, turning it to steam • The steam turns a turbine, generating electricity Tro: Chemistry: A Molecular Approach 74 Copyright 2011 Pearson Education, Inc.

Nuclear Power Plants vs. Coal-Burning Power Plants • Use about 2 million kg • Use about 50 kg of • fuel to generate enough electricity for 1 million people No air pollution • • Tro: Chemistry: A Molecular Approach 75 of fuel to generate enough electricity for 1 million people Produce NO 2 and SOx that add to acid rain Produce CO 2 that adds to the greenhouse effect Copyright 2011 Pearson Education, Inc.

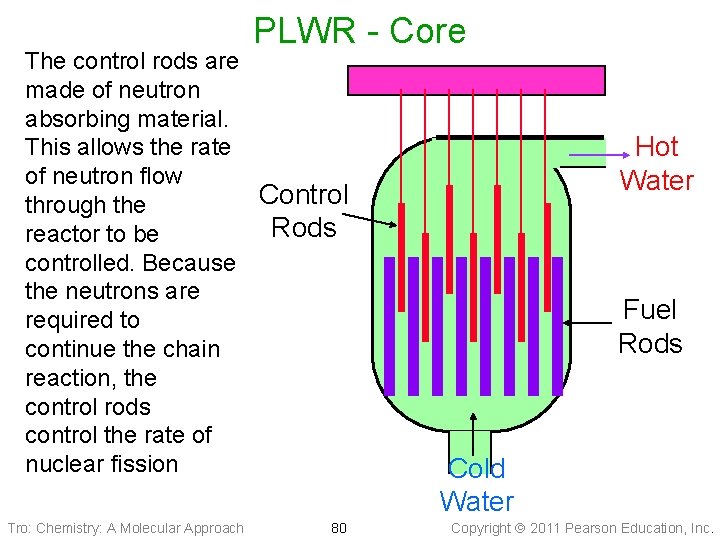
Nuclear Power Plants - Core • The fissionable material is stored in long tubes, called fuel rods, arranged in a matrix ü subcritical • Between the fuel rods are control rods made of neutron-absorbing material ü B and/or Cd ü neutrons needed to sustain the chain reaction • The rods are placed in a material to slow down the ejected neutrons, called a moderator ü allows chain reaction to occur below critical mass Tro: Chemistry: A Molecular Approach 76 Copyright 2011 Pearson Education, Inc.

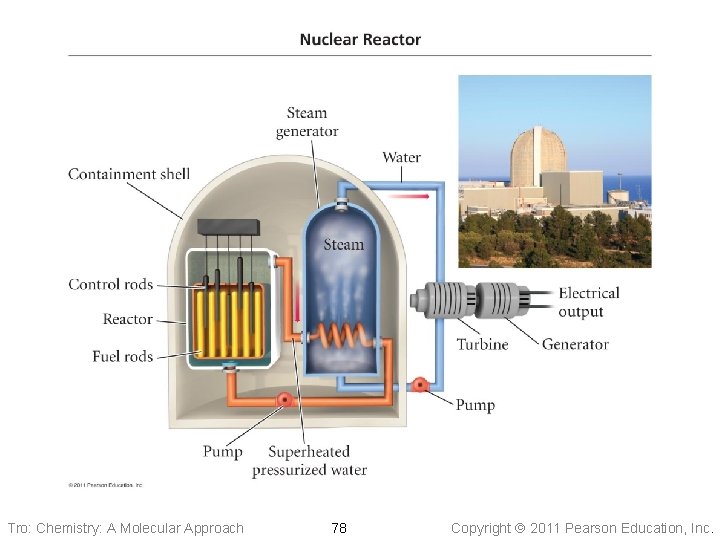
Pressurized Light Water Reactor • Design used in United States (GE, • • • Westinghouse) Water is both the coolant and moderator Water in core kept under pressure to keep it from boiling Fuel is enriched uranium ü subcritical • Containment dome of concrete Tro: Chemistry: A Molecular Approach 77 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 78 Copyright 2011 Pearson Education, Inc.

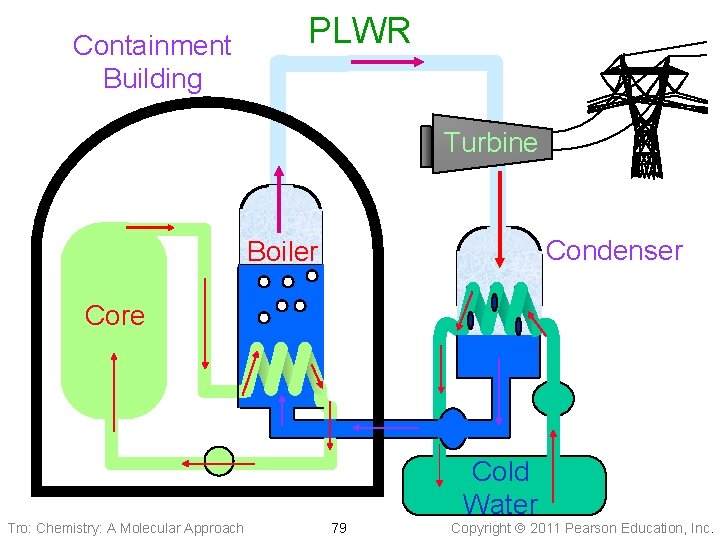
Containment Building PLWR Turbine Condenser Boiler Core Cold Water Tro: Chemistry: A Molecular Approach 79 Copyright 2011 Pearson Education, Inc.

