National 5 Chemistry Unit 2 Natures Chemistry Section






































- Slides: 80

National 5 Chemistry Unit 2 – Nature’s Chemistry Section 6 – Homologous Series

Section 6 – Homologous Series Pupils should be able to… • State that hydrocarbons are compounds of hydrogen and carbon • Define a homologous series • State that alkanes are a homologous series of saturated hydrocarbons • Define saturated hydrocarbons as having single carbon-carbon bonds • Represent the alkanes by a general formula • Describe the pattern of physical properties of a homologous series such as melting and boiling points. • State common uses of alkanes, chemical and physical properties • Explain this pattern of physical properties in relation to the increasing strength of intermolecular forces as molecular size increases

What are Hydrocarbons? • A hydrocarbon is a molecule which contains only the elements Carbon and Hydrogen. • Hydrocarbons are obtained from the Fractional Distillation of crude oil. We use them for fuels in cars (octane) and in cookers (methane).

How do we Group all these Hydrocarbons Together?

What is a Homologous Series? Homologous Series • A group of chemically similar compounds which can be represented by a general formula. • Physical properties change gradually as you go down the series There are three groups that we are going to look at 1. Alkanes 2. Alkenes 3. Cycloalkanes

Alkanes Methane Monsters Ethane Eat Propane Pupils Butane But Pentane Prefer Hexane Hairy Heptane Haggis Octane Occasionally

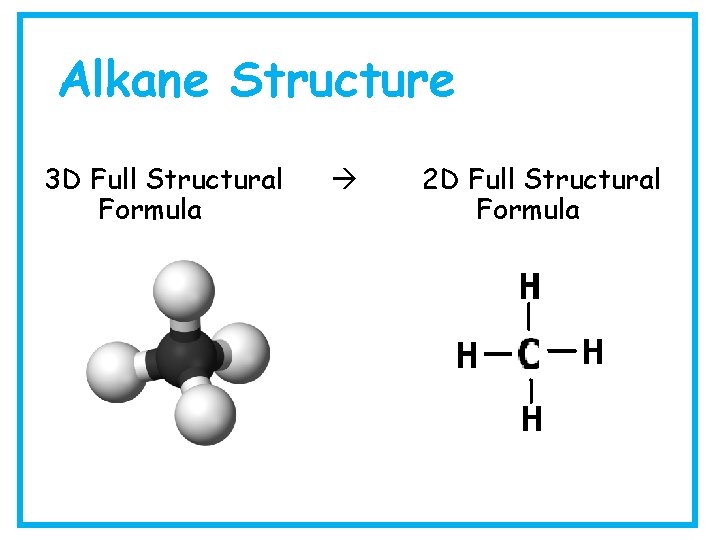
Alkane Structure 3 D Full Structural Formula 2 D Full Structural Formula

Alkanes • Each member can be represented in three different ways: Methane Propane • Full Structural Formula • Shortened Structural Formula • Molecular Formula

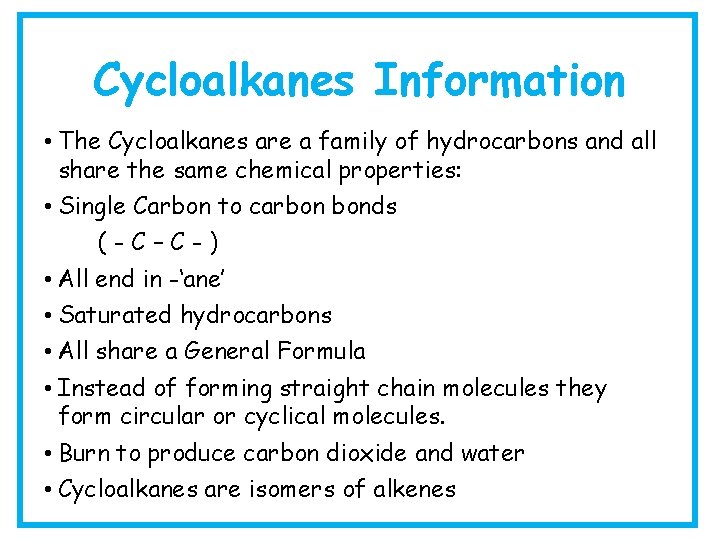
Alkane Information • The Alkanes are a family of hydrocarbons and all share the same chemical properties: • Single Carbon to carbon bonds (- C – C -) • They are insoluble in water • Burn in oxygen to produce carbon dioxide and water • All end in -‘ane’ • Saturated hydrocarbons • All share a General Formula

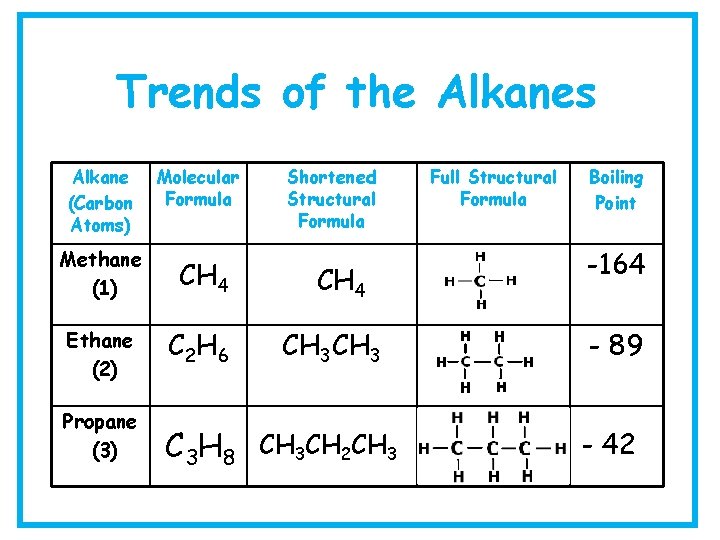
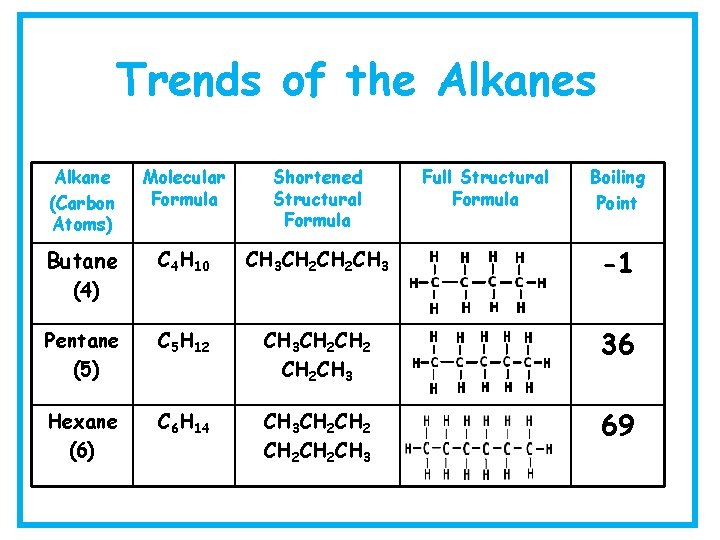
Trends of the Alkanes Alkane (Carbon Atoms) Molecular Formula Methane (1) CH 4 Ethane (2) C 2 H 6 Propane (3) Shortened Structural Formula CH 4 CH 3 C 3 H 8 CH 3 CH 2 CH 3 Full Structural Formula Boiling Point -164 - 89 - 42

Trends of the Alkanes Alkane (Carbon Atoms) Molecular Formula Shortened Structural Formula Butane C 4 H 10 CH 3 CH 2 CH 3 -1 Pentane (5) C 5 H 12 CH 3 CH 2 CH 3 36 Hexane (6) C 6 H 14 CH 3 CH 2 CH 3 69 (4) Full Structural Formula Boiling Point

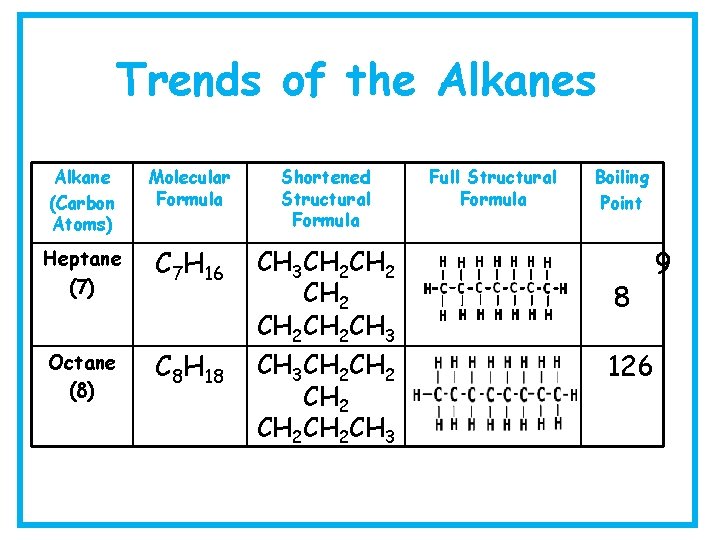
Trends of the Alkanes Alkane (Carbon Atoms) Molecular Formula Shortened Structural Formula Heptane (7) C 7 H 16 Octane (8) C 8 H 18 CH 3 CH 2 CH 2 CH 2 CH 2 CH 3 Full Structural Formula Boiling Point 8 126 9

