Chemistry A Molecular Approach 2 nd Ed Nivaldo





































- Slides: 140

Chemistry: A Molecular Approach, 2 nd Ed. Nivaldo Tro Chapter 11 Liquids, Solids, and Intermolecular Forces Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA Copyright 2011 Pearson Education, Inc.

Climbing Geckos • Geckos can adhere to almost any surface • Recent studies indicate that this amazing ability • is related to intermolecular attractive forces Geckos have millions of tiny hairs on their feet that branch out and flatten out on the end ü setae = hairs, spatulae = flat ends • This structure allows the gecko to have unusually close contact to the surface – allowing the intermolecular attractive forces to act strongly Tro: Chemistry: A Molecular Approach, 2/e 2 Copyright 2011 Pearson Education, Inc.

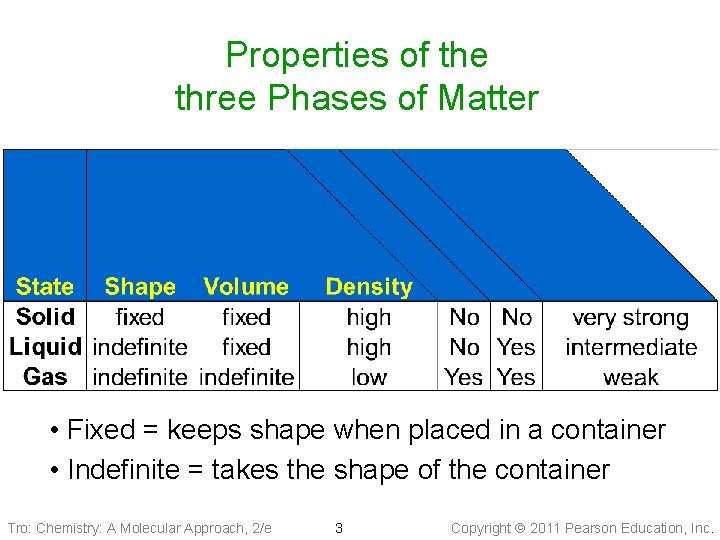
Properties of the three Phases of Matter • Fixed = keeps shape when placed in a container • Indefinite = takes the shape of the container Tro: Chemistry: A Molecular Approach, 2/e 3 Copyright 2011 Pearson Education, Inc.

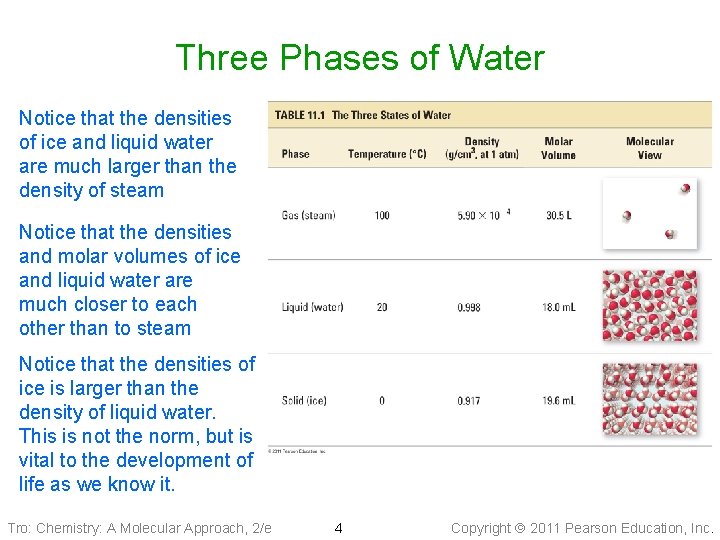
Three Phases of Water Notice that the densities of ice and liquid water are much larger than the density of steam Notice that the densities and molar volumes of ice and liquid water are much closer to each other than to steam Notice that the densities of ice is larger than the density of liquid water. This is not the norm, but is vital to the development of life as we know it. Tro: Chemistry: A Molecular Approach, 2/e 4 Copyright 2011 Pearson Education, Inc.

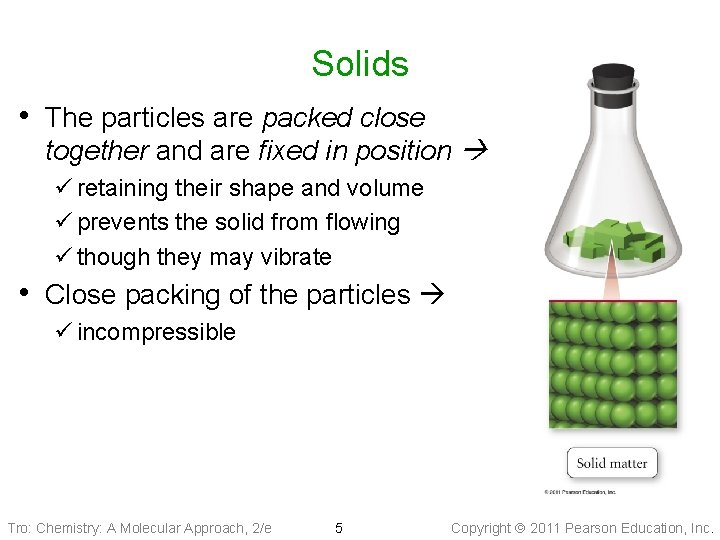
Solids • The particles are packed close together and are fixed in position ü retaining their shape and volume ü prevents the solid from flowing ü though they may vibrate • Close packing of the particles ü incompressible Tro: Chemistry: A Molecular Approach, 2/e 5 Copyright 2011 Pearson Education, Inc.

Solids • Crystalline solids: particles arranged in an orderly geometric pattern ü salt and diamonds • Amorphous solids: particles do not show a regular geometric pattern over a long range ü plastic and glass Tro: Chemistry: A Molecular Approach, 2/e 6 Copyright 2011 Pearson Education, Inc.

Liquids • Particles closely packed ü Incompressible ü take the shape of their container ü can flow • Not enough freedom to escape or expand to fill the container Tro: Chemistry: A Molecular Approach, 2/e 7 Copyright 2011 Pearson Education, Inc.

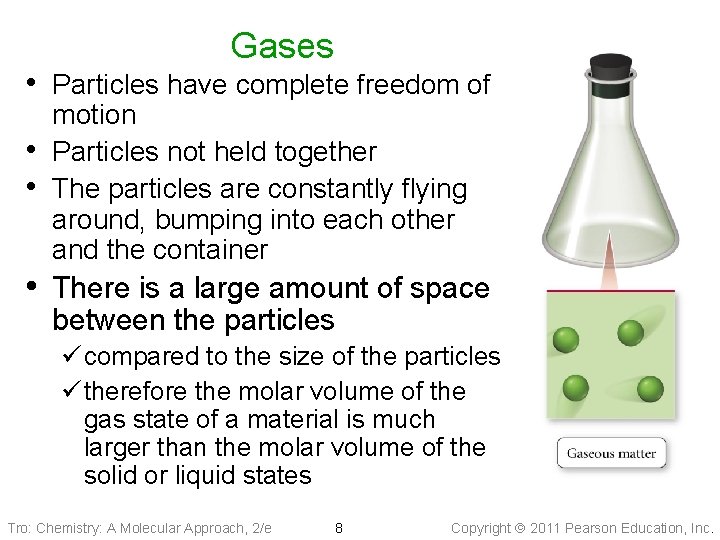
Gases • Particles have complete freedom of • • motion Particles not held together The particles are constantly flying around, bumping into each other and the container • There is a large amount of space between the particles ü compared to the size of the particles ü therefore the molar volume of the gas state of a material is much larger than the molar volume of the solid or liquid states Tro: Chemistry: A Molecular Approach, 2/e 8 Copyright 2011 Pearson Education, Inc.

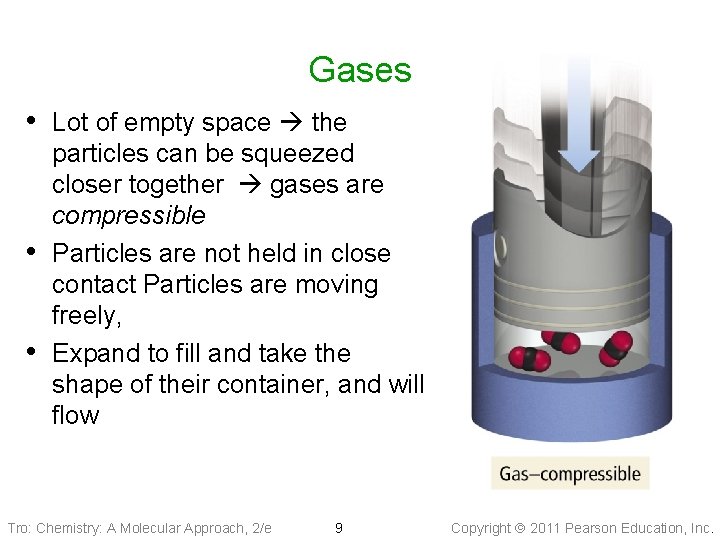
Gases • Lot of empty space the • • particles can be squeezed closer together gases are compressible Particles are not held in close contact Particles are moving freely, Expand to fill and take the shape of their container, and will flow Tro: Chemistry: A Molecular Approach, 2/e 9 Copyright 2011 Pearson Education, Inc.

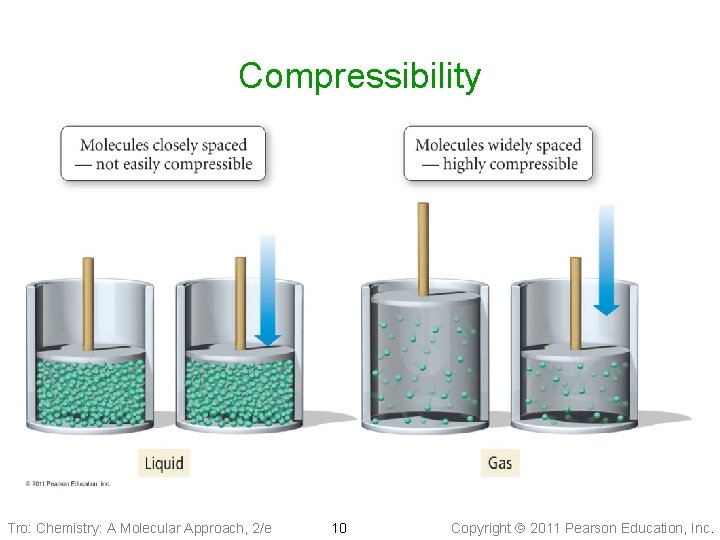
Compressibility Tro: Chemistry: A Molecular Approach, 2/e 10 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach 11 Copyright 2011 Pearson Education, Inc.

Attractive Forces • The particles are attracted to each other by • • • electrostatic forces The strength of the attractive forces varies, some are small and some are large The strength of the attractive forces depends on the kind(s) of particles The stronger the attractive forces between the particles, the more they resist moving ü though no material completely lacks particle motion Tro: Chemistry: A Molecular Approach, 2/e 12 Copyright 2011 Pearson Education, Inc.