PLWR - Core The control rods are made of neutron absorbing material. This allows the rate of neutron flow Control through the Rods reactor to be controlled. Because the neutrons are required to continue the chain reaction, the control rods control the rate of nuclear fission Tro: Chemistry: A Molecular Approach 80 Hot Water Fuel Rods Cold Water Copyright 2011 Pearson Education, Inc.

Concerns about Nuclear Power • Core melt-down ü water loss from core, heat melts core ü China Syndrome ü Chernobyl • Waste disposal ü waste highly radioactive ü reprocessing, underground storage? ü Federal High Level Radioactive Waste Storage Facility at Yucca Mountain, Nevada • Transporting waste • How do we deal with nuclear power plants that are no longer safe to operate? ü Yankee Rowe in Massachusetts Tro: Chemistry: A Molecular Approach 81 Copyright 2011 Pearson Education, Inc.

Where Does the Energy from Fission Come from? • During nuclear fission, some of the mass of the nucleus is converted into energy ü E = mc 2 • Each mole of U– 235 that fissions produces about 1. 7 x 1013 J of energy ü a very exothermic chemical reaction produces 106 J per mole Tro: Chemistry: A Molecular Approach 82 Copyright 2011 Pearson Education, Inc.

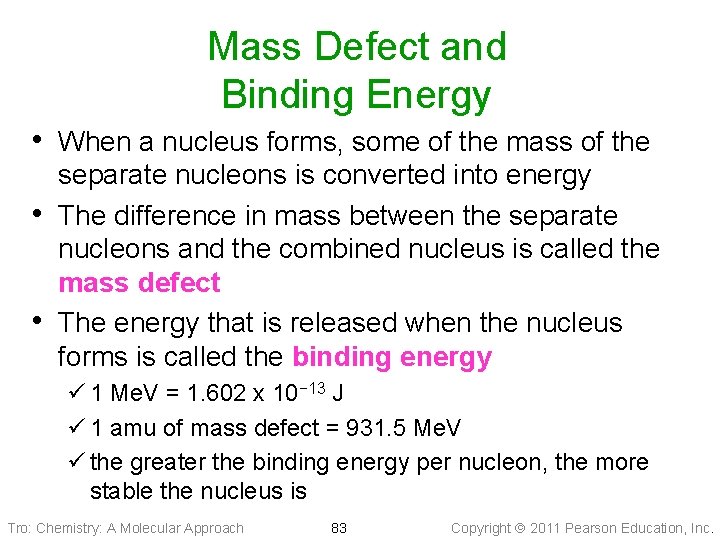
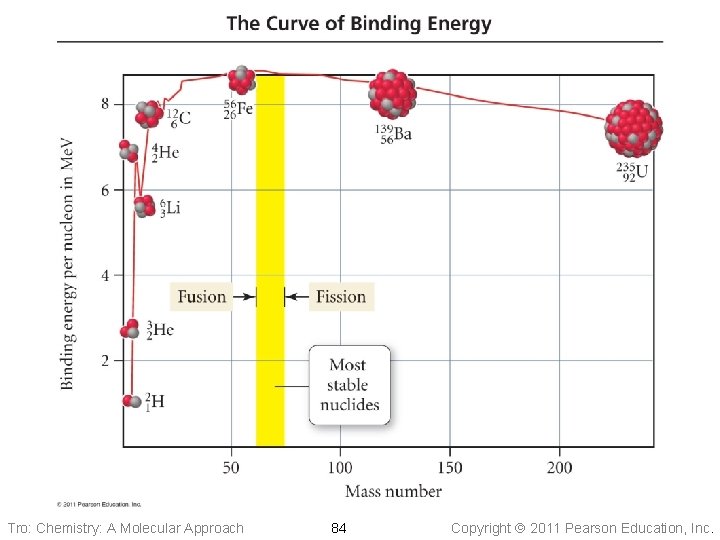
Mass Defect and Binding Energy • When a nucleus forms, some of the mass of the • • separate nucleons is converted into energy The difference in mass between the separate nucleons and the combined nucleus is called the mass defect The energy that is released when the nucleus forms is called the binding energy ü 1 Me. V = 1. 602 x 10− 13 J ü 1 amu of mass defect = 931. 5 Me. V ü the greater the binding energy per nucleon, the more stable the nucleus is Tro: Chemistry: A Molecular Approach 83 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 84 Copyright 2011 Pearson Education, Inc.