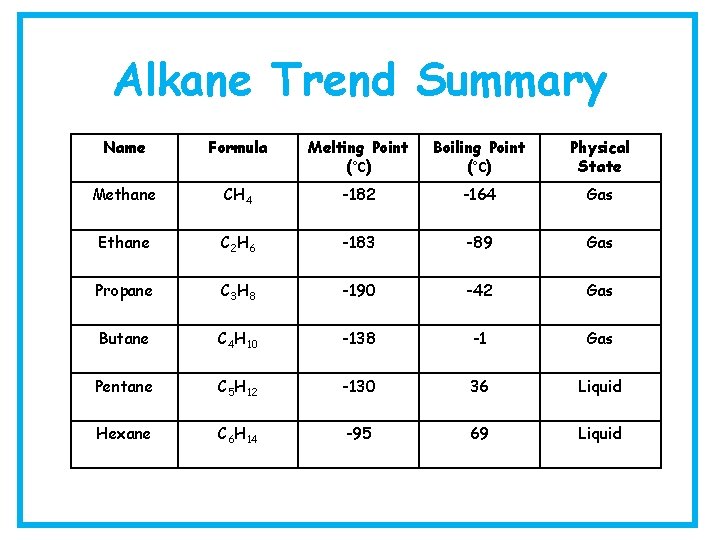
Alkane Trend Summary Name Formula Melting Point (°C) Boiling Point (°C) Physical State Methane CH 4 -182 -164 Gas Ethane C 2 H 6 -183 -89 Gas Propane C 3 H 8 -190 -42 Gas Butane C 4 H 10 -138 -1 Gas Pentane C 5 H 12 -130 36 Liquid Hexane C 6 H 14 -95 69 Liquid

Alkanes General Formula The no. of Hydrogen atoms = 2 times the number of Carbon atoms plus 2 General Formula for alkanes is Cn. H 2 n+2

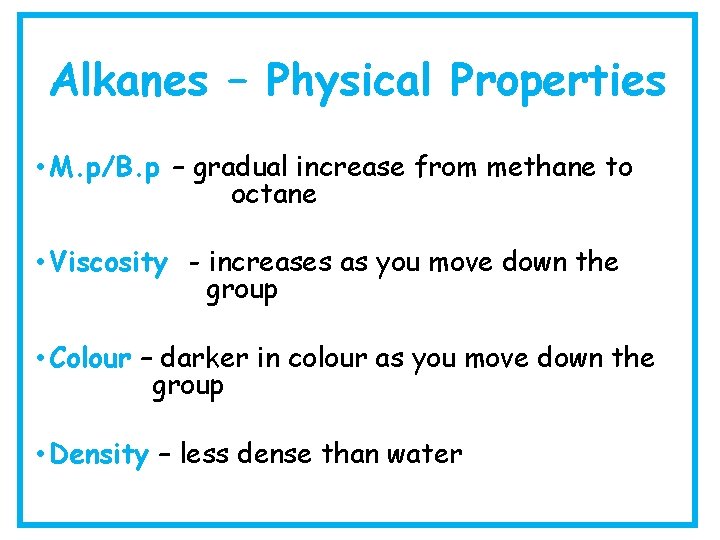
Alkanes – Physical Properties • M. p/B. p – gradual increase from methane to octane • Viscosity - increases as you move down the group • Colour – darker in colour as you move down the group • Density – less dense than water

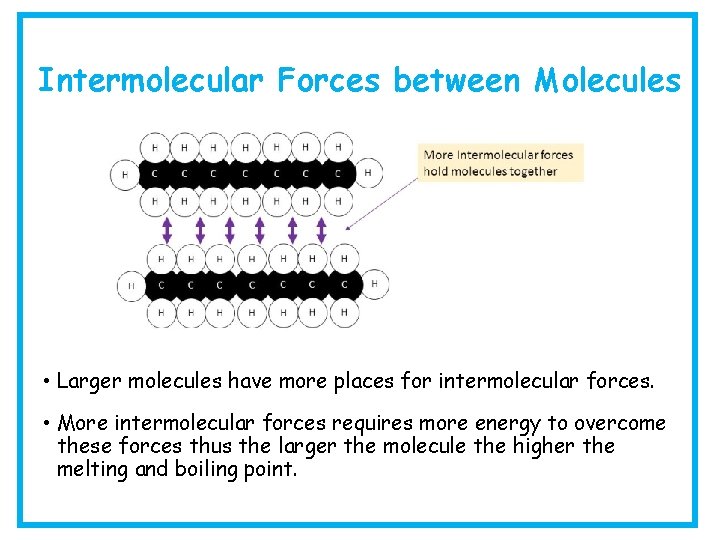
Intermolecular Forces between Molecules • Larger molecules have more places for intermolecular forces. • More intermolecular forces requires more energy to overcome these forces thus the larger the molecule the higher the melting and boiling point.

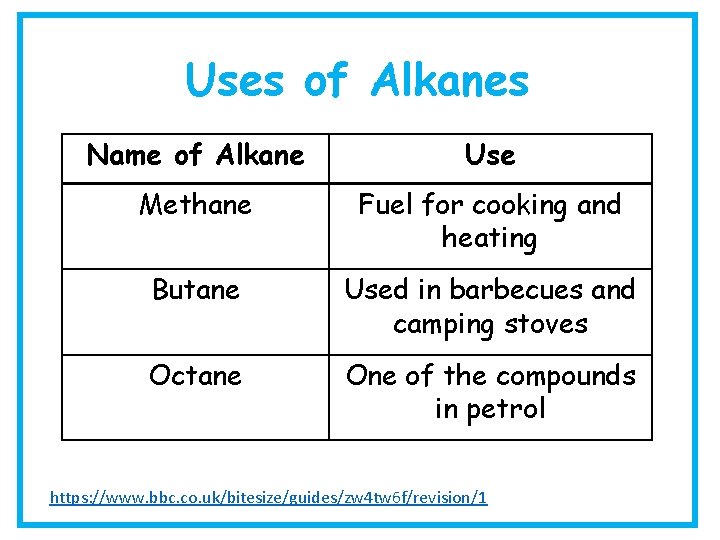
Uses of Alkanes Name of Alkane Use Methane Fuel for cooking and heating Butane Used in barbecues and camping stoves Octane One of the compounds in petrol https: //www. bbc. co. uk/bitesize/guides/zw 4 tw 6 f/revision/1

Section 6 – Homologous Series Pupils should be able to… • State that alkenes are a homologous series of unsaturated hydrocarbons • State that alkenes contain the C = C double bond • State common uses of alkenes, chemical and physical properties • Represent the alkenes by a general formula • Write molecular as full and shortened formula

Alkenes X Ethene Propene Butene Pentene Hexene Heptene Octene Monsters Eat Pupils But Prefer Hairy Haggis Occasionally

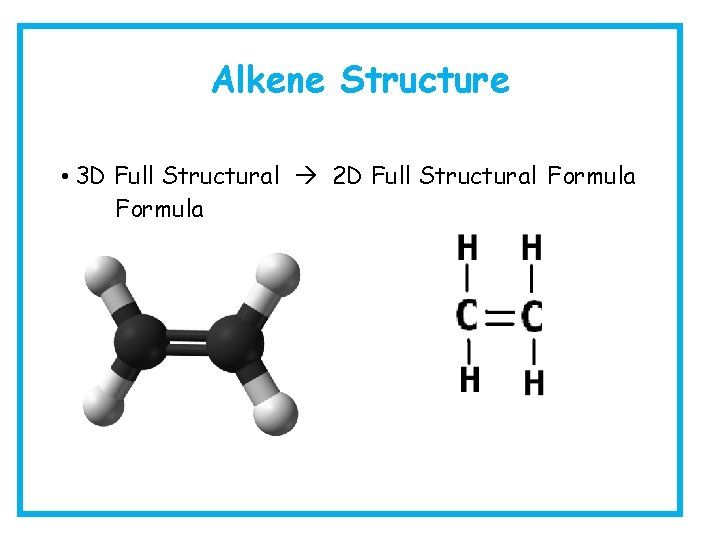
Alkene Structure • 3 D Full Structural 2 D Full Structural Formula