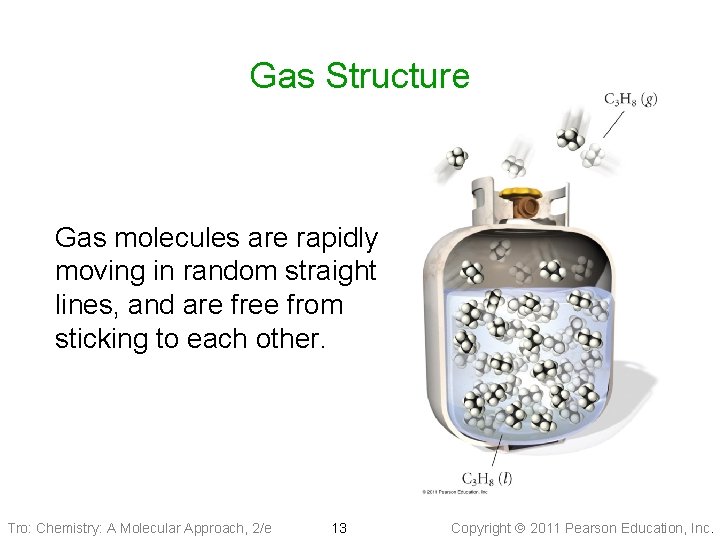
Gas Structure Gas molecules are rapidly moving in random straight lines, and are free from sticking to each other. Tro: Chemistry: A Molecular Approach, 2/e 13 Copyright 2011 Pearson Education, Inc.

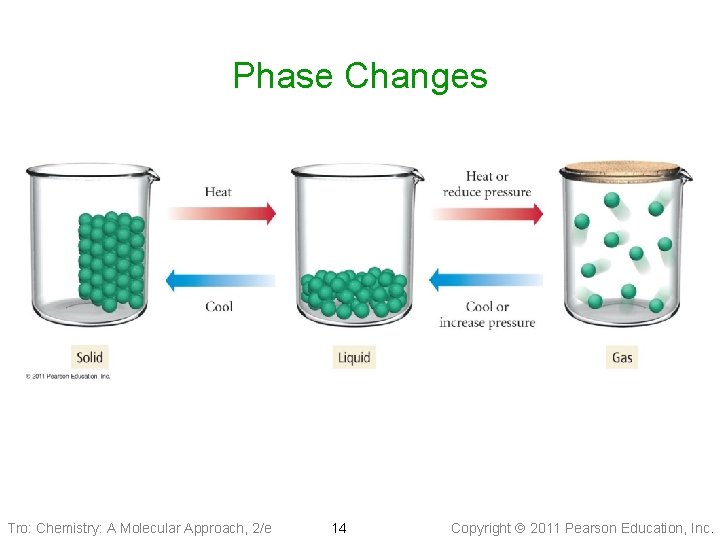
Phase Changes Tro: Chemistry: A Molecular Approach, 2/e 14 Copyright 2011 Pearson Education, Inc.

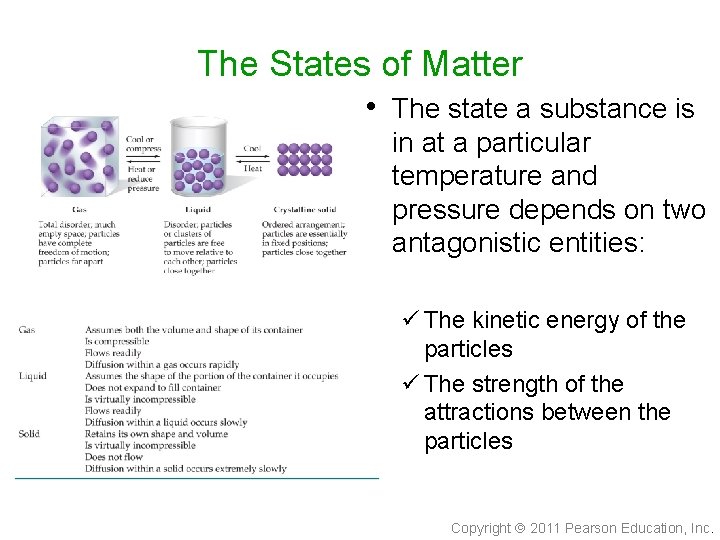
The States of Matter • The state a substance is in at a particular temperature and pressure depends on two antagonistic entities: ü The kinetic energy of the particles ü The strength of the attractions between the particles Copyright 2011 Pearson Education, Inc.

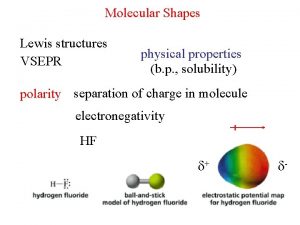
Intermolecular Forces Intramolecular forces are interactions of atoms within a molecule. Intermolecular forces are interactions between molecules. Among other things, intermolecular forces are responsible for condensation of gases, and freezing of liquids into solids. Examples of intermolecular forces in liquids; 1) Surface tension 2) Viscosity 3) Vapour pressure Intermolecular Copyright 2011 Pearson Education, Inc.

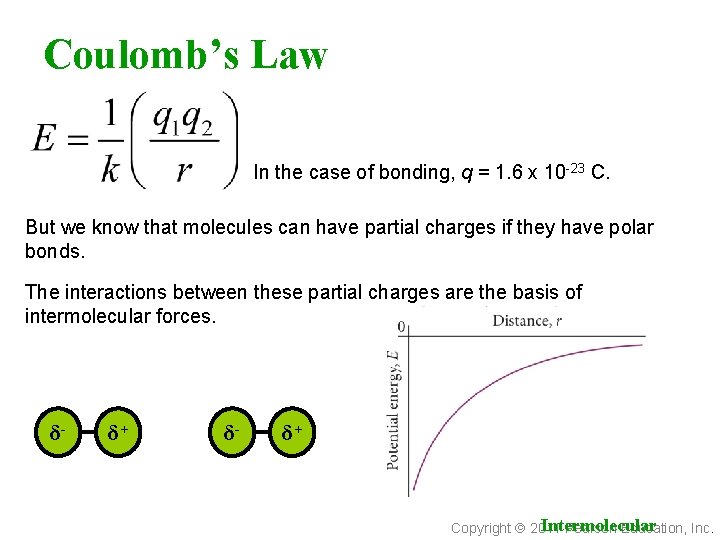
Coulomb’s Law In the case of bonding, q = 1. 6 x 10 -23 C. But we know that molecules can have partial charges if they have polar bonds. The interactions between these partial charges are the basis of intermolecular forces. δ- δ+ Intermolecular Copyright 2011 Pearson Education, Inc.

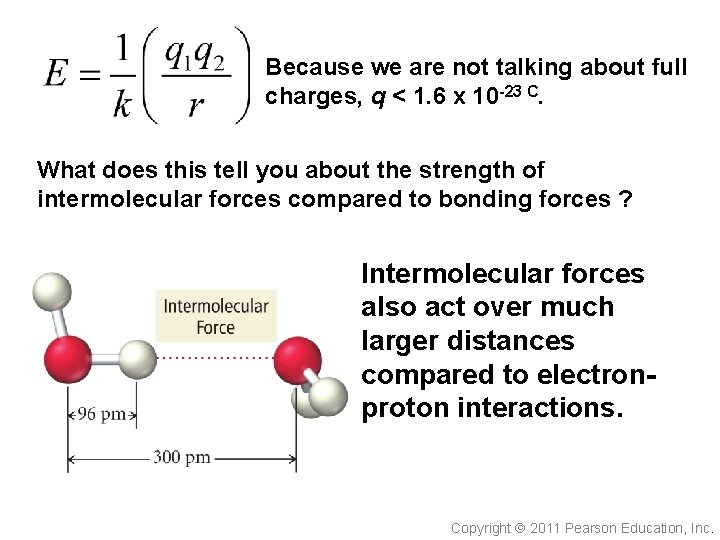
Because we are not talking about full charges, q < 1. 6 x 10 -23 C. What does this tell you about the strength of intermolecular forces compared to bonding forces ? Intermolecular forces also act over much larger distances compared to electronproton interactions. Copyright 2011 Pearson Education, Inc.

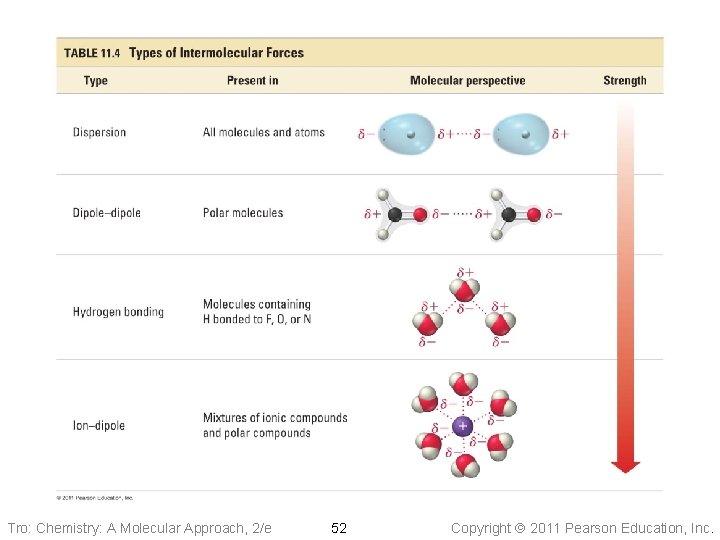
Types of IM Forces There are four categories of IM forces that we will consider. 1. Dispersion Forces 2. Dipole-Dipole Forces 3. Hydrogen Bonding 4. Ion-Dipole Forces Copyright 2011 Pearson Education, Inc.

Types of IM Forces There are four categories of IM forces that we will consider. 1. Dispersion Forces 2. Dipole-Dipole Forces 3. Hydrogen Bonding 4. Ion-Dipole Forces Copyright 2011 Pearson Education, Inc.

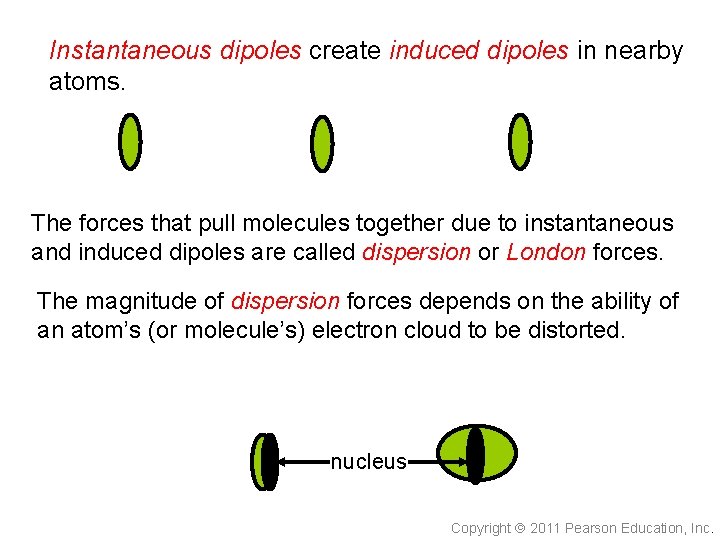
Instantaneous dipoles create induced dipoles in nearby atoms. The forces that pull molecules together due to instantaneous and induced dipoles are called dispersion or London forces. The magnitude of dispersion forces depends on the ability of an atom’s (or molecule’s) electron cloud to be distorted. nucleus Copyright 2011 Pearson Education, Inc.