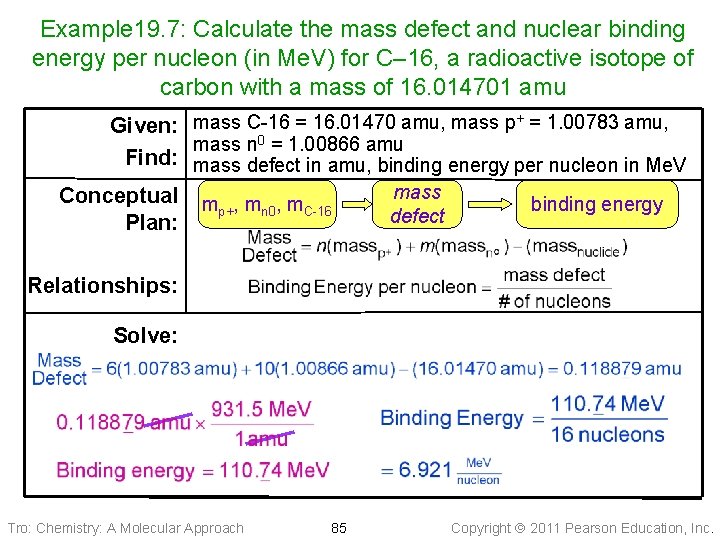
Example 19. 7: Calculate the mass defect and nuclear binding energy per nucleon (in Me. V) for C– 16, a radioactive isotope of carbon with a mass of 16. 014701 amu Given: mass C-16 = 16. 01470 amu, mass p+ = 1. 00783 amu, mass n 0 = 1. 00866 amu Find: mass defect in amu, binding energy per nucleon in Me. V Conceptual Plan: mp+, mn 0, m. C-16 mass defect binding energy Relationships: Solve: Tro: Chemistry: A Molecular Approach 85 Copyright 2011 Pearson Education, Inc.

Practice – Calculate the binding energy per nucleon in Fe– 56 Tro: Chemistry: A Molecular Approach 86 Copyright 2011 Pearson Education, Inc.

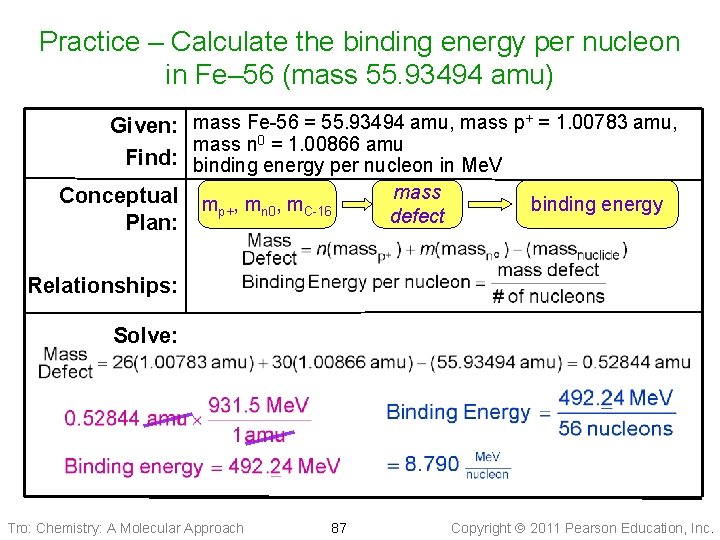
Practice – Calculate the binding energy per nucleon in Fe– 56 (mass 55. 93494 amu) Given: mass Fe-56 = 55. 93494 amu, mass p+ = 1. 00783 amu, mass n 0 = 1. 00866 amu Find: binding energy per nucleon in Me. V mass Conceptual m , m binding energy p+ n 0 C-16 defect Plan: Relationships: Solve: Tro: Chemistry: A Molecular Approach 87 Copyright 2011 Pearson Education, Inc.

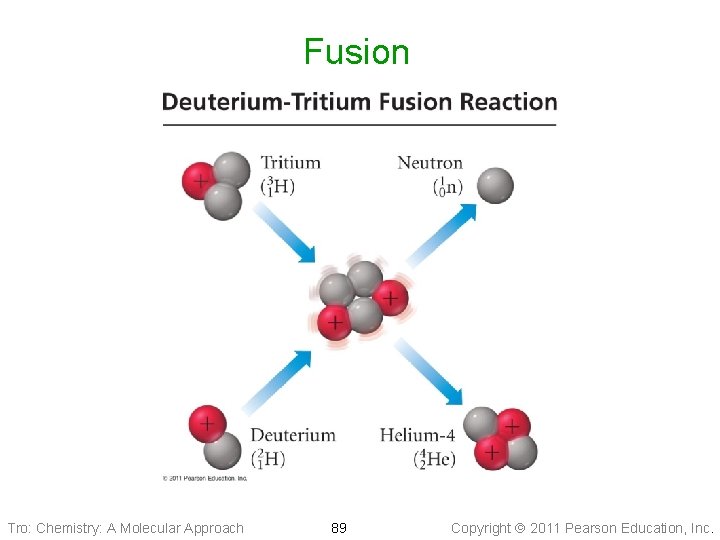
Nuclear Fusion • Fusion is the combining of light nuclei to make a • • heavier, more stable nuclide The Sun uses the fusion of hydrogen isotopes to make helium as a power source Requires high input of energy to initiate the process ü because need to overcome repulsion of positive nuclei • Produces 10 x the energy per gram as fission • No radioactive byproducts • Unfortunately, the only currently working application is the H-bomb Tro: Chemistry: A Molecular Approach 88 Copyright 2011 Pearson Education, Inc.

Fusion Tro: Chemistry: A Molecular Approach 89 Copyright 2011 Pearson Education, Inc.

Tokamak Fusion Reactor Tro: Chemistry: A Molecular Approach 90 Copyright 2011 Pearson Education, Inc.