Alkenes • Each member can be represented in three different ways: Ethene Propene • Full Structural Formula • Shortened Structural Formula • Molecular Formula

Alkenes • The Alkenes are a family of hydrocarbons and all share the same chemical properties: • Double Carbon to carbon bonds (-C=C-) • All end in -‘ene’ • Unsaturated hydrocarbons • All share a General Formula

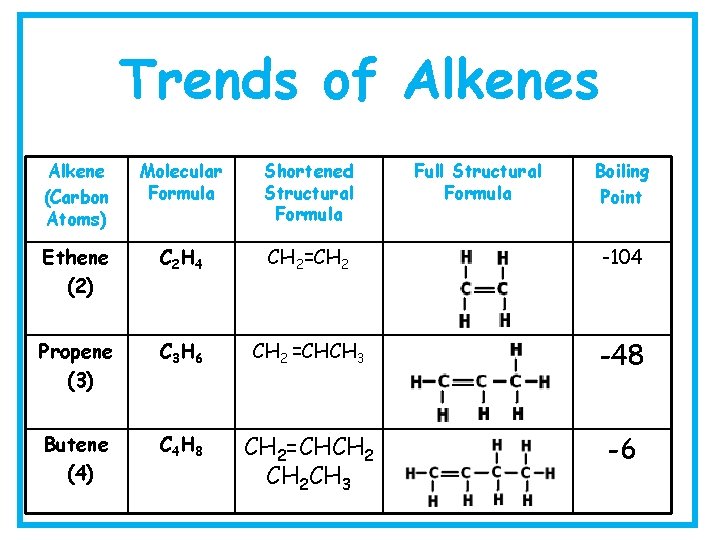
Trends of Alkenes Alkene (Carbon Atoms) Molecular Formula Shortened Structural Formula Full Structural Formula Boiling Point Ethene (2) C 2 H 4 CH 2=CH 2 -104 Propene (3) C 3 H 6 CH 2 =CHCH 3 -48 Butene (4) C 4 H 8 CH 2=CHCH 2 CH 3 -6

Trends of Alkenes Alkene (Carbon Atoms) Molecular Formula Shortened Structural Formula Full Structural Formula Boiling Point Pentene (5) C 5 H 10 CH 2=CHCH 2 CH 3 30 Hexene (6) C 6 H 12 CH 2=CHCH 2 CH 3 63

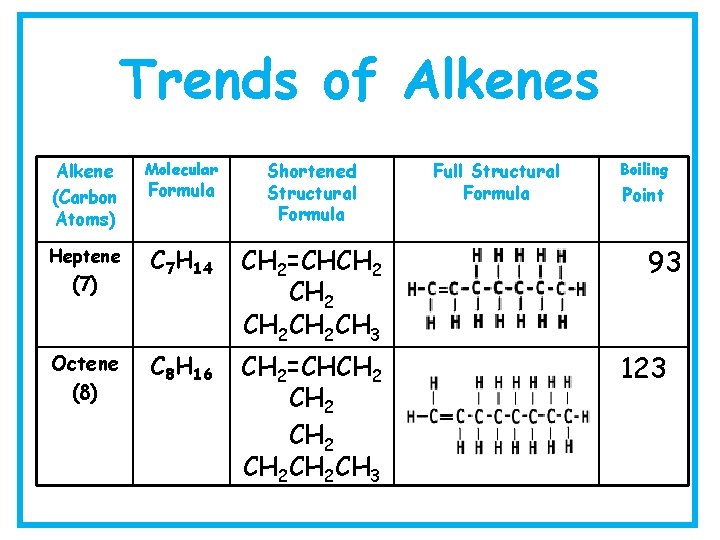
Trends of Alkenes Alkene (Carbon Atoms) Molecular Heptene C 7 H 14 CH 2=CHCH 2 CH 3 C 8 H 16 CH 2=CHCH 2 CH 2 CH 3 (7) Octene (8) Formula Shortened Structural Formula Full Structural Formula Boiling Point 93 123

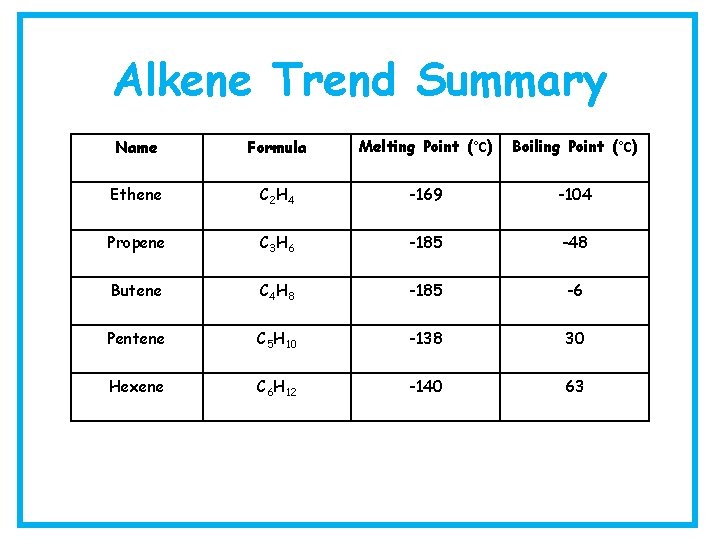
Alkene Trend Summary Name Formula Melting Point (°C) Boiling Point (°C) Ethene C 2 H 4 -169 -104 Propene C 3 H 6 -185 -48 Butene C 4 H 8 -185 -6 Pentene C 5 H 10 -138 30 Hexene C 6 H 12 -140 63

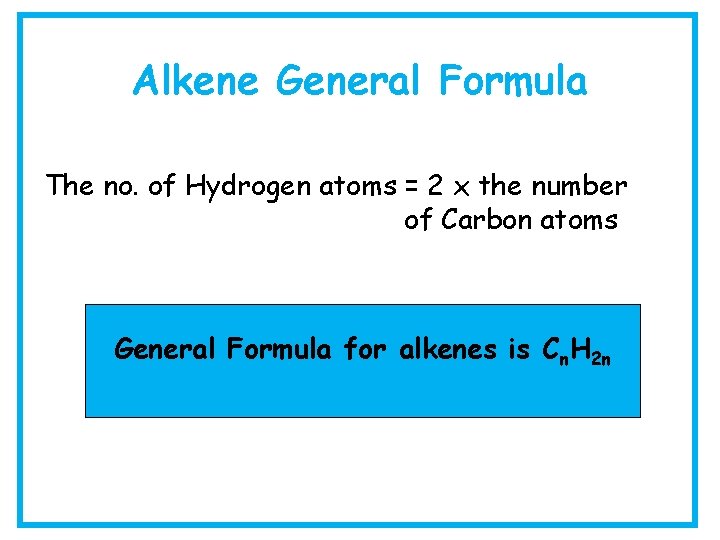
Alkene General Formula The no. of Hydrogen atoms = 2 x the number of Carbon atoms General Formula for alkenes is Cn. H 2 n

Properties of Alkenes • Physical Properties • The alkenes increase in size down the group • Their melting and boiling points increase down the group • Chemical Properties • All burn completely to produce carbon dioxide and water • Undergo addition reactions

Uses of Alkenes • Making polymers (plastics) • Industrial production of ethanol • Fuels • Solvents https: //www. bbc. co. uk/bitesize/guides/zw 4 tw 6 f/revision/3

Section 6 – Homologous Series Pupils should be able to… • State that cycloalkanes are a homologous series of saturated cyclic hydrocarbons • State common uses of cycloalkanes, chemical and physical properties • Represent the cycloalkanes by a general formula

Cycloalkanes Information • Cycloalkanes are a homologous series of cyclic hydrocarbon Uses Cyclohexane for making nylon Solvents for compounds that don’t dissolve in water Used in motor oil, kerosine, diesel and other heavy oils