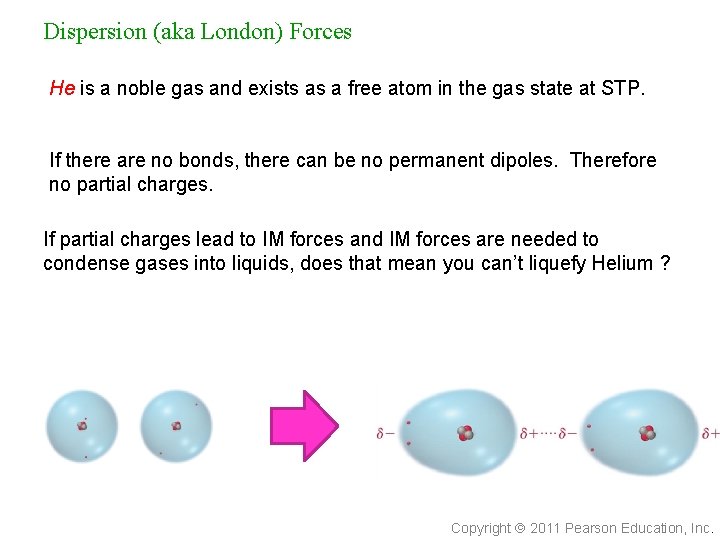
Dispersion (aka London) Forces He is a noble gas and exists as a free atom in the gas state at STP. If there are no bonds, there can be no permanent dipoles. Therefore no partial charges. If partial charges lead to IM forces and IM forces are needed to condense gases into liquids, does that mean you can’t liquefy Helium ? Copyright 2011 Pearson Education, Inc.

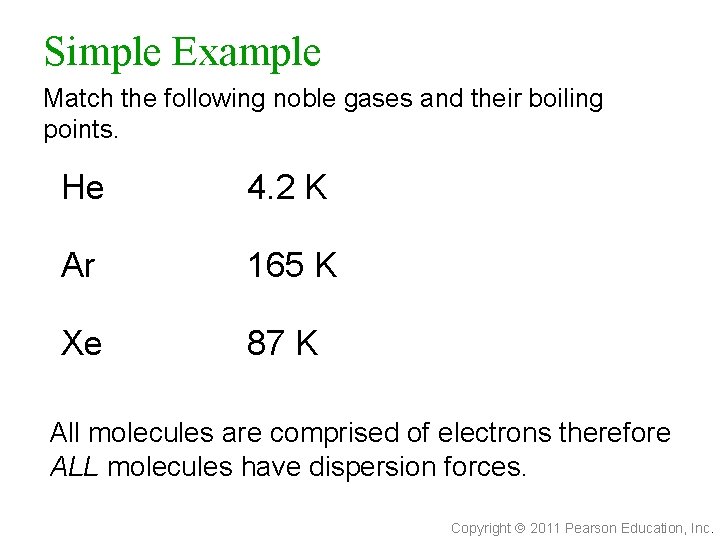
Simple Example Match the following noble gases and their boiling points. He 4. 2 K Ar 165 K Xe 87 K All molecules are comprised of electrons therefore ALL molecules have dispersion forces. Copyright 2011 Pearson Education, Inc.

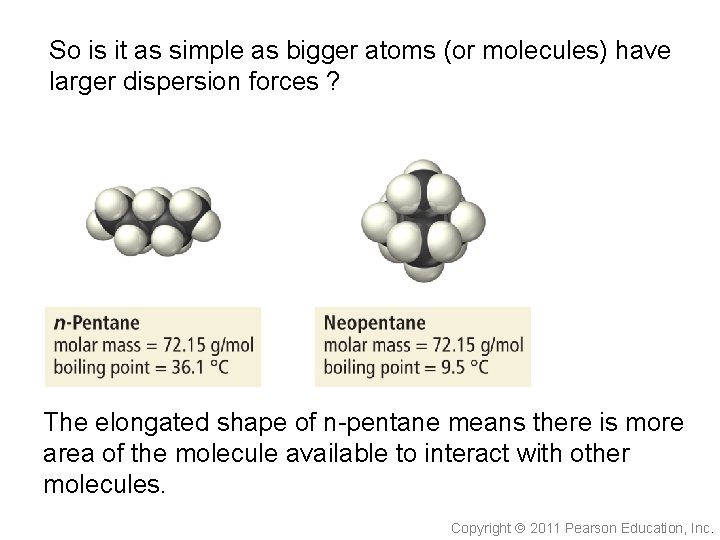
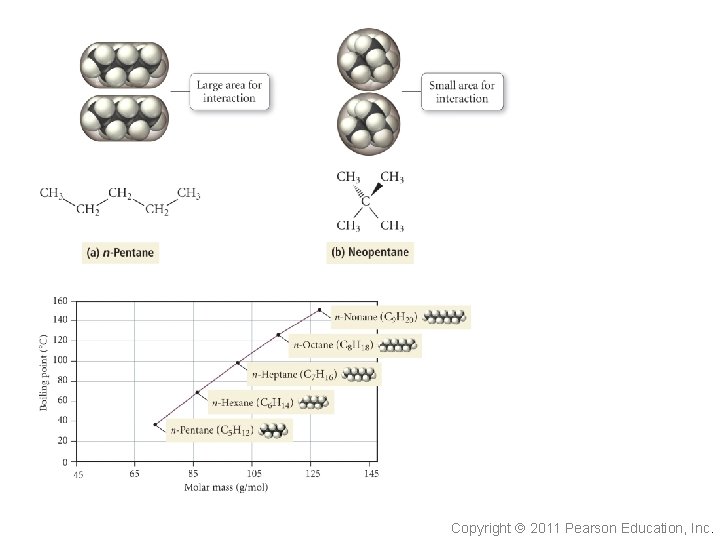
So is it as simple as bigger atoms (or molecules) have larger dispersion forces ? The elongated shape of n-pentane means there is more area of the molecule available to interact with other molecules. Copyright 2011 Pearson Education, Inc.

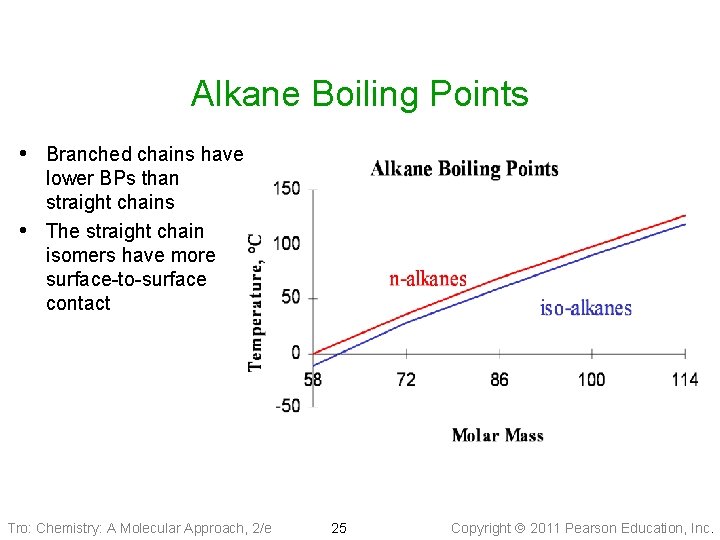
Alkane Boiling Points • Branched chains have • lower BPs than straight chains The straight chain isomers have more surface-to-surface contact Tro: Chemistry: A Molecular Approach, 2/e 25 Copyright 2011 Pearson Education, Inc.

Copyright 2011 Pearson Education, Inc.

Types of IM Forces There are four categories of IM forces that we will consider. 1. Dispersion Forces 2. Dipole-Dipole Forces 3. Hydrogen Bonding 4. Ion-Dipole Forces Copyright 2011 Pearson Education, Inc.

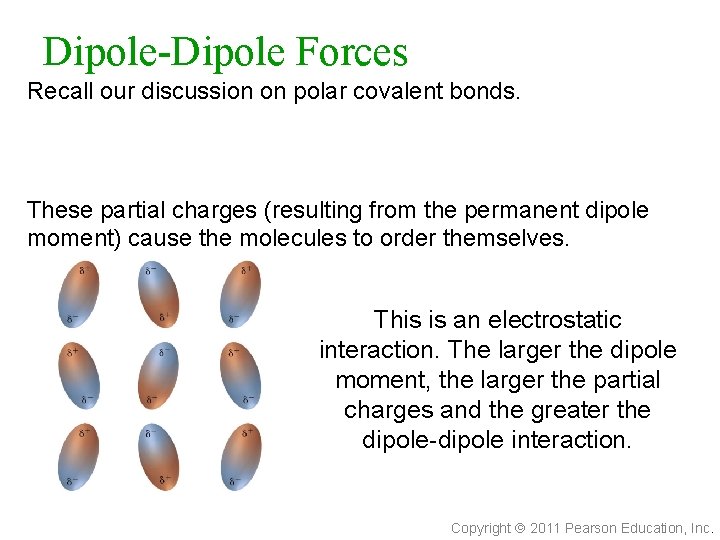
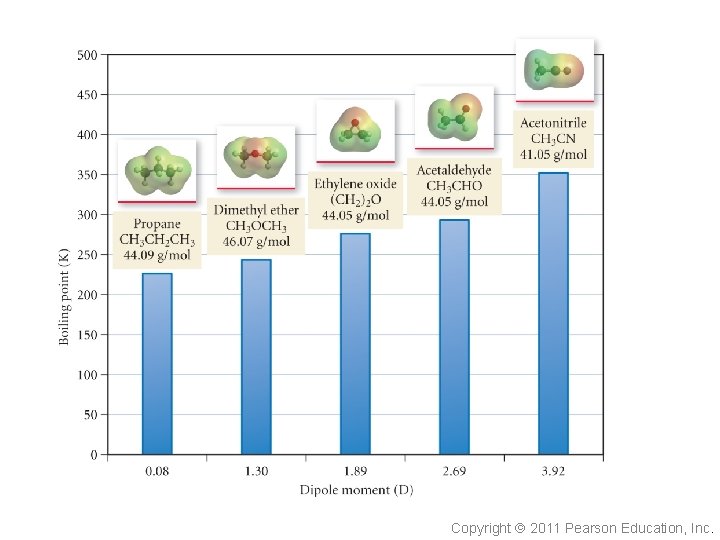
Dipole-Dipole Forces Recall our discussion on polar covalent bonds. These partial charges (resulting from the permanent dipole moment) cause the molecules to order themselves. This is an electrostatic interaction. The larger the dipole moment, the larger the partial charges and the greater the dipole-dipole interaction. Copyright 2011 Pearson Education, Inc.

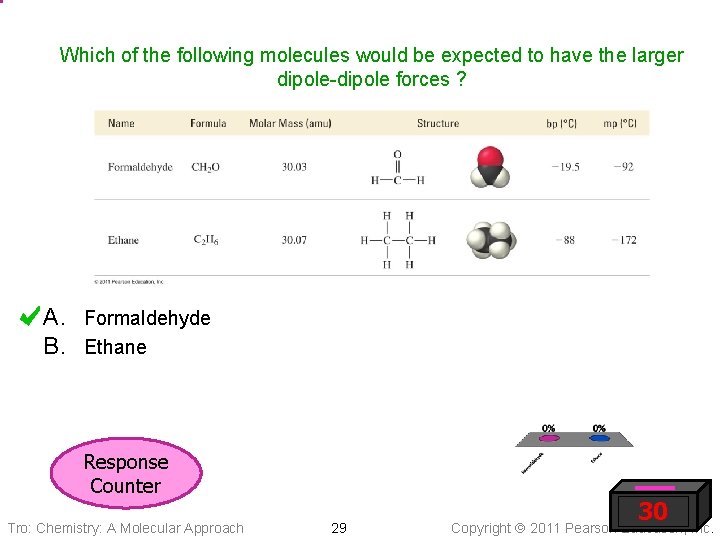
Which of the following molecules would be expected to have the larger dipole-dipole forces ? A. Formaldehyde B. Ethane Response Counter Tro: Chemistry: A Molecular Approach 29 30 Copyright 2011 Pearson Education, Inc.

Copyright 2011 Pearson Education, Inc.