Making New Elements: Artificial Transmutation • High energy particles can be smashed into target nuclei, resulting in the production of new nuclei • The particles may be radiation from another radionuclide, or charged particles that are accelerated ü Rutherford made O– 17 bombarding N– 14 with alpha rays from radium ü Cf– 244 is made by bombarding U– 238 with C– 12 in a particle accelerator Tro: Chemistry: A Molecular Approach 91 Copyright 2011 Pearson Education, Inc.

Artificial Transmutation • Bombardment of one nucleus with another causing new atoms to be made ü can also bombard with neutrons • Reaction done in a particle accelerator ü linear ü cyclotron Tc-97 is made by bombarding Mo-96 with deuterium, releasing a neutron Tro: Chemistry: A Molecular Approach 92 Copyright 2011 Pearson Education, Inc.

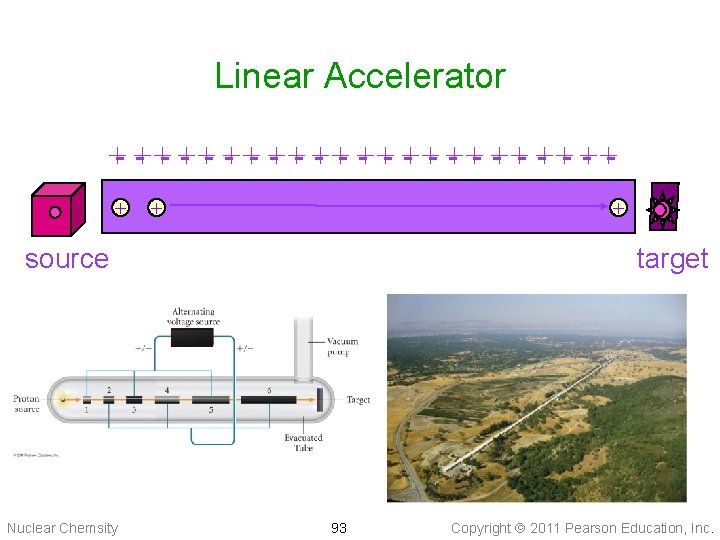
Linear Accelerator +- + - +- + - +- + - ++ + + source Nuclear Chemsity target 93 Copyright 2011 Pearson Education, Inc.

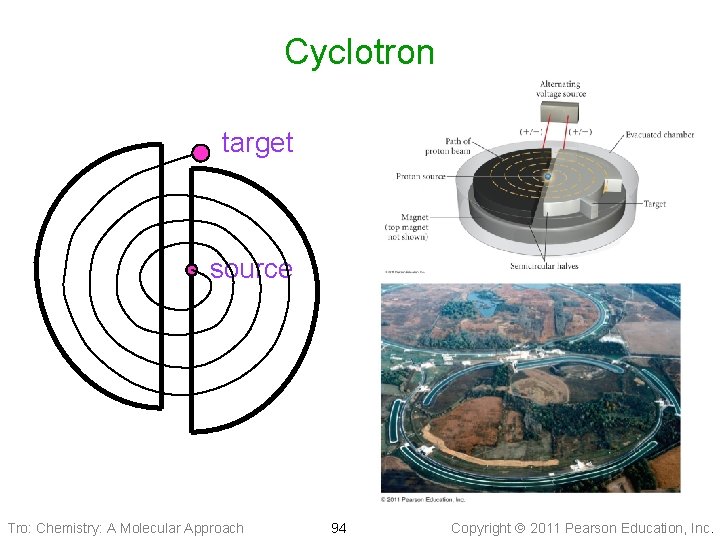
Cyclotron target source Tro: Chemistry: A Molecular Approach 94 Copyright 2011 Pearson Education, Inc.

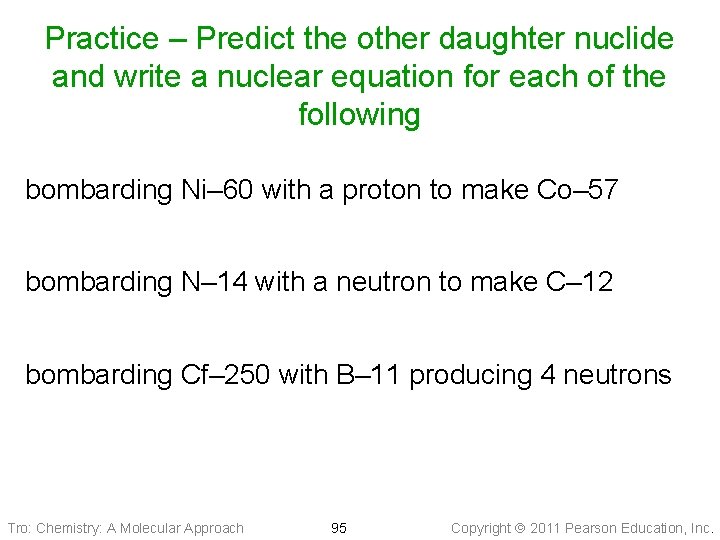
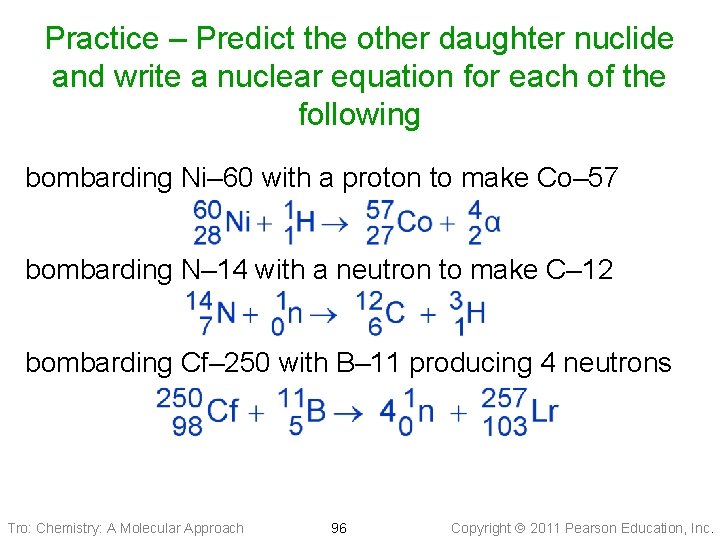
Practice – Predict the other daughter nuclide and write a nuclear equation for each of the following bombarding Ni– 60 with a proton to make Co– 57 bombarding N– 14 with a neutron to make C– 12 bombarding Cf– 250 with B– 11 producing 4 neutrons Tro: Chemistry: A Molecular Approach 95 Copyright 2011 Pearson Education, Inc.