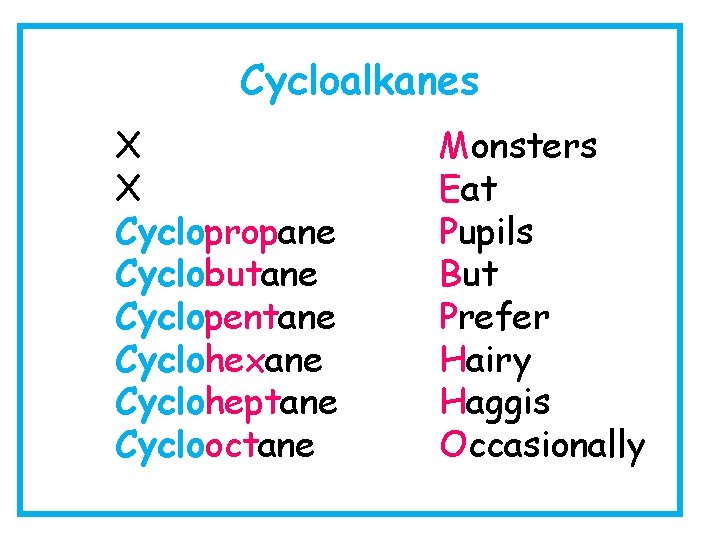
Cycloalkanes X X Cyclopropane Cyclobutane Cyclopentane Cyclohexane Cycloheptane Cyclooctane Monsters Eat Pupils But Prefer Hairy Haggis Occasionally

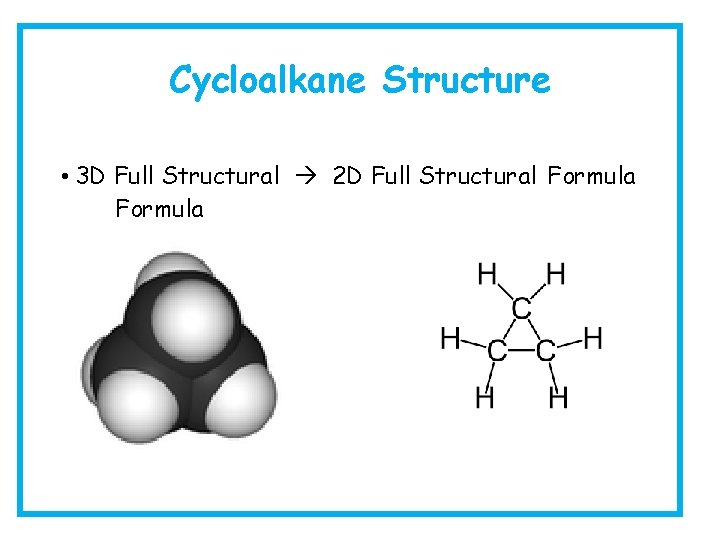
Cycloalkane Structure • 3 D Full Structural 2 D Full Structural Formula

Cycloalkanes • Each member can be represented in three different ways: Cyclopropane • Full Structural Formula • Shortened Structural Formula • Molecular Formula

Cycloalkanes Information • The Cycloalkanes are a family of hydrocarbons and all share the same chemical properties: • Single Carbon to carbon bonds (-C–C-) • All end in -‘ane’ • Saturated hydrocarbons • All share a General Formula • Instead of forming straight chain molecules they form circular or cyclical molecules. • Burn to produce carbon dioxide and water • Cycloalkanes are isomers of alkenes

Physical Properties • They increase in size • The m. p/b. p increases as the size increases

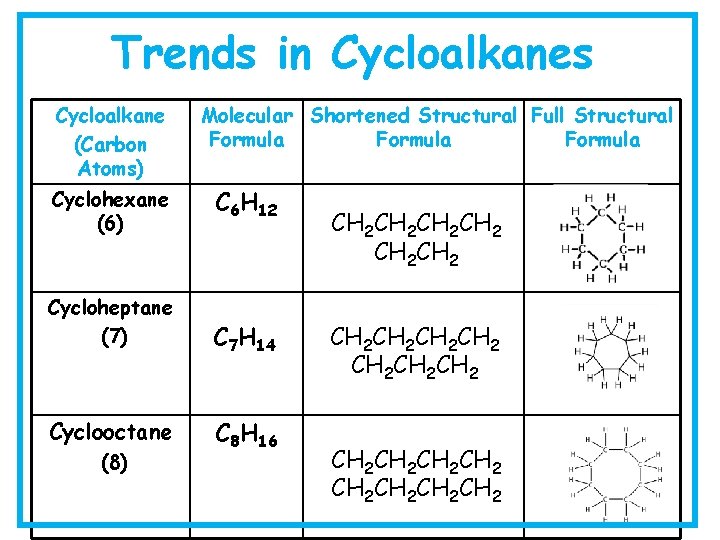
Trends in Cycloalkanes Cycloalkane (Carbon Atoms) Molecular Formula Shortened Structural Formula C 3 H 6 CH 2 CH 2 Cyclobutane (4) C 4 H 8 CH 2 CH 2 Cyclopentane C 5 H 10 Cyclopropane (3) (5) CH 2 CH 2 Full Structural Formula

Trends in Cycloalkanes Cycloalkane (Carbon Atoms) Cyclohexane (6) Molecular Shortened Structural Full Structural Formula C 6 H 12 Cycloheptane (7) C 7 H 14 Cyclooctane C 8 H 16 (8) CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH 2

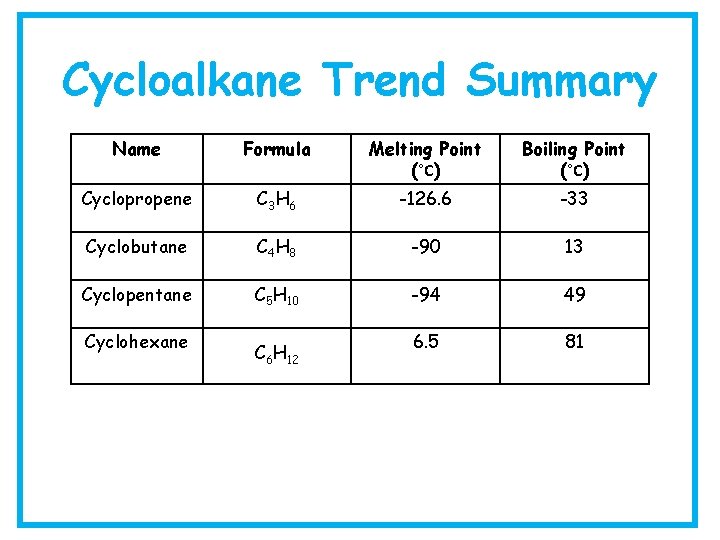
Cycloalkane Trend Summary Name Formula Melting Point (°C) Boiling Point (°C) Cyclopropene C 3 H 6 -126. 6 -33 Cyclobutane C 4 H 8 -90 13 Cyclopentane C 5 H 10 -94 49 6. 5 81 Cyclohexane C 6 H 12

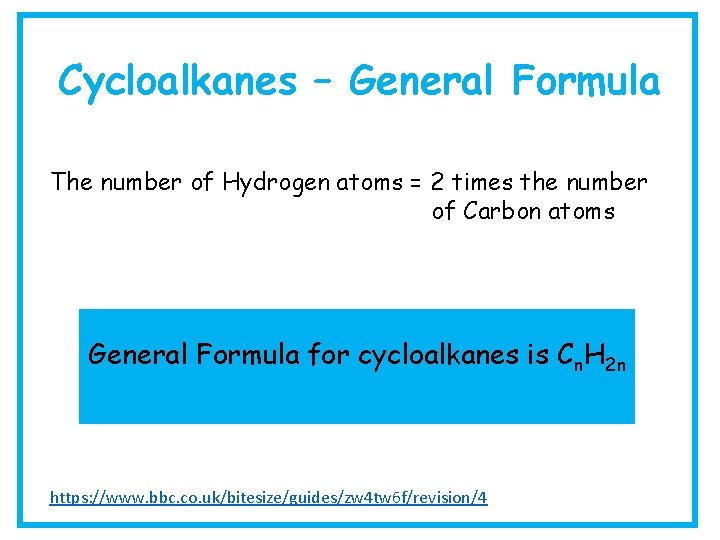
Cycloalkanes – General Formula The number of Hydrogen atoms = 2 times the number of Carbon atoms General Formula for cycloalkanes is Cn. H 2 n https: //www. bbc. co. uk/bitesize/guides/zw 4 tw 6 f/revision/4

Section 6 – Homologous Series Pupils should be able to… • Describe how to distinguish an unsaturated and saturated compound using bromine solution • State that alkenes can undergo addition reactons: • With hydrogen forming alkanes, known as hydrogenation • With halogens forming dihaloalkanes • With water forming alcohols known as hydration

How can we tell these Hydrocarbons apart? We need to be able to experimentally tell whether a hydrocarbon is an alkane, an alkene or even a cycloalkane so how might we do this? What chemical property do they have that is different that we may be able to exploit? Answer: the reactive C=C bond which we can break. We use the Bromine Test.