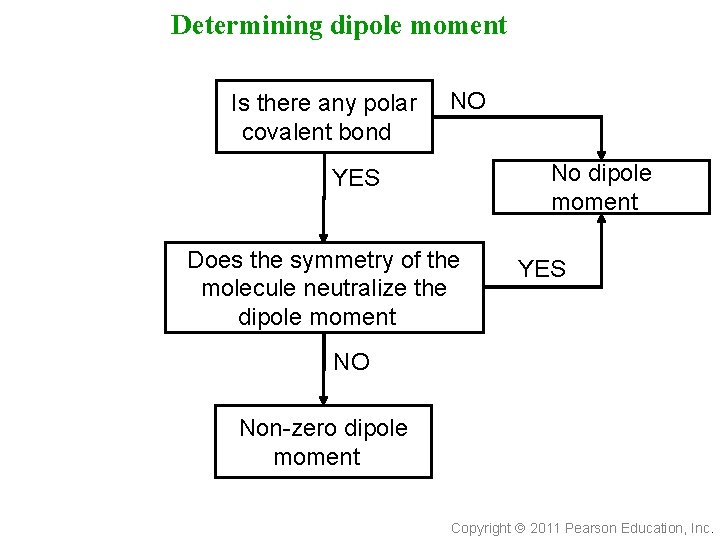
Determining dipole moment Is there any polar covalent bond? NO No dipole moment? YES Does the symmetry of the molecule neutralize the dipole moment? YES NO Non-zero dipole moment? Copyright 2011 Pearson Education, Inc.

Which of the following molecules will have dipole forces ? A. B. C. D. E. F. CO 2 CH 2 Cl 2 CH 4 CCl 4 O 2 SO 2 Response Counter Tro: Chemistry: A Molecular Approach 120 32 Copyright 2011 Pearson Education, Inc.

Types of IM Forces There are four categories of IM forces that we will consider. 1. Dispersion Forces 2. Dipole-Dipole Forces 3. Hydrogen Bonding 4. Ion-Dipole Forces Copyright 2011 Pearson Education, Inc.

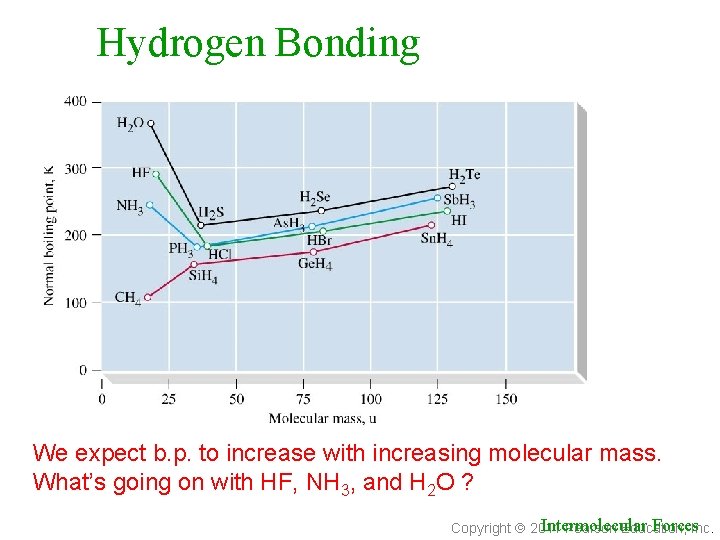
Hydrogen Bonding We expect b. p. to increase with increasing molecular mass. What’s going on with HF, NH 3, and H 2 O ? Intermolecular Forces. Inc. Copyright 2011 Pearson Education,

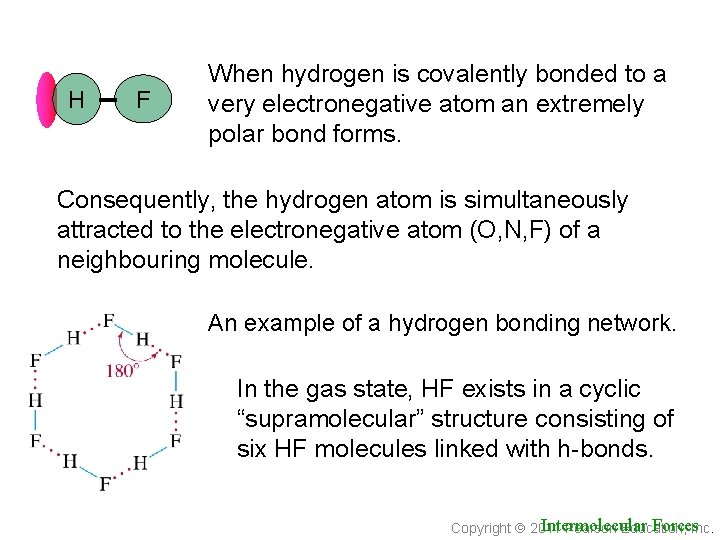
H F When hydrogen is covalently bonded to a very electronegative atom an extremely polar bond forms. Consequently, the hydrogen atom is simultaneously attracted to the electronegative atom (O, N, F) of a neighbouring molecule. An example of a hydrogen bonding network. In the gas state, HF exists in a cyclic “supramolecular” structure consisting of six HF molecules linked with h-bonds. Intermolecular Forces. Inc. Copyright 2011 Pearson Education,

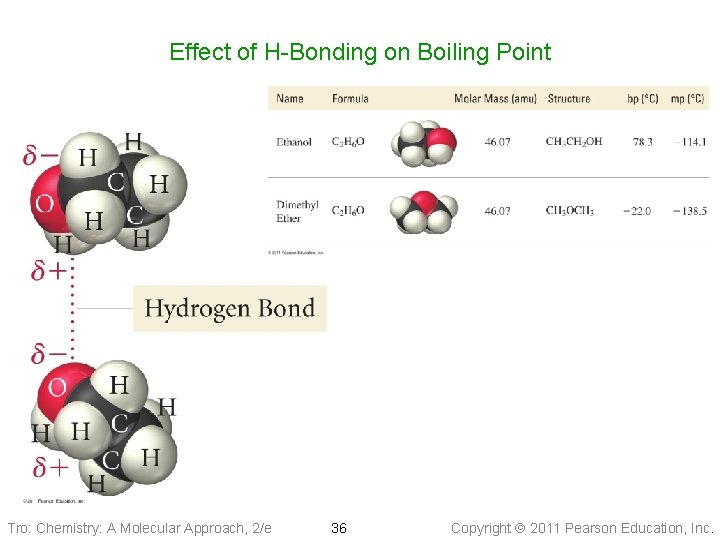
Effect of H-Bonding on Boiling Point Tro: Chemistry: A Molecular Approach, 2/e 36 Copyright 2011 Pearson Education, Inc.

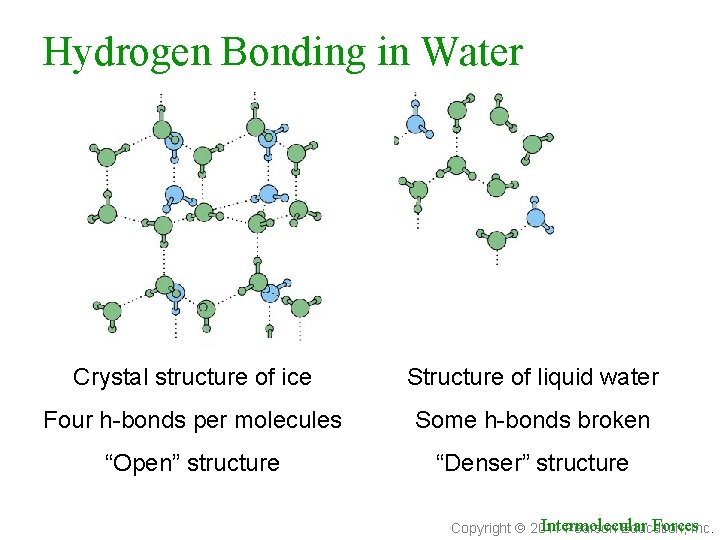
Hydrogen Bonding in Water Crystal structure of ice Structure of liquid water Four h-bonds per molecules Some h-bonds broken “Open” structure “Denser” structure Intermolecular Forces. Inc. Copyright 2011 Pearson Education,

Types of IM Forces There are four categories of IM forces that we will consider. 1. Dispersion Forces 2. Dipole-Dipole Forces 3. Hydrogen Bonding 4. Ion-Dipole Forces Copyright 2011 Pearson Education, Inc.

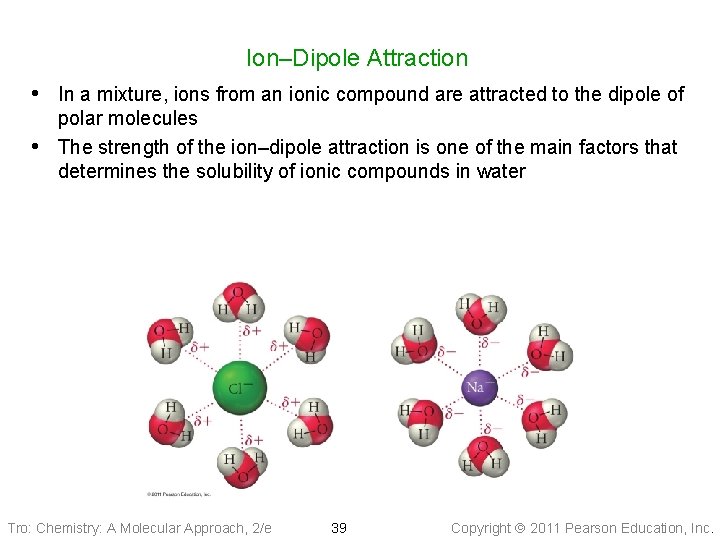
Ion–Dipole Attraction • In a mixture, ions from an ionic compound are attracted to the dipole of • polar molecules The strength of the ion–dipole attraction is one of the main factors that determines the solubility of ionic compounds in water Tro: Chemistry: A Molecular Approach, 2/e 39 Copyright 2011 Pearson Education, Inc.

How Strong are Intermolecular Forces ? Ionic bond Covalent bond Ion-Dipole Hydrogen bonding Perm. Dipole – Perm. Dipole Induced dipole forces Intermolecular Forces. Inc. Copyright 2011 Pearson Education,

Examples 2. Arrange the following in order of increasing boiling point: He, Ne, O 3, O 2, Cl 2, (CH 3)2 C=O 3. Arrange the following in order of increasing boiling point: C 6 H 6 ; C 6 H 5 Cl ; C 6 H 5 Br ; C 6 H 5 OH Intermolecular Forces. Inc. Copyright 2011 Pearson Education,

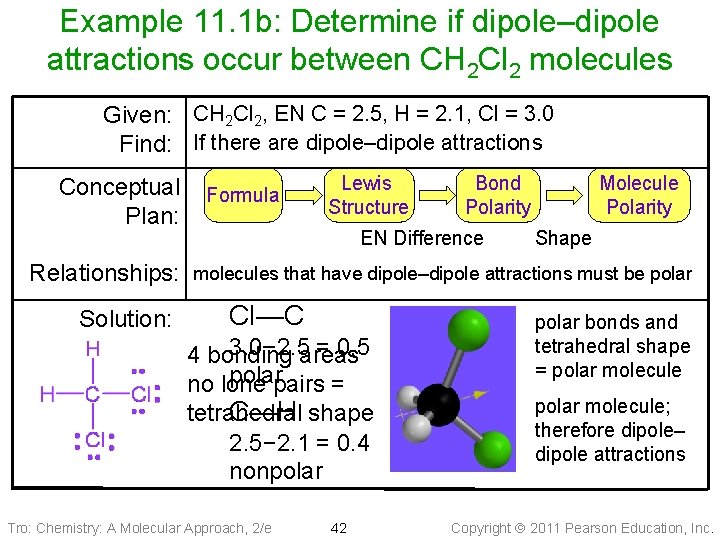
Example 11. 1 b: Determine if dipole–dipole attractions occur between CH 2 Cl 2 molecules Given: CH 2 Cl 2, EN C = 2. 5, H = 2. 1, Cl = 3. 0 Find: If there are dipole–dipole attractions Conceptual Plan: Relationships: Solution: Formula Lewis Structure Bond Polarity EN Difference Molecule Polarity Shape molecules that have dipole–dipole attractions must be polar Cl—C 3. 0− 2. 5 areas = 0. 5 4 bonding polarpairs = no lone tetrahedral C—H shape 2. 5− 2. 1 = 0. 4 nonpolar Tro: Chemistry: A Molecular Approach, 2/e 42 polar bonds and tetrahedral shape = polar molecule; therefore dipole– dipole attractions Copyright 2011 Pearson Education, Inc.