Practice – Predict the other daughter nuclide and write a nuclear equation for each of the following bombarding Ni– 60 with a proton to make Co– 57 bombarding N– 14 with a neutron to make C– 12 bombarding Cf– 250 with B– 11 producing 4 neutrons Tro: Chemistry: A Molecular Approach 96 Copyright 2011 Pearson Education, Inc.

Biological Effects of Radiation • Radiation has high energy, energy enough to knock electrons from molecules and break bonds ü ionizing radiation • Energy transferred to cells can damage biological molecules and cause malfunction of the cell Tro: Chemistry: A Molecular Approach 97 Copyright 2011 Pearson Education, Inc.

Acute Effects of Radiation • High levels of radiation over a short period of time kill large numbers of cells ü from a nuclear blast or exposed reactor core • Causes weakened immune system and lower ability to absorb nutrients from food ü may result in death, usually from infection Tro: Chemistry: A Molecular Approach 98 Copyright 2011 Pearson Education, Inc.

Chronic Effects • Low doses of radiation over a period of time show an increased risk for the development of cancer ü radiation damages DNA that may not get repaired properly • Low doses over time may damage • reproductive organs, which may lead to sterilization Damage to reproductive cells may lead to genetic defects in offspring Tro: Chemistry: A Molecular Approach 99 Copyright 2011 Pearson Education, Inc.

Measuring Radiation Exposure • The curie (Ci) is an exposure of 3. 7 x 1010 events per second ü no matter the kind of radiation • The gray (Gy) measures the amount of energy absorbed by body tissue from radiation ü 1 Gy = 1 J/kg body tissue • The rad also measures the amount of energy absorbed by body tissue from radiation ü 1 rad = 0. 01 Gy • A correction factor is used to account for a number of factors that affect the result of the exposure – this biological effectiveness factor is the RBE, and the result is the dose in rems ü rads x RBE = rems ü rem = roentgen equivalent man Tro: Chemistry: A Molecular Approach 100 Copyright 2011 Pearson Education, Inc.

Factors that Determine the Biological Effects of Radiation 1. The more energy the radiation has, the larger its effect can be 2. The better the ionizing radiation penetrates human tissue, the deeper effect it can have ü Gamma >> Beta > Alpha 3. The more ionizing the radiation, the larger the effect of the radiation ü Alpha > Beta > Gamma 4. The radioactive half-life of the radionuclide 5. The biological half-life of the element 6. The physical state of the radioactive material Tro: Chemistry: A Molecular Approach 101 Copyright 2011 Pearson Education, Inc.

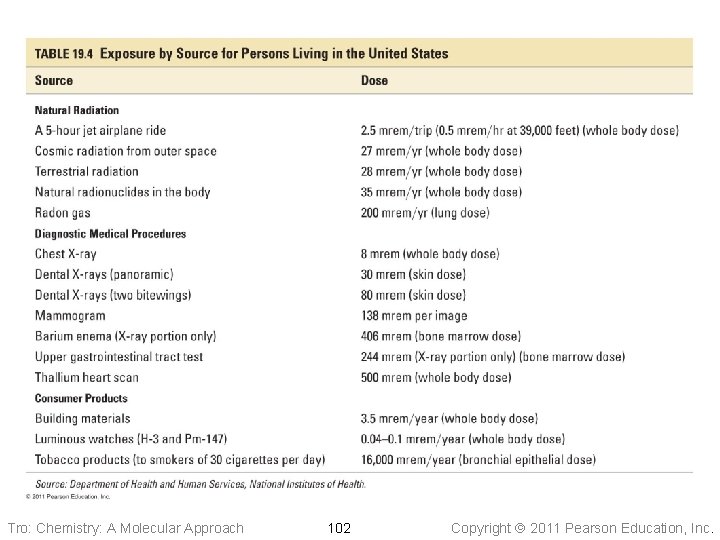
Tro: Chemistry: A Molecular Approach 102 Copyright 2011 Pearson Education, Inc.