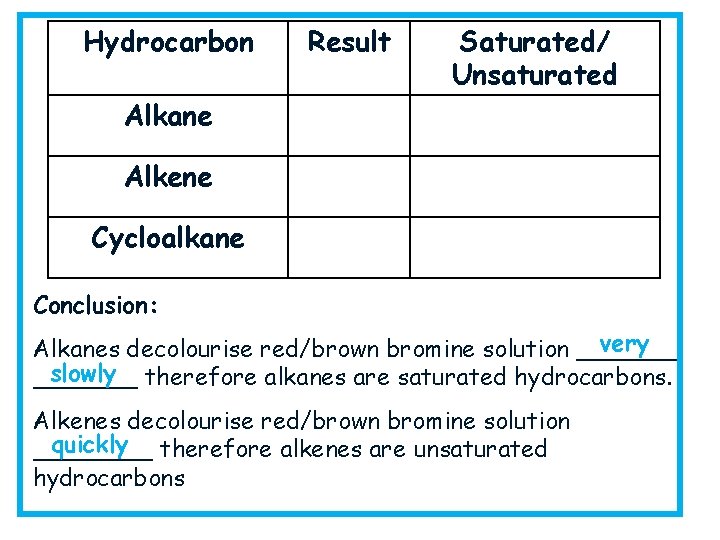
Testing for Saturation Collect: goggles, three test tubes, bromine solution, test tube rack, an alkane, alkene and cycloalkane for testing. Method: add 2 cm 3 alkane to a test tube then add in a few drops of bromine water, shake and observe what happens Repeat with the alkene and the cycloalkane then record your results in a table.

Hydrocarbon Result Saturated/ Unsaturated Alkane Alkene Cycloalkane Conclusion: very Alkanes decolourise red/brown bromine solution _______ slowly therefore alkanes are saturated hydrocarbons. _______ Alkenes decolourise red/brown bromine solution quickly therefore alkenes are unsaturated ____ hydrocarbons

Testing for Unsaturation Alkane + Bromine Cycloalkane + Bromine Alkene + Bromine

Why?

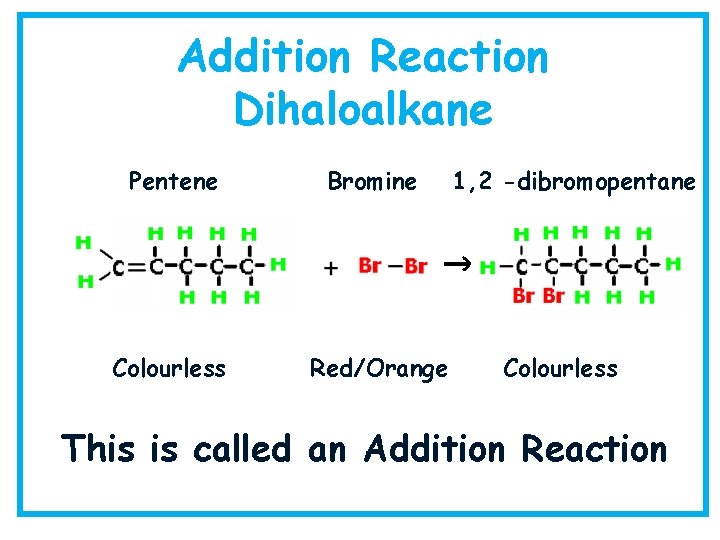
Addition Reaction Dihaloalkane Pentene Bromine Colourless Red/Orange 1, 2 -dibromopentane Colourless This is called an Addition Reaction

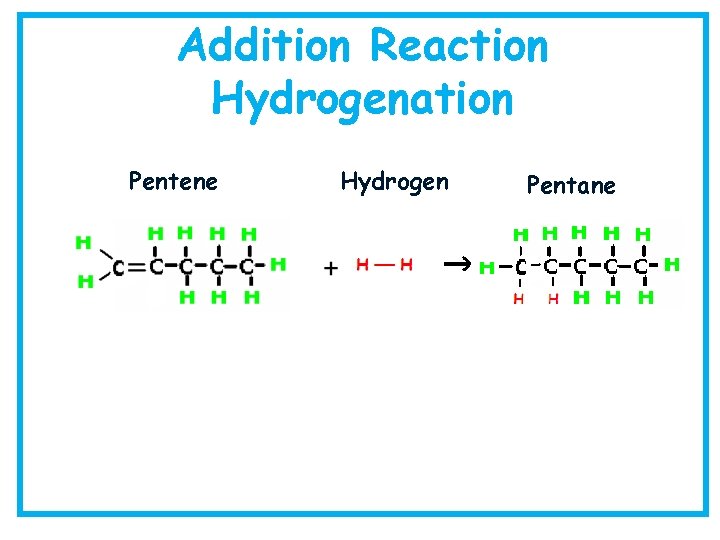
Addition Reaction Hydrogenation Pentene Hydrogen Pentane

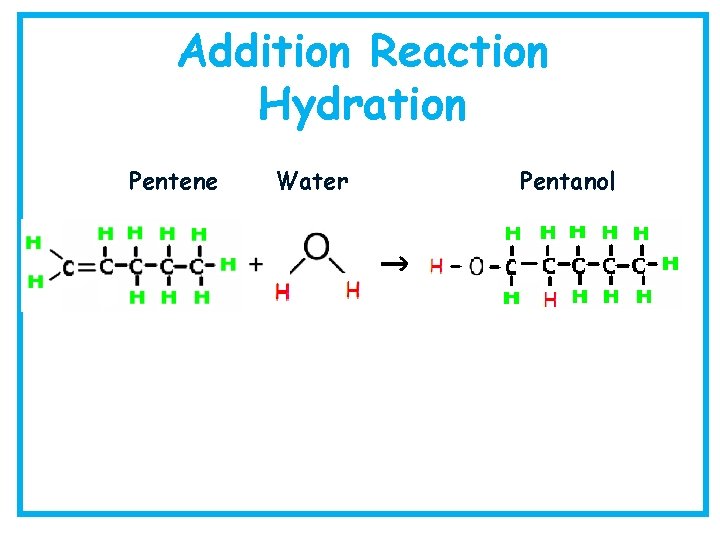
Addition Reaction Hydration Pentene Water Pentanol

Saturation Summary • We can use Bromine solution to test for unsaturation in an addition reaction • Alkanes are saturated hydrocarbons. Alkanes decolourise red/brown bromine solution very slowly • Alkenes are unsaturated hydrocarbons. Alkenes decolourise red/brown bromine solution quickly

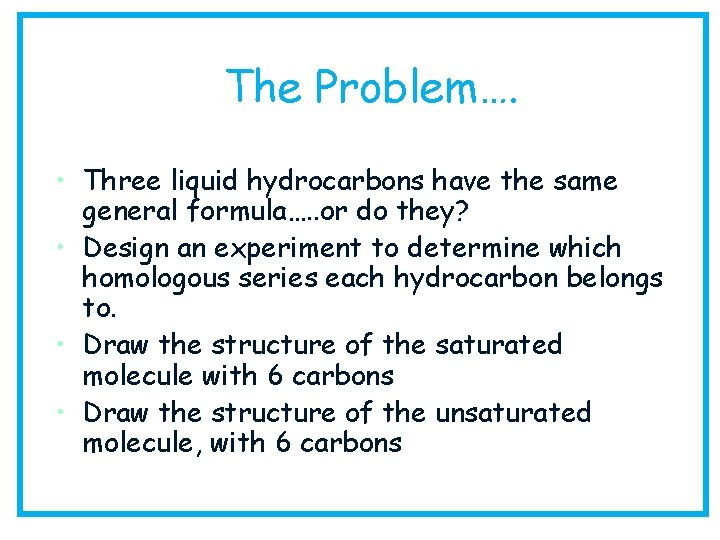
The Problem…. • Three liquid hydrocarbons have the same general formula…. . or do they? • Design an experiment to determine which homologous series each hydrocarbon belongs to. • Draw the structure of the saturated molecule with 6 carbons • Draw the structure of the unsaturated molecule, with 6 carbons

Section 6 – Homologous Series Pupils should be able to… • Systematically name straight and branched chain alkanes and alkenes • Draw structural formula from the systematic names of straight chain and branched alkanes and alkenes • Systematically name non-branched cycloalkanes

Naming Alkanes 1. Find the longest chain which will form the last part of the name. 2. Number the main chain so that it gives the lower number over positions to the side groups. 3. Side branch names end in “yl” and depend on the number of carbon atoms in them; 1 = methyl 2 = ethyl 3 = propyl etc 4. Alphabetically order is used if different side branches appear in the same structure e. g. ethyl before methyl 5. Hyphens are used before or after numbers that come next to letters within a name e. g. (2 -ethyl-3 -methyl…. . ) 6. Commas are used between numbers if there is more than one of the same side branch (e. g. 2, 3, 3 – trimethyl…. )