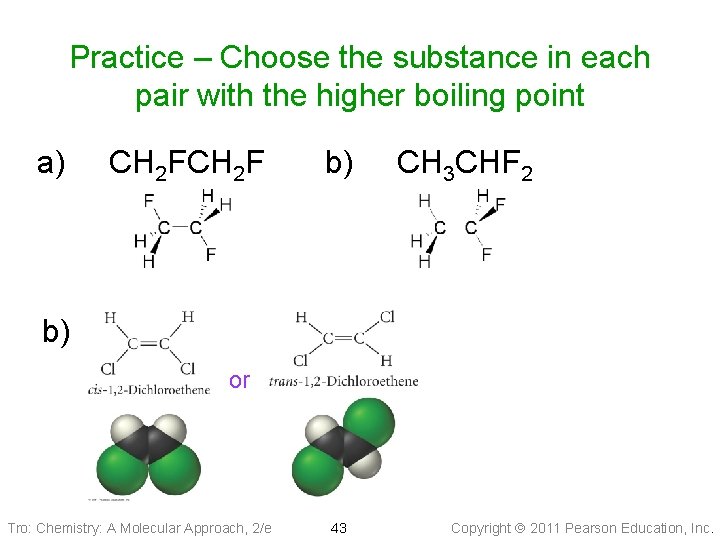
Practice – Choose the substance in each pair with the higher boiling point a) CH 2 F b) CH 3 CHF 2 b) or Tro: Chemistry: A Molecular Approach, 2/e 43 Copyright 2011 Pearson Education, Inc.

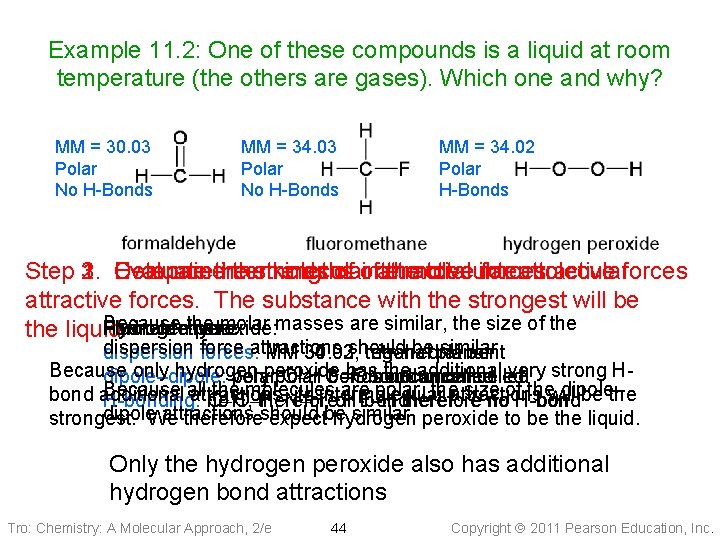
Example 11. 2: One of these compounds is a liquid at room temperature (the others are gases). Which one and why? MM = 30. 03 Polar No H-Bonds MM = 34. 02 Polar H-Bonds Step 2. 3. Compare 1. Evaluate the Determine intermolecular thestrengths kinds of of intermolecular attractive the total intermolecular forces attractive forces. The substance with the strongest will be Because the molar masses are similar, the size of the Formaldehyde: Fluoromethane: Hydrogen peroxide: the liquid. dispersion forces: force attractions should be planar similar dispersion MM 34. 02, 30. 03, 34. 03, trigonal tetrahedral bent Because only hydrogen has the additional very strong Hdipole–dipole: veryperoxide polar O–H C–F bonds bond uncancelled C=O bond uncancelled Because all the molecules are polar, theattractions size of thewill dipole– bond additional attractions, its intermolecular be the H-bonding: no O–H, therefore N–H, or H-bond F–H therefore no H-bond dipole. We attractions similar peroxide to be the liquid. strongest. thereforeshould expectbe hydrogen Only the hydrogen peroxide also has additional hydrogen bond attractions Tro: Chemistry: A Molecular Approach, 2/e 44 Copyright 2011 Pearson Education, Inc.

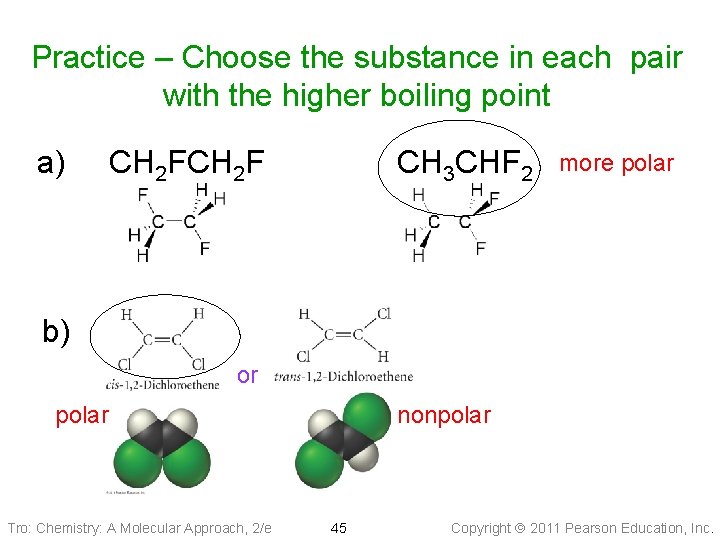
Practice – Choose the substance in each pair with the higher boiling point a) CH 2 F CH 3 CHF 2 more polar b) or polar Tro: Chemistry: A Molecular Approach, 2/e nonpolar 45 Copyright 2011 Pearson Education, Inc.

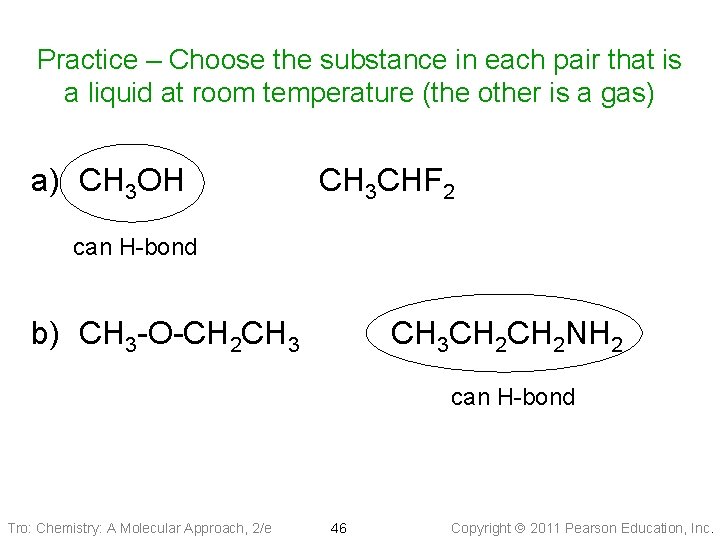
Practice – Choose the substance in each pair that is a liquid at room temperature (the other is a gas) a) CH 3 OH CH 3 CHF 2 can H-bond b) CH 3 -O-CH 2 CH 3 CH 2 NH 2 can H-bond Tro: Chemistry: A Molecular Approach, 2/e 46 Copyright 2011 Pearson Education, Inc.

Attractive Forces and Solubility • • Solubility depends, in part, on the attractive forces of the solute and solvent molecules ü like dissolves like ü miscible liquids will always dissolve in each other Polar substances dissolve in polar solvents ü hydrophilic groups = OH, CHO, C=O, COOH, NH 2, Cl Nonpolar molecules dissolve in nonpolar solvents ü hydrophobic groups = C-H, C-C Many molecules have both hydrophilic and hydrophobic parts – solubility in water becomes a competition between the attraction of the polar groups for the water and the attraction of the nonpolar groups for their own kind Tro: Chemistry: A Molecular Approach, 2/e 47 Copyright 2011 Pearson Education, Inc.

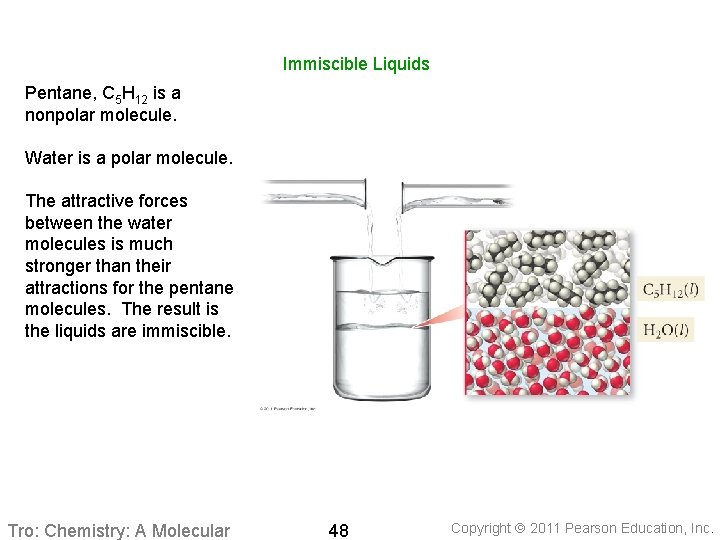
Immiscible Liquids Pentane, C 5 H 12 is a nonpolar molecule. Water is a polar molecule. The attractive forces between the water molecules is much stronger than their attractions for the pentane molecules. The result is the liquids are immiscible. Tro: Chemistry: A Molecular 48 Copyright 2011 Pearson Education, Inc.

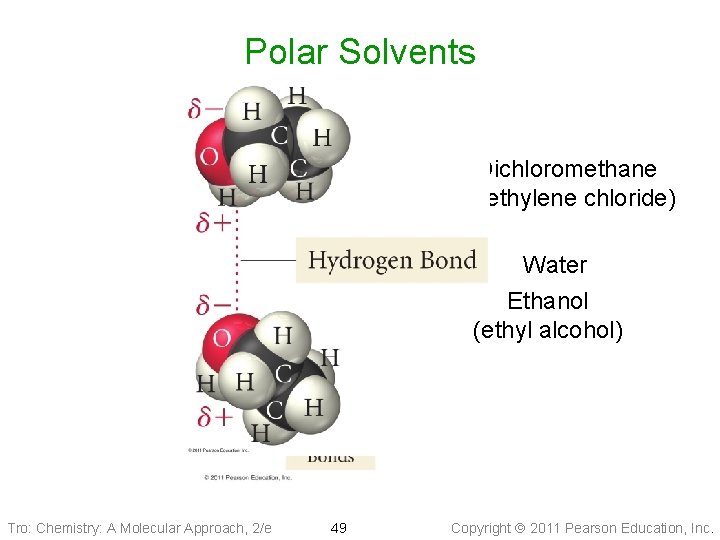
Polar Solvents Dichloromethane (methylene chloride) Water Ethanol (ethyl alcohol) Tro: Chemistry: A Molecular Approach, 2/e 49 Copyright 2011 Pearson Education, Inc.