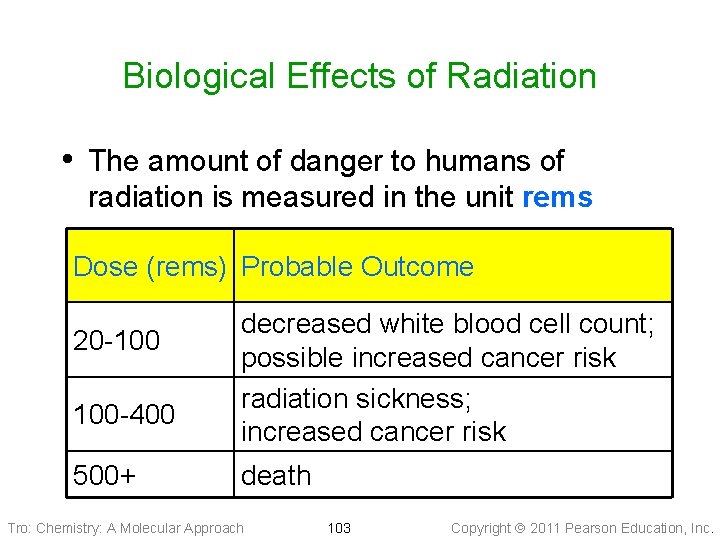
Biological Effects of Radiation • The amount of danger to humans of radiation is measured in the unit rems Dose (rems) Probable Outcome 20 -100 100 -400 500+ decreased white blood cell count; possible increased cancer risk radiation sickness; increased cancer risk death Tro: Chemistry: A Molecular Approach 103 Copyright 2011 Pearson Education, Inc.

Medical Uses of Radioisotopes, Diagnosis • Radiotracers ü certain organs absorb most or all of a particular element ü you can measure the amount absorbed by using tagged isotopes of the element and a Geiger counter Øtagged = radioisotope that can then be detected and measured ü use radioisotope with a short half-life ü use radioisotope that is low ionizing Øbeta or gamma Tro: Chemistry: A Molecular Approach 104 Copyright 2011 Pearson Education, Inc.

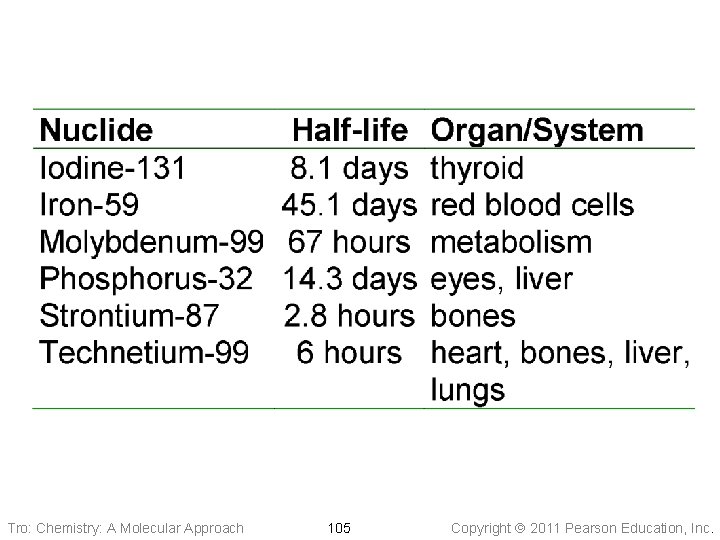
Tro: Chemistry: A Molecular Approach 105 Copyright 2011 Pearson Education, Inc.

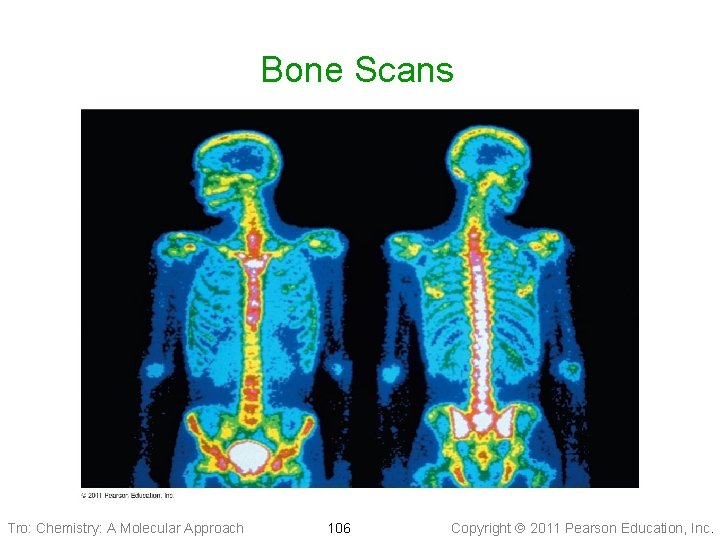
Bone Scans Tro: Chemistry: A Molecular Approach 106 Copyright 2011 Pearson Education, Inc.