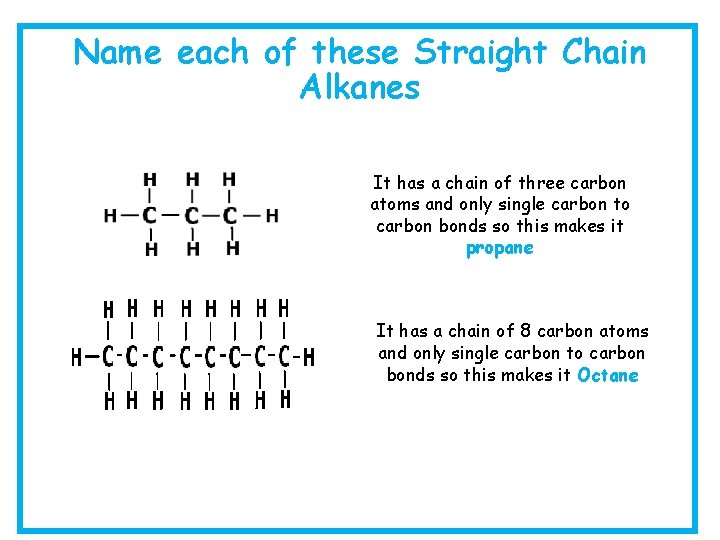
Name each of these Straight Chain Alkanes It has a chain of three carbon atoms and only single carbon to carbon bonds so this makes it propane It has a chain of 8 carbon atoms and only single carbon to carbon bonds so this makes it Octane

Naming Straight Chain Alkenes 1. Find the longest chain which contains the double bond, this will form the last part of the name. 2. Number the main chain so that it gives the lowest number for the double bond over positions to the side groups. 3. Side branch names end in “yl” and depend on the number of carbon atoms in them; 1 = methyl 2 = ethyl 3 = propyl etc 4. Alphabetically order is used if different side branches appear in the same structure e. g. ethyl before methyl 5. Hyphens are used before or after numbers that come next to letters within a name e. g. (2 -ethyl-3 -methyl…. . ) 6. Commas are used between numbers if there is more than one of the same side branch (e. g. 2, 3, 3 – trimethyl…. )

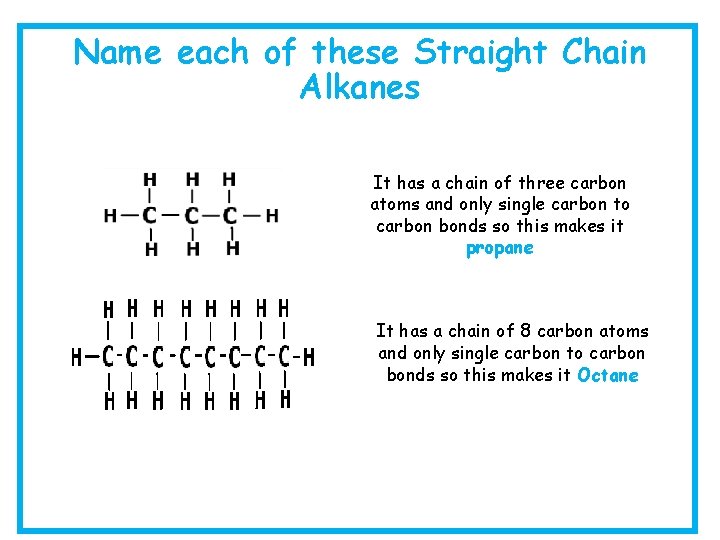
Name each of these Straight Chain Alkanes It has a chain of three carbon atoms and only single carbon to carbon bonds so this makes it propane It has a chain of 8 carbon atoms and only single carbon to carbon bonds so this makes it Octane

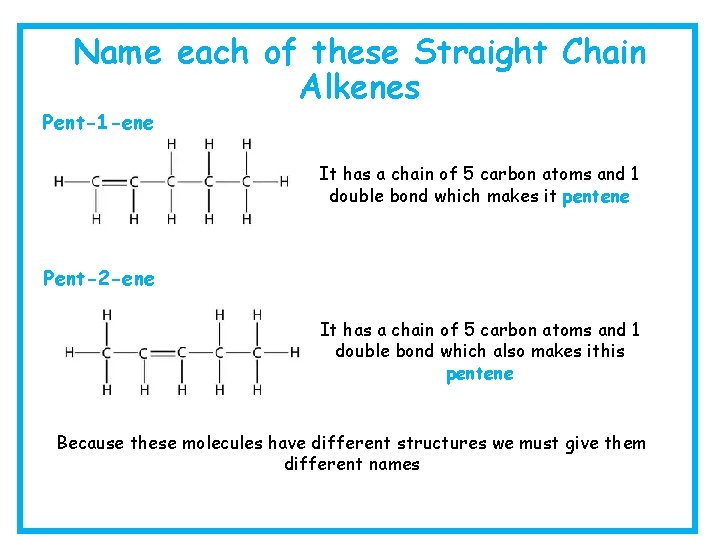
Name each of these Straight Chain Alkenes Pent-1 -ene It has a chain of 5 carbon atoms and 1 double bond which makes it pentene Pent-2 -ene It has a chain of 5 carbon atoms and 1 double bond which also makes ithis pentene Because these molecules have different structures we must give them different names

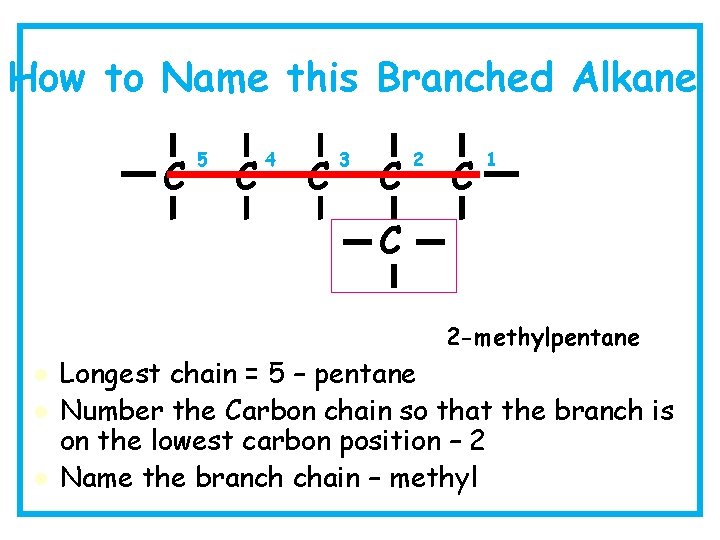
How to Name this Branched Alkane C 5 C 4 C 3 C 2 C 1 C 2 -methylpentane l l l Longest chain = 5 – pentane Number the Carbon chain so that the branch is on the lowest carbon position – 2 Name the branch chain – methyl

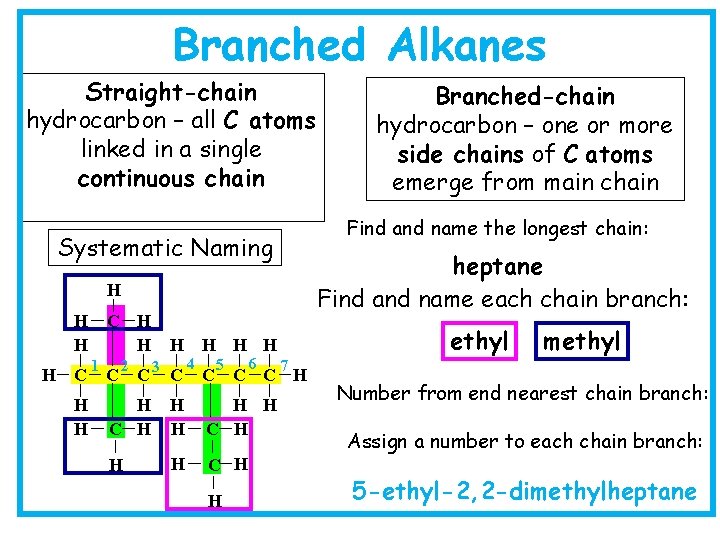
Branched Alkanes Straight-chain hydrocarbon – all C atoms linked in a single continuous chain Find and name the longest chain: Systematic Naming heptane Find and name each chain branch: H H C H H 1 2 C C H 3 C 4 ethyl H H H 5 6 H H C H H methyl 7 C C C H H C H Branched-chain hydrocarbon – one or more side chains of C atoms emerge from main chain Number from end nearest chain branch: Assign a number to each chain branch: 5 -ethyl-2, 2 -dimethylheptane