Nonpolar Solvents Tro: Chemistry: A Molecular Approach, 2/e 50 Copyright 2011 Pearson Education, Inc.

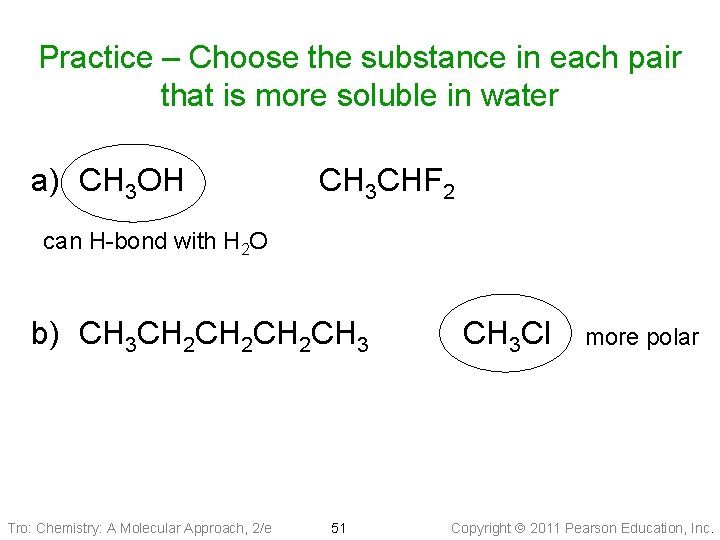
Practice – Choose the substance in each pair that is more soluble in water a) CH 3 OH CH 3 CHF 2 can H-bond with H 2 O b) CH 3 CH 2 CH 2 CH 3 Tro: Chemistry: A Molecular Approach, 2/e 51 CH 3 Cl more polar Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 52 Copyright 2011 Pearson Education, Inc.

Liquids Properties & Structure Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

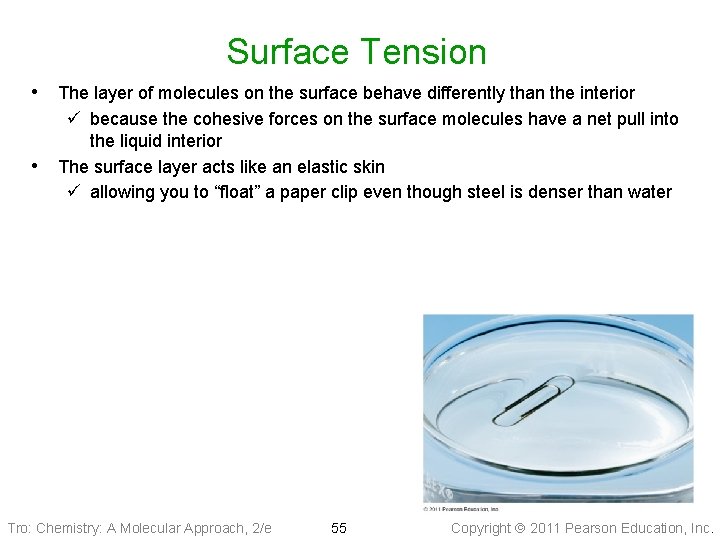
Surface Tension • Surface tension is a property of liquids that results from the tendency • of liquids to minimize their surface area To minimize their surface area, liquids form drops that are spherical ü as long as there is no gravity Tro: Chemistry: A Molecular Approach, 2/e 54 Copyright 2011 Pearson Education, Inc.

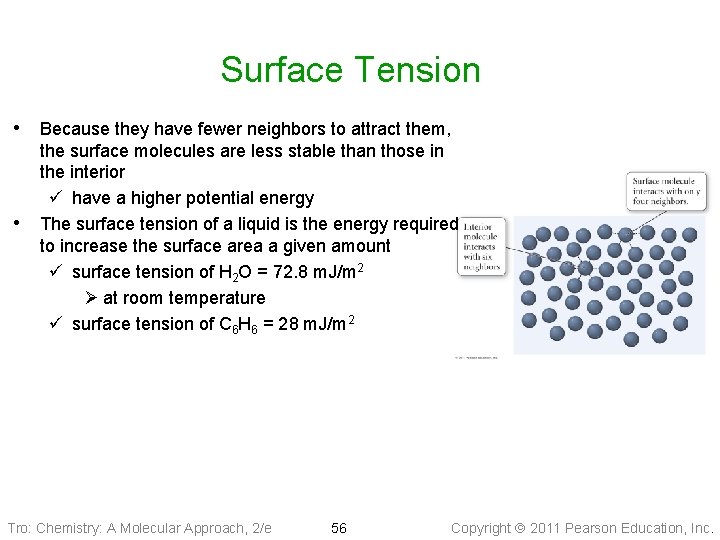
Surface Tension • The layer of molecules on the surface behave differently than the interior • ü because the cohesive forces on the surface molecules have a net pull into the liquid interior The surface layer acts like an elastic skin ü allowing you to “float” a paper clip even though steel is denser than water Tro: Chemistry: A Molecular Approach, 2/e 55 Copyright 2011 Pearson Education, Inc.

Surface Tension • Because they have fewer neighbors to attract them, • the surface molecules are less stable than those in the interior ü have a higher potential energy The surface tension of a liquid is the energy required to increase the surface area a given amount ü surface tension of H 2 O = 72. 8 m. J/m 2 Ø at room temperature ü surface tension of C 6 H 6 = 28 m. J/m 2 Tro: Chemistry: A Molecular Approach, 2/e 56 Copyright 2011 Pearson Education, Inc.

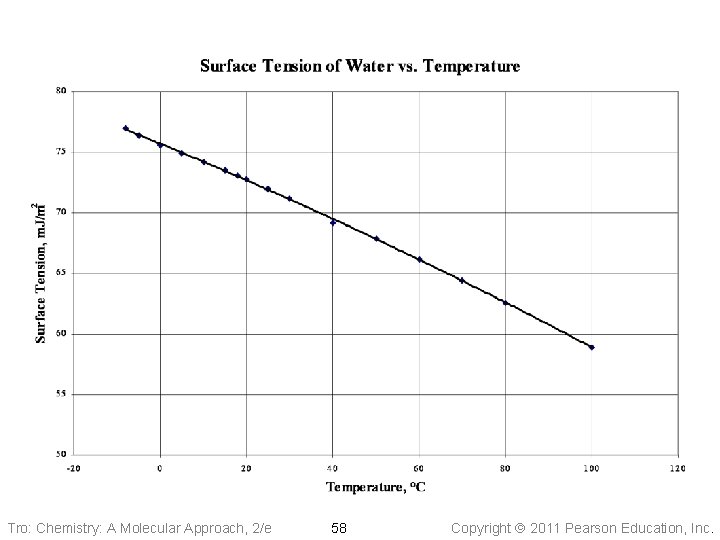
Factors Affecting Surface Tension • The stronger the intermolecular attractive forces, the higher the surface • tension will be Raising the temperature of a liquid reduces its surface tension ü raising the temperature of the liquid increases the average kinetic energy of the molecules ü the increased molecular motion makes it easier to stretch the surface Tro: Chemistry: A Molecular Approach, 2/e 57 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 58 Copyright 2011 Pearson Education, Inc.

Viscosity • Viscosity is the resistance of a liquid to flow • ü 1 poise = 1 P = 1 g/cm∙s ü often given in centipoise, c. P Ø H 2 O = 1 c. P at room temperature Larger intermolecular attractions = larger viscosity Tro: Chemistry: A Molecular Approach, 2/e 59 Copyright 2011 Pearson Education, Inc.

Factors Affecting Viscosity • The stronger the intermolecular attractive forces, the higher the liquid’s viscosity will • • be The more spherical the molecular shape, the lower the viscosity will be ü molecules roll more easily ü less surface-to-surface contact lowers attractions Raising the temperature of a liquid reduces its viscosity ü raising the temperature of the liquid increases the average kinetic energy of the molecules ü the increased molecular motion makes it easier to overcome the intermolecular attractions and flow Tro: Chemistry: A Molecular Approach, 2/e 60 Copyright 2011 Pearson Education, Inc.

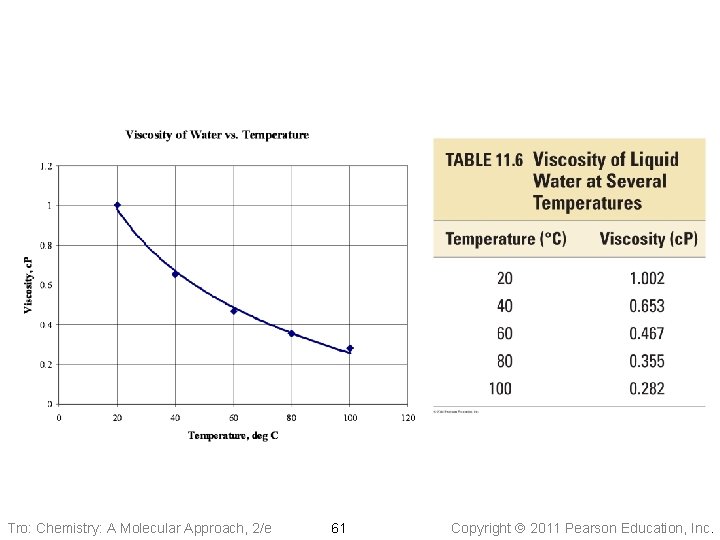
Insert Table 11. 6 Tro: Chemistry: A Molecular Approach, 2/e 61 Copyright 2011 Pearson Education, Inc.

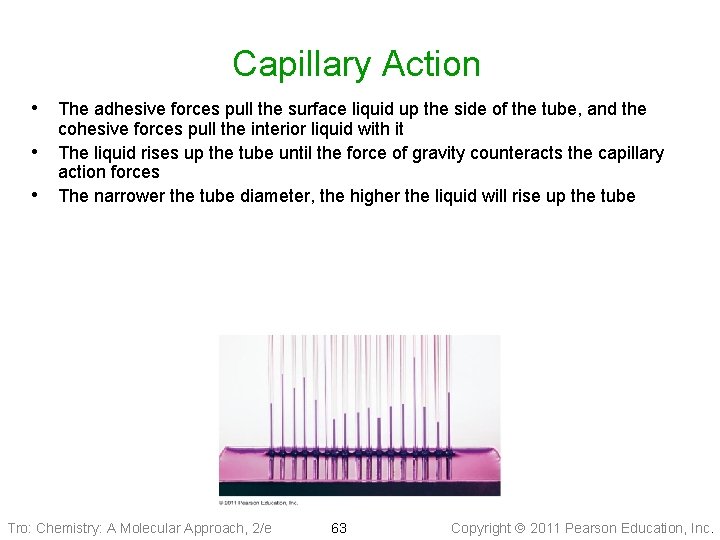
Capillary Action • Capillary action is the ability of a liquid to flow up a thin tube against the • influence of gravity ü the narrower the tube, the higher the liquid rises Capillary action is the result of two forces working in conjunction, the cohesive and adhesive forces ü cohesive forces hold the liquid molecules together ü adhesive forces attract the outer liquid molecules to the tube’s surface Tro: Chemistry: A Molecular Approach, 2/e 62 Copyright 2011 Pearson Education, Inc.

Capillary Action • The adhesive forces pull the surface liquid up the side of the tube, and the • • cohesive forces pull the interior liquid with it The liquid rises up the tube until the force of gravity counteracts the capillary action forces The narrower the tube diameter, the higher the liquid will rise up the tube Tro: Chemistry: A Molecular Approach, 2/e 63 Copyright 2011 Pearson Education, Inc.