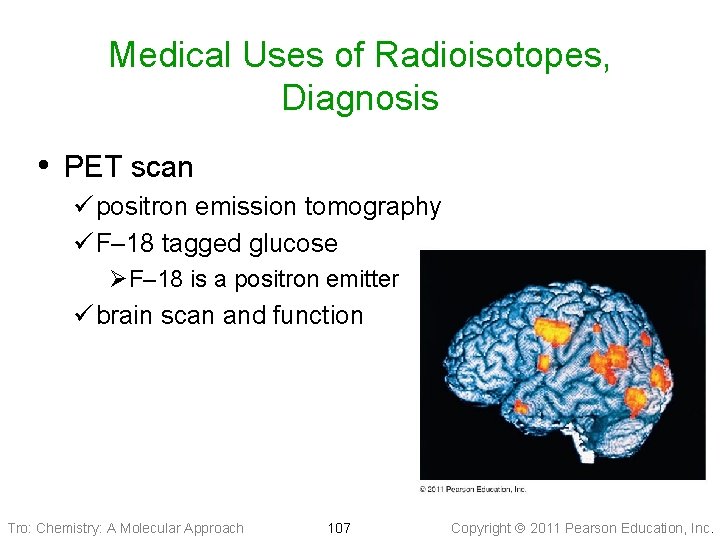
Medical Uses of Radioisotopes, Diagnosis • PET scan ü positron emission tomography ü F– 18 tagged glucose ØF– 18 is a positron emitter ü brain scan and function Tro: Chemistry: A Molecular Approach 107 Copyright 2011 Pearson Education, Inc.

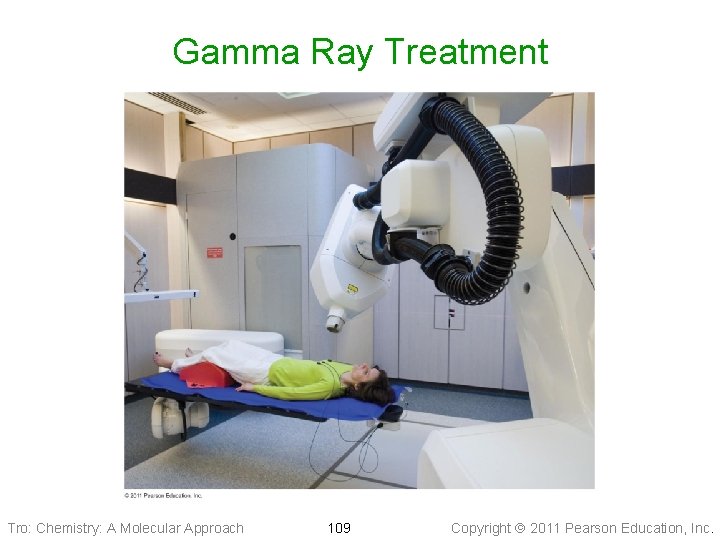
Medical Uses of Radioisotopes, Treatment – Radiotherapy • Cancer treatment ü cancer cells more sensitive to radiation than healthy cells – use radiation to kill cancer cells without doing significant damage ü brachytherapy Ø place radioisotope directly at site of cancer ü teletherapy Ø use gamma radiation from Co– 60 outside to penetrate inside Ø IMRT ü radiopharmaceutical therapy Ø use radioisotopes that concentrate in one area of the body Tro: Chemistry: A Molecular Approach 108 Copyright 2011 Pearson Education, Inc.

Gamma Ray Treatment Tro: Chemistry: A Molecular Approach 109 Copyright 2011 Pearson Education, Inc.

Nonmedical Uses of Radioactive Isotopes • Smoke detectors ü Am– 241 ü smoke blocks ionized air, breaks circuit • Insect control ü sterilize males • Food preservation • Radioactive tracers ü follow progress of a “tagged” atom in a reaction • Chemical analysis ü neutron activation analysis Tro: Chemistry: A Molecular Approach 110 Copyright 2011 Pearson Education, Inc.

Nonmedical Uses of Radioactive Isotopes • Authenticating art object ü many older pigments and ceramics were made from minerals with small amounts of radioisotopes • Crime scene investigation • Measure thickness or condition of industrial materials ü corrosion ü track flow through process ü gauges in high temp processes ü weld defects in pipelines ü road thickness Tro: Chemistry: A Molecular Approach 111 Copyright 2011 Pearson Education, Inc.