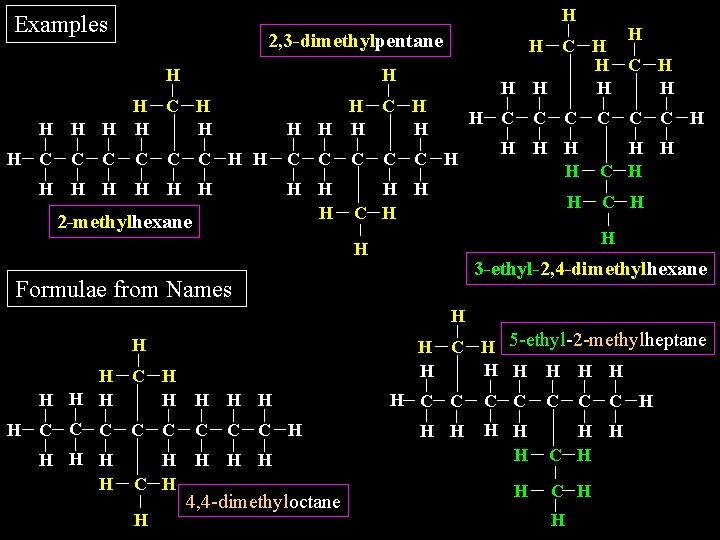
H Examples H H C H H H H C C H H H C H 2, 3 -dimethylpentane H H H H C H H H H H C C C H H H C H 2 -methylhexane H C C H C H H H 3 -ethyl-2, 4 -dimethylhexane Formulae from Names H H C H 5 -ethyl-2 -methylheptane H H H C H H H C C C C H H H 4, 4 -dimethyloctane H C C C H H H H C C H H

Naming Branched Alkenes Isomers are compounds with the same molecular formula but different structural formulae Systematic Naming H H 4 3 2 H H C C H H 1 Find the longest chain containing the C=C and but give its prefix: Number from the end nearest C=C: Insert lowest number for C=C between prefix and the ending ‘ene’: but-1 -ene Number and name any branches as for alkanes: Isomers of but-1 -ene H H H C C H H H but-2 -ene H C H C C H H 2 -methylpropene

Section 6 – Homologous Series Pupils should be able to… • State the definition of an isomer • Understand that an isomer may belong to different homologous series • Describe how isomers may have different physical properties • Be given the structural formula for a compound, be able to draw an isomer • Use a molecular formula to draw isomers

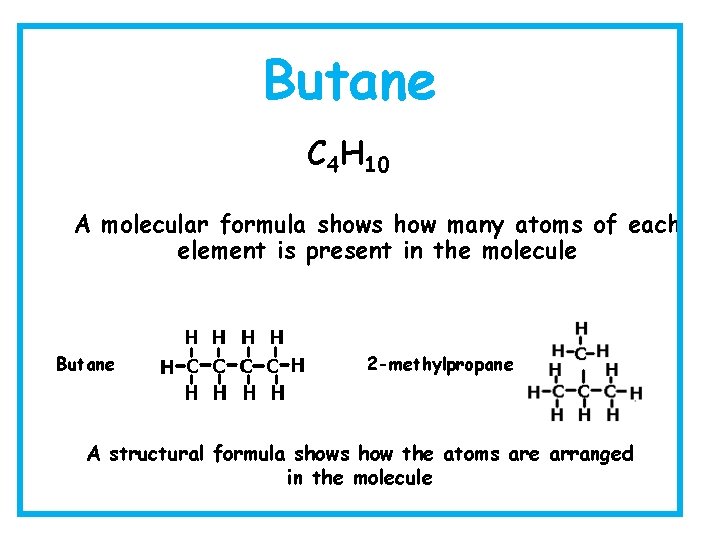
Isomers of Alkanes Isomers have the same molecular formula but different structural formulas A molecular formula shows how many atoms of each element is present in the molecule A structural formula shows how the atoms are arranged in the molecule

Butane C 4 H 10 A molecular formula shows how many atoms of each element is present in the molecule Butane 2 -methylpropane A structural formula shows how the atoms are arranged in the molecule

Isomers of Butane 2 -methylpropane are • Butane Bothand Molecules contain isomers of each other as they have 4 carbon atomsformula but the same molecular different structural formulas • Both Molecules contain 10 hydrogen atoms 2 -methylpropane

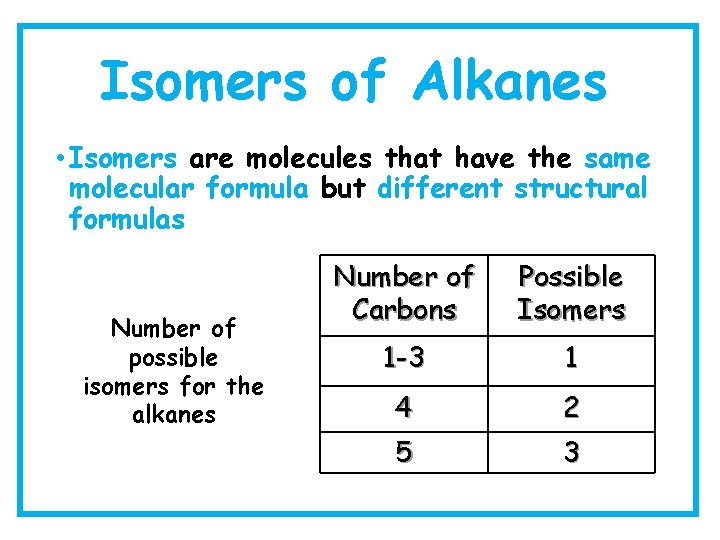
Isomers of Alkanes • Isomers are molecules that have the same molecular formula but different structural formulas Number of possible isomers for the alkanes Number of Carbons Possible Isomers 1 -3 1 4 2 5 3

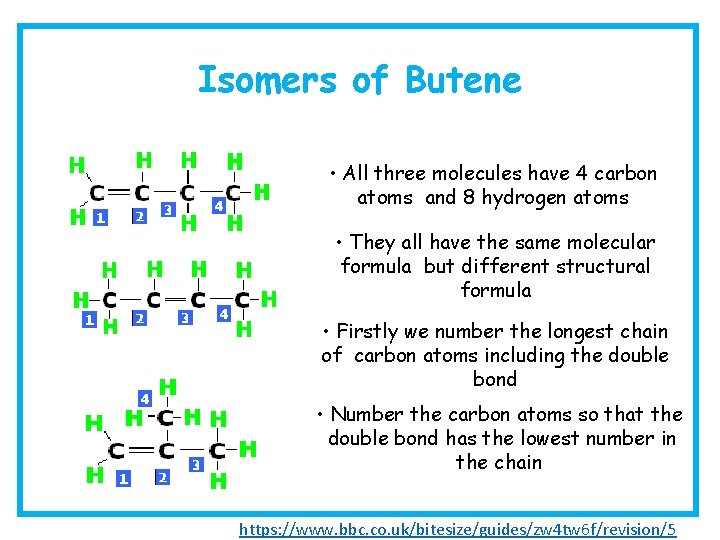
Isomers of Alkenes e. g. Butene C 4 H 8 A molecular formula shows how many atoms of each element is present in the molecule A structural formula shows how the atoms are arranged in the molecule

Isomers of Butene • All three molecules have 4 carbon atoms and 8 hydrogen atoms 1 -Butene 2 -Butene • They all have the same molecular formula but different structural formula • Firstly we number the longest chain of carbon atoms including the double bond • Number the carbon atoms so that the double bond has the lowest number in the chain 2 -methyl-1 -propene https: //www. bbc. co. uk/bitesize/guides/zw 4 tw 6 f/revision/5




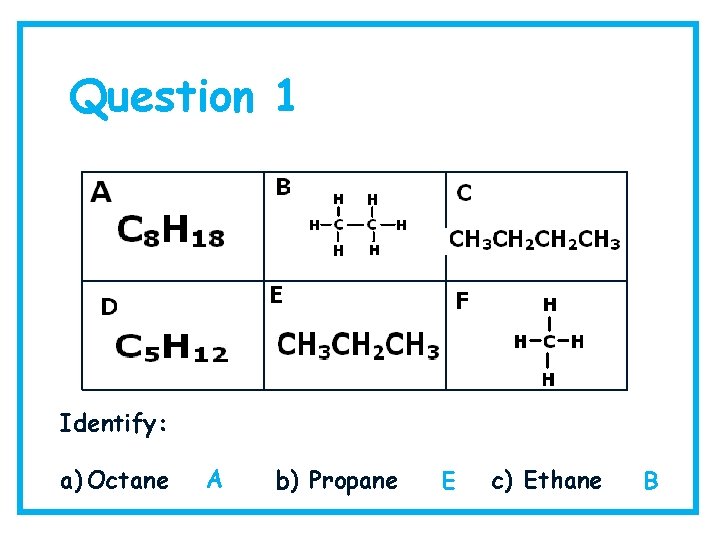
Question 1 Identify: a) Octane A b) Propane E c) Ethane B