Meniscus • The curving of the liquid surface in a thin tube is due to the • • competition between adhesive and cohesive forces The meniscus of water is concave in a glass tube because its adhesion to the glass is stronger than its cohesion for itself The meniscus of mercury is convex in a glass tube because its cohesion for itself is stronger than its adhesion for the glass ü metallic bonds are stronger than intermolecular attractions Tro: Chemistry: A Molecular Approach, 2/e 64 Copyright 2011 Pearson Education, Inc.

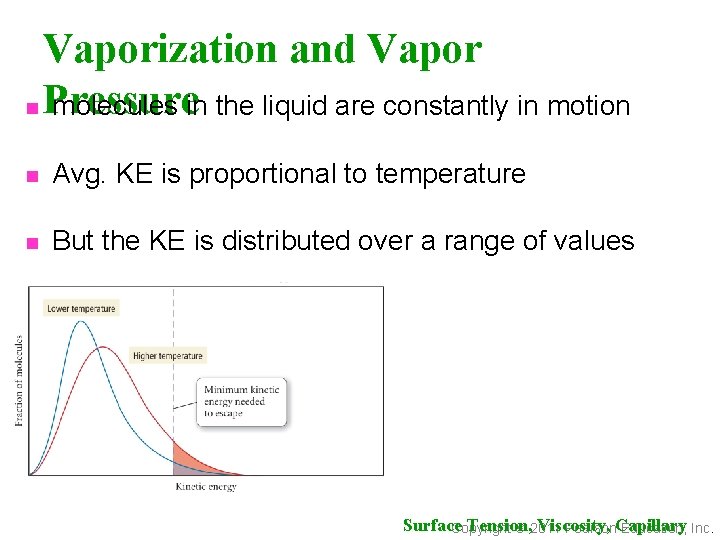
Vaporization and Vapor n Pressure molecules in the liquid are constantly in motion n Avg. KE is proportional to temperature n But the KE is distributed over a range of values Surface Tension, Viscosity, Copyright 2011 Pearson. Capillary Education, Inc.

if these molecules are at the surface, they can escape from the liquid and become a vapor At a given temperature, the larger the surface area, the faster the rate of evaporation As the temperature increases, a greater fraction of water molecules have this minimum energy faster rate of evaporation The stronger the IMFs, the slower the rate of vaporization Copyright 2011 Pearson Education, Inc.

What happens to the molecules that have escaped ? n n n some molecules of the vapor will lose energy through molecular collisions the result will be that some of the molecules will get captured back into the liquid when they collide with it also some may stick and gather together to form droplets of liquid q particularly on surrounding surfaces Copyright 2011 Pearson Education, Inc.

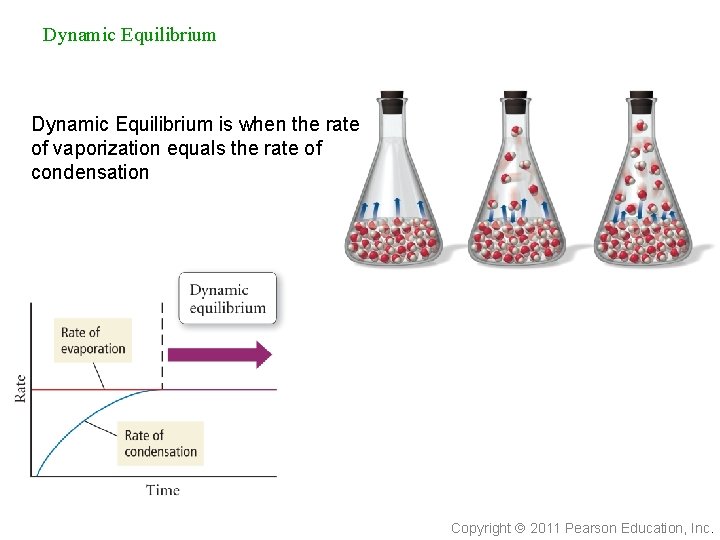
Evaporation vs. Condensation n n vaporization and condensation are opposite processes in an open container, the vapor molecules generally spread out faster than they can condense. Net result is a loss of liquid. however, in a closed container, the vapor is not allowed to spread out indefinitely the net result in a closed container is that at some time the rates of vaporization and condensation will be equal Copyright 2011 Pearson Education, Inc.

Energetics of Vaporization n when the high energy molecules are lost from the liquid, it lowers the average kinetic energy if energy is not drawn back into the liquid, its temperature will decrease – therefore, vaporization requires input of energy to overcome the attractions between molecules Copyright 2011 Pearson Education, Inc.

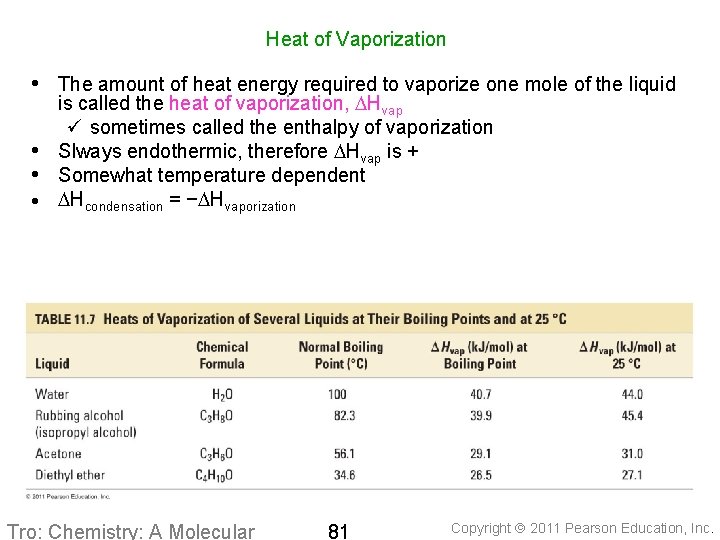
Heat of Vaporization n the amount of heat energy required to vaporize one mole of the liquid is called the Heat of Vaporization, DHvap q sometimes called the enthalpy of vaporization always endothermic, therefore DHvap is + somewhat temperature dependent Copyright 2011 Pearson Education, Inc.

Dynamic Equilibrium is when the rate of vaporization equals the rate of condensation Copyright 2011 Pearson Education, Inc.

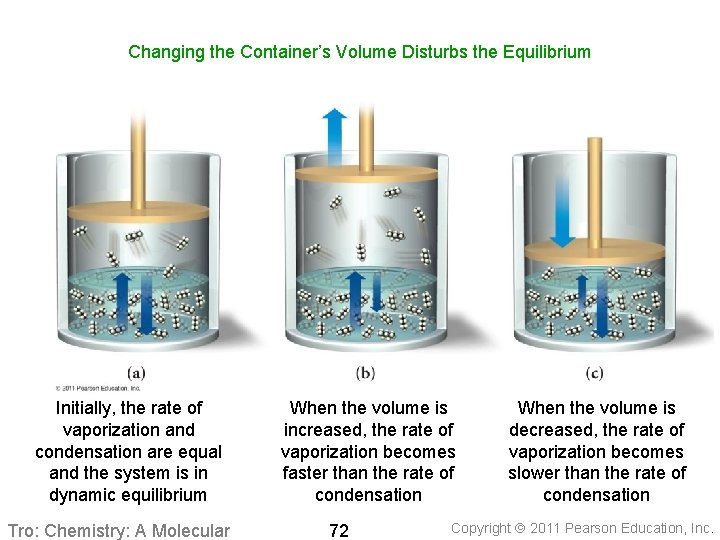
Changing the Container’s Volume Disturbs the Equilibrium Initially, the rate of vaporization and condensation are equal and the system is in dynamic equilibrium Tro: Chemistry: A Molecular When the volume is increased, the rate of vaporization becomes faster than the rate of condensation 72 When the volume is decreased, the rate of vaporization becomes slower than the rate of condensation Copyright 2011 Pearson Education, Inc.

Effect of Intermolecular Attraction on Evaporation and Condensation • The weaker the attractive forces between molecules, the less energy they will need • • • to vaporize Also, weaker attractive forces means that more energy will need to be removed from the vapor molecules before they can condense The net result will be more molecules in the vapor phase, and a liquid that evaporates faster – the weaker the attractive forces, the faster the rate of evaporation Liquids that evaporate easily are said to be volatile ü e. g. , gasoline, fingernail polish remover ü liquids that do not evaporate easily are called nonvolatile Ø e. g. , motor oil Tro: Chemistry: A Molecular 73 Copyright 2011 Pearson Education, Inc.

Boiling Point n when the temperature of a liquid reaches a point where its vapor pressure is the same as the external pressure, vapor bubbles can form anywhere in the liquid n this phenomenon is what is called boiling and the temperature required to have the vapor pressure = external pressure is the boiling point At the summit of Mt. Everest, water boils at 78 o. C. Why ? Copyright 2011 Pearson Education, Inc.

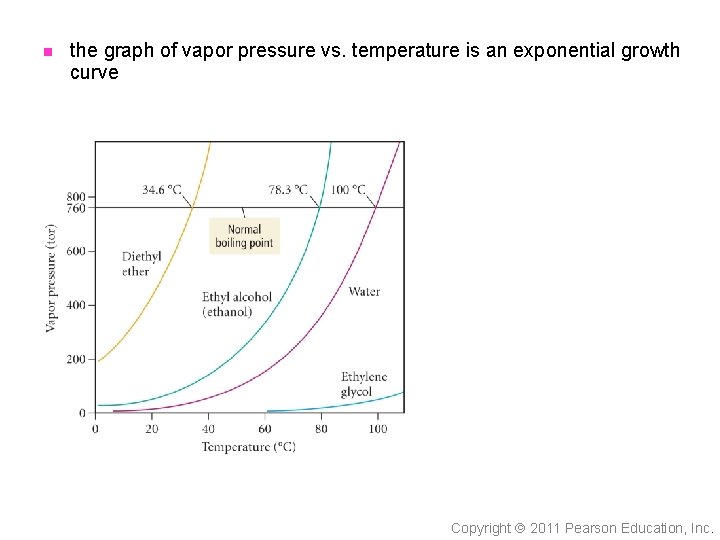
n the graph of vapor pressure vs. temperature is an exponential growth curve Copyright 2011 Pearson Education, Inc.

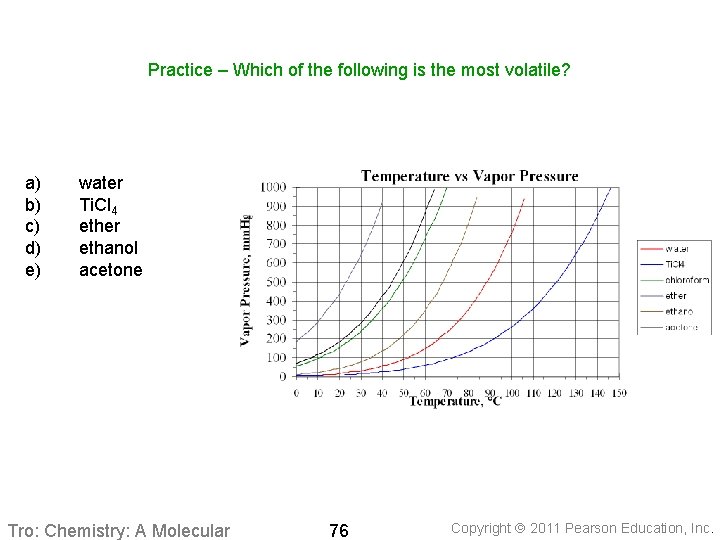
Practice – Which of the following is the most volatile? a) b) c) d) e) water Ti. Cl 4 ether ethanol acetone Tro: Chemistry: A Molecular 76 Copyright 2011 Pearson Education, Inc.