Nonmedical Uses of Radioactive Isotopes • Agribusiness ü develop disease-resistant crops ü trace fertilizer use • Treat computer disks to enhance data integrity • Nonstick pan coatings ü initiates polymerization • Photocopiers to help keep paper from jamming • Sterilize cosmetics, hair products and contact lens solutions and other personal hygiene products Tro: Chemistry: A Molecular Approach 112 Copyright 2011 Pearson Education, Inc.
 Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Democritus atomic model diagram
Democritus atomic model diagram Covalent bond melting point
Covalent bond melting point Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Ap chemistry unit 9 notes
Ap chemistry unit 9 notes Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Approach chemistry chalk chapter
Approach chemistry chalk chapter Difference between datagram and virtual circuit operation
Difference between datagram and virtual circuit operation Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Fine grained screening
Fine grained screening Multiple approach-avoidance conflict
Multiple approach-avoidance conflict Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Research approach example
Research approach example Traditional approach to systems implementation
Traditional approach to systems implementation Tony wagner's seven survival skills
Tony wagner's seven survival skills Pg 91
Pg 91 Chemistry geometry
Chemistry geometry Molecular geometry of pf3
Molecular geometry of pf3 Molecular biology crash course
Molecular biology crash course Molecular level vs cellular level
Molecular level vs cellular level Molecular absorption
Molecular absorption Ab6 molecular geometry
Ab6 molecular geometry Kinetic molecular theory
Kinetic molecular theory Covalent molecular and covalent network
Covalent molecular and covalent network Kinetic molecular theory of solid
Kinetic molecular theory of solid Significance of chemical formula
Significance of chemical formula Clasificacion molecular cancer de endometrio
Clasificacion molecular cancer de endometrio Unit chemical bonding molecular geometry
Unit chemical bonding molecular geometry Palindromic sequence
Palindromic sequence Molecular rebar
Molecular rebar Covalent molecular substances
Covalent molecular substances Empirical formula of haemoglobin
Empirical formula of haemoglobin Polydispersity
Polydispersity Molecular clock theory
Molecular clock theory How to calculate empirical formula using percentages
How to calculate empirical formula using percentages How to get empirical formula from percentages
How to get empirical formula from percentages Potassium permanganate percent composition
Potassium permanganate percent composition How to find empirical formula from percent
How to find empirical formula from percent Molecular modelling laboratory
Molecular modelling laboratory Molecular replacement method
Molecular replacement method Cycloalkanes
Cycloalkanes Naming compounds and writing formulas
Naming compounds and writing formulas Binary molecule
Binary molecule N srinivasan mbu
N srinivasan mbu Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Number-average molecular weight
Number-average molecular weight Molecular shape for ch2o
Molecular shape for ch2o Molecular shapes quiz
Molecular shapes quiz Molecular shape finder
Molecular shape finder Molecular replacement method
Molecular replacement method Patterson
Patterson Carbon dioxide orbital diagram
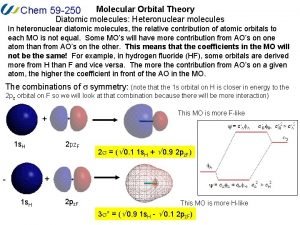
Carbon dioxide orbital diagram Heteronuclear diatomic molecules molecular orbital diagram
Heteronuclear diatomic molecules molecular orbital diagram B2 molecular orbital diagram
B2 molecular orbital diagram Imotinib
Imotinib Hnhhh
Hnhhh Define microbiology
Define microbiology Vsepr axe chart
Vsepr axe chart No2-1 molecular geometry
No2-1 molecular geometry Lewis structures and molecular geometry
Lewis structures and molecular geometry Scl molecular geometry
Scl molecular geometry Molecular geometry and bonding theories
Molecular geometry and bonding theories Abe3 molecular geometry
Abe3 molecular geometry Molecular gastronomy restaurants
Molecular gastronomy restaurants How to find empirical formula
How to find empirical formula Molecular farming definition
Molecular farming definition Molecular ecological network analyses
Molecular ecological network analyses Unit of molar mass
Unit of molar mass How to find molecular concentration
How to find molecular concentration Cintico
Cintico Membrane osmometry molecular weight
Membrane osmometry molecular weight Molécula sales minerales
Molécula sales minerales Molecular dynamics limitations
Molecular dynamics limitations Molecular geometry of icl4-
Molecular geometry of icl4- I3- point group
I3- point group Lentils examples
Lentils examples Kinetic molecular theory of gases
Kinetic molecular theory of gases Molecular theory of gases and liquids
Molecular theory of gases and liquids The kinetic molecular theory
The kinetic molecular theory Nocl lewis structure shape
Nocl lewis structure shape Application of uv visible spectroscopy
Application of uv visible spectroscopy Molecular replacement method
Molecular replacement method Covalent network solid vs molecular solid
Covalent network solid vs molecular solid Bio molecular systems ltd
Bio molecular systems ltd Icl2 molecular geometry
Icl2 molecular geometry Section 14-3 human molecular genetics answers
Section 14-3 human molecular genetics answers Molecular gastronomy history
Molecular gastronomy history Hidrogenul molecular
Hidrogenul molecular Molecular biology evidence of evolution
Molecular biology evidence of evolution Formula mass vs molecular mass
Formula mass vs molecular mass Pbr3 geometria molecular
Pbr3 geometria molecular Geometria molecular
Geometria molecular Geometría molecular angular
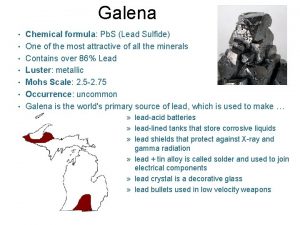
Geometría molecular angular What is galena chemical formula
What is galena chemical formula Formula mass vs molecular mass
Formula mass vs molecular mass What is atomicity
What is atomicity Dan dial molecular enhancer
Dan dial molecular enhancer What is the percent of iodine in ointment
What is the percent of iodine in ointment Fuentes de ionizacion duras y blandas
Fuentes de ionizacion duras y blandas Covalent molecular and covalent network
Covalent molecular and covalent network Silicon carbide covalent network
Silicon carbide covalent network Carbon dioxide symbol
Carbon dioxide symbol Empirical formula c5h12
Empirical formula c5h12 Empirical and molecular formula worksheet
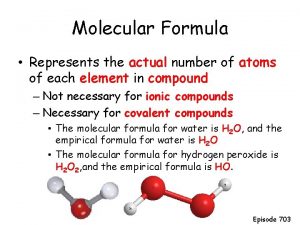
Empirical and molecular formula worksheet Molecular formula
Molecular formula Empirical formula vs
Empirical formula vs Empirical vs molecular formula
Empirical vs molecular formula Which formula is an empirical formula?
Which formula is an empirical formula? Molecular formula example
Molecular formula example