Question 2 Explain what is meant by: a) A saturated hydrocarbon b) An unsaturated hydrocarbon

Question 3 Consider this list of hydrocarbons Butane, Ethene, Octane, Methane, Butene Name the hydrocarbons which are a) Saturated b) Unsaturated

Question 4 State the molecular formula for each of the following straight chain hydrocarbons a) Octane b) Hexene c) The saturated hydrocarbon with 12 carbon atoms per molecule d) The unsaturated hydrocarbon with 10 carbon atoms per molecule

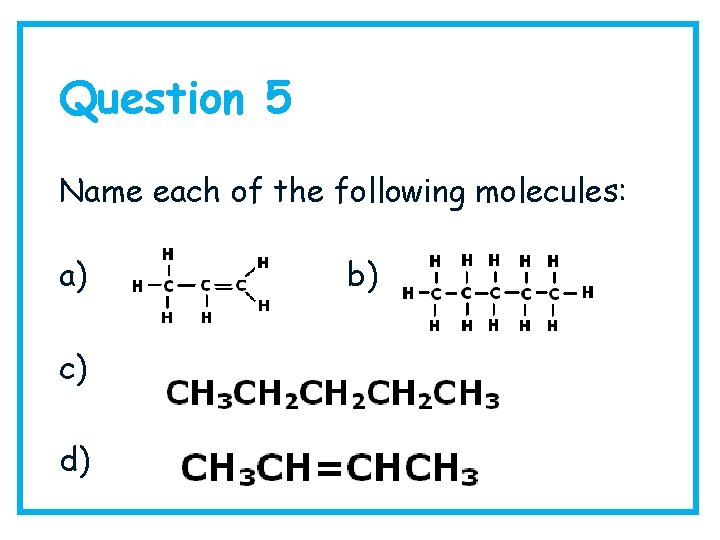
Question 5 Name each of the following molecules: a) c) d) b)

Question 6 • Which bond is present in molecules of heptene but not those in of heptane?

Question 7 • Two bottles contain a different hydrocarbon, one is saturated and the other is unsaturated. Explain how to find out which is which.

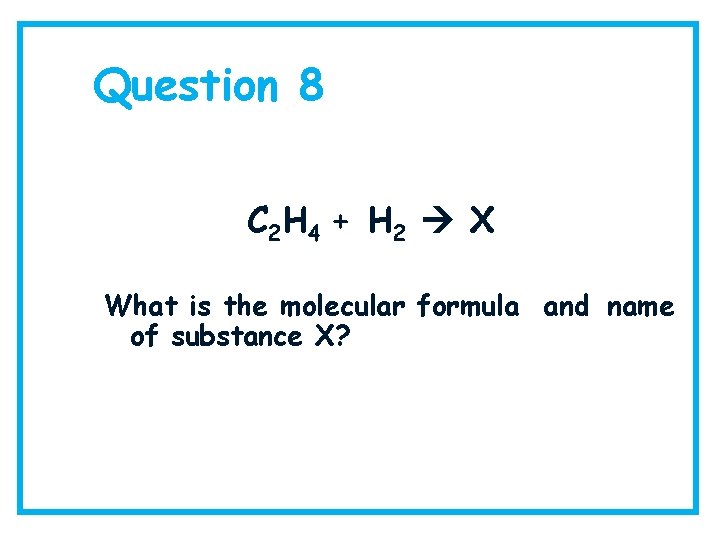
Question 8 C 2 H 4 + H 2 X What is the molecular formula and name of substance X?

Question 9 a) Explain the meaning of the following terms: i) Isomer ii) Homologous series b) Give an example of a reaction that butene and cyclobutane would undergo
 Alliteration in nothing gold can stay
Alliteration in nothing gold can stay Natures%20truth
Natures%20truth Discuss the nature of educational psychology
Discuss the nature of educational psychology Nothing gold can stay theme
Nothing gold can stay theme Natures pure purifier
Natures pure purifier Unit 6 review questions
Unit 6 review questions National unification and the national state
National unification and the national state National 3 chemistry
National 3 chemistry Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Chapter 10 section 1 the national legislature
Chapter 10 section 1 the national legislature A new national identity section 1
A new national identity section 1 Article 1 section 1 national territory
Article 1 section 1 national territory Chapter 7 section 4 states rights and the national bank
Chapter 7 section 4 states rights and the national bank A new national identity section 1
A new national identity section 1 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Section 4 the building blocks of life
Section 4 the building blocks of life Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Chapter 2 the chemistry of life section 2-3 answer key
Chapter 2 the chemistry of life section 2-3 answer key Carboxylic acid structure
Carboxylic acid structure Introduction to chemistry section 3 scientific methods
Introduction to chemistry section 3 scientific methods Chemistry in biology chapter 6 section 1 answer key
Chemistry in biology chapter 6 section 1 answer key Chemistry in biology section 3 water and solutions
Chemistry in biology section 3 water and solutions National risk and resilience unit
National risk and resilience unit Canadas physical regions
Canadas physical regions Chemistry unit 9 lesson 4
Chemistry unit 9 lesson 4 Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq Chemistry unit 7 molarity
Chemistry unit 7 molarity Unit 9 thermochemistry
Unit 9 thermochemistry Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Molal units
Molal units Wjec chemistry unit 2
Wjec chemistry unit 2 Unit 8 ap chemistry
Unit 8 ap chemistry Chemical reactions grade 11
Chemical reactions grade 11 Chemistry unit 2 study guide answer key
Chemistry unit 2 study guide answer key Chemistry review answer key
Chemistry review answer key Chemistry grade 10 unit 4
Chemistry grade 10 unit 4 Unit 6 chemistry review
Unit 6 chemistry review Ap chemistry unit 9 notes
Ap chemistry unit 9 notes Higher chemistry unit 3 notes
Higher chemistry unit 3 notes Wjec chemistry unit 2
Wjec chemistry unit 2 Chemistry unit 6 sticky tape post lab
Chemistry unit 6 sticky tape post lab Living by chemistry unit 2 smells answers
Living by chemistry unit 2 smells answers Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Ap chemistry unit 3
Ap chemistry unit 3 Ap chemistry unit 2
Ap chemistry unit 2 Honors chemistry unit 4 review answers
Honors chemistry unit 4 review answers Three characteristics of metals
Three characteristics of metals Ap chemistry unit 3
Ap chemistry unit 3 Ounce and pounds
Ounce and pounds Conversion problems with answers
Conversion problems with answers Chemistry unit 6
Chemistry unit 6 Chemistry
Chemistry Sectional views reveal
Sectional views reveal Cross section view
Cross section view Showing one-half of the view in section
Showing one-half of the view in section Describing energy section 2 answers
Describing energy section 2 answers Section quick check chapter 10 section 1 meiosis answer key
Section quick check chapter 10 section 1 meiosis answer key Unit 6 section 2
Unit 6 section 2 Unit 4 section 1
Unit 4 section 1 Unit 3 statistical studies answers
Unit 3 statistical studies answers Unit 4 section 1
Unit 4 section 1 Unit surplus dan unit defisit
Unit surplus dan unit defisit How to label hyp opp adj
How to label hyp opp adj English system of measurement
English system of measurement Algebra 2 unit 1 test
Algebra 2 unit 1 test Contoh soal perhitungan unit cost rumah sakit
Contoh soal perhitungan unit cost rumah sakit Unit process and unit operation
Unit process and unit operation Difference between unit process and unit operation
Difference between unit process and unit operation Setiap unit akuntansi dianggap sebagai unit yang mandiri
Setiap unit akuntansi dianggap sebagai unit yang mandiri Alpha kappa alpha membership intake process manual
Alpha kappa alpha membership intake process manual Yuriy fedkovych chernivtsi national university
Yuriy fedkovych chernivtsi national university Seo for youth sports clubs
Seo for youth sports clubs Skypak hvac replacement
Skypak hvac replacement Numeracy continuum qld
Numeracy continuum qld National meteorological and hydrological services
National meteorological and hydrological services Whybuyonline
Whybuyonline Nsf aimsweb
Nsf aimsweb Armenian national corpus
Armenian national corpus Gdp per capita formula
Gdp per capita formula