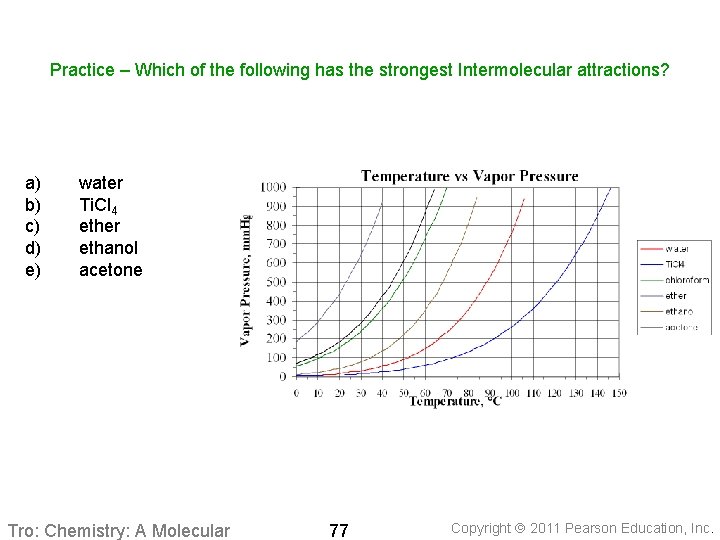
Practice – Which of the following has the strongest Intermolecular attractions? a) b) c) d) e) water Ti. Cl 4 ether ethanol acetone Tro: Chemistry: A Molecular 77 Copyright 2011 Pearson Education, Inc.

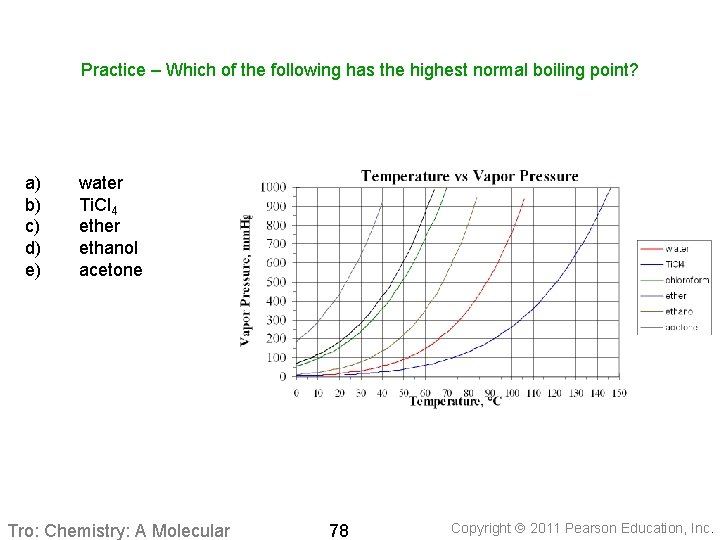
Practice – Which of the following has the highest normal boiling point? a) b) c) d) e) water Ti. Cl 4 ether ethanol acetone Tro: Chemistry: A Molecular 78 Copyright 2011 Pearson Education, Inc.

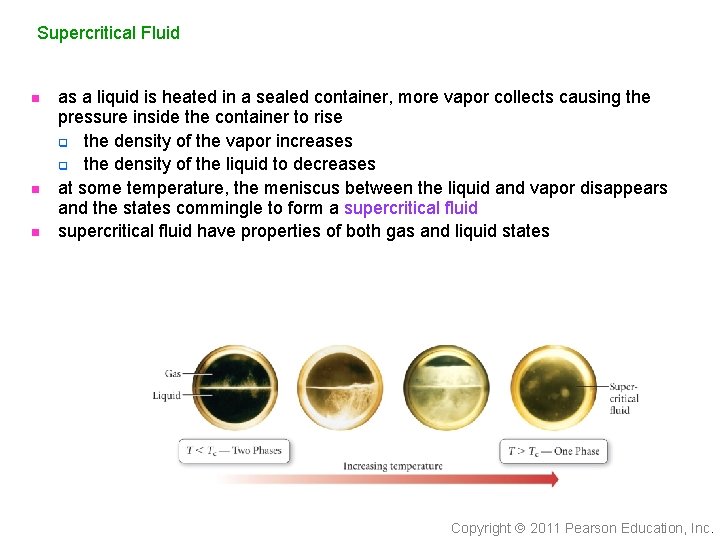
Supercritical Fluid n n n as a liquid is heated in a sealed container, more vapor collects causing the pressure inside the container to rise q the density of the vapor increases q the density of the liquid to decreases at some temperature, the meniscus between the liquid and vapor disappears and the states commingle to form a supercritical fluid have properties of both gas and liquid states Copyright 2011 Pearson Education, Inc.

The Critical Point n n n the temperature required to produce a supercritical fluid is called the critical temperature the pressure at the critical temperature is called the critical pressure at the critical temperature or higher temperatures, the gas cannot be condensed to a liquid, no matter how high the pressure gets Copyright 2011 Pearson Education, Inc.

Heat of Vaporization • The amount of heat energy required to vaporize one mole of the liquid is called the heat of vaporization, DHvap ü sometimes called the enthalpy of vaporization • Slways endothermic, therefore DHvap is + • Somewhat temperature dependent · DHcondensation = −DHvaporization Tro: Chemistry: A Molecular 81 Copyright 2011 Pearson Education, Inc.

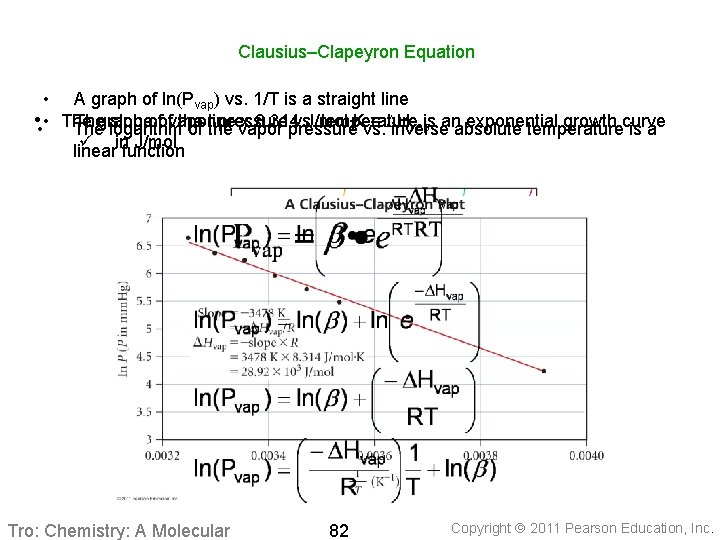
Clausius–Clapeyron Equation • A graph of ln(Pvap) vs. 1/T is a straight line Thegraph slopeofofvapor the line x 8. 314 J/mol∙K = DH • • • The pressure vs. temperature is anabsolute exponential growth curve vap The logarithm of the vapor pressure vs. inverse temperature is a ü infunction J/mol linear Tro: Chemistry: A Molecular 82 Copyright 2011 Pearson Education, Inc.

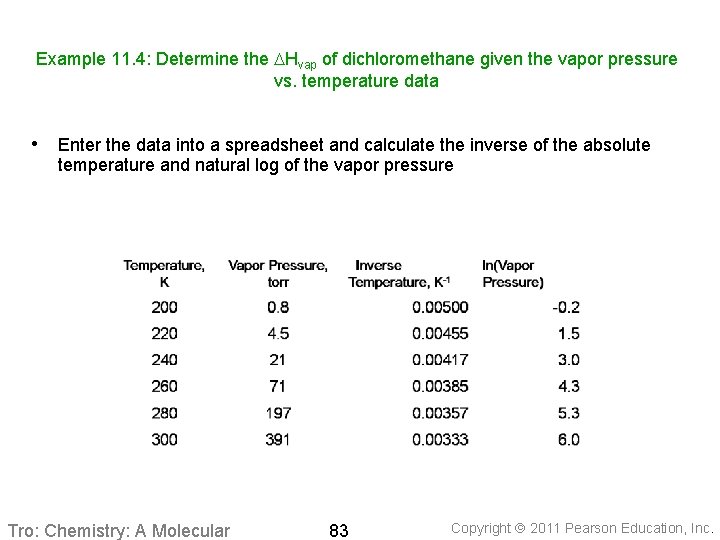
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Enter the data into a spreadsheet and calculate the inverse of the absolute temperature and natural log of the vapor pressure Tro: Chemistry: A Molecular 83 Copyright 2011 Pearson Education, Inc.

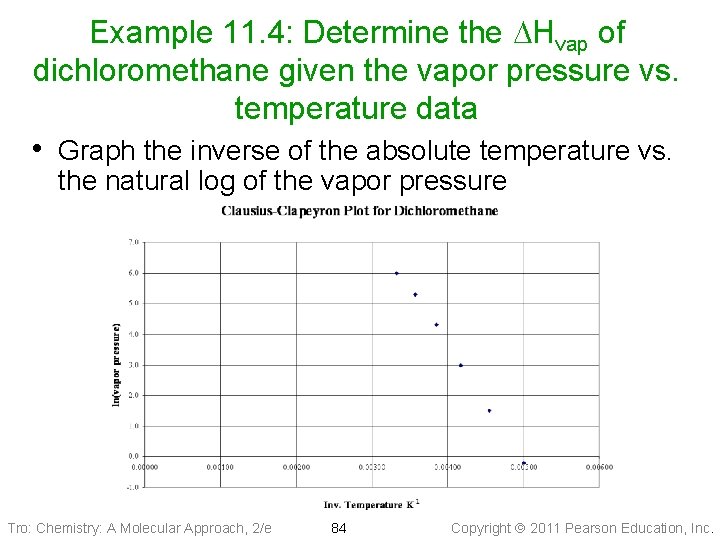
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Graph the inverse of the absolute temperature vs. the natural log of the vapor pressure Tro: Chemistry: A Molecular Approach, 2/e 84 Copyright 2011 Pearson Education, Inc.

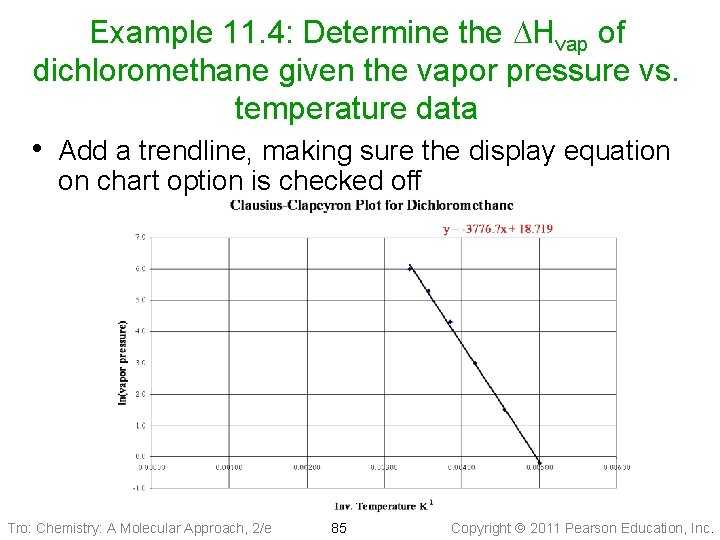
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Add a trendline, making sure the display equation on chart option is checked off Tro: Chemistry: A Molecular Approach, 2/e 85 Copyright 2011 Pearson Education, Inc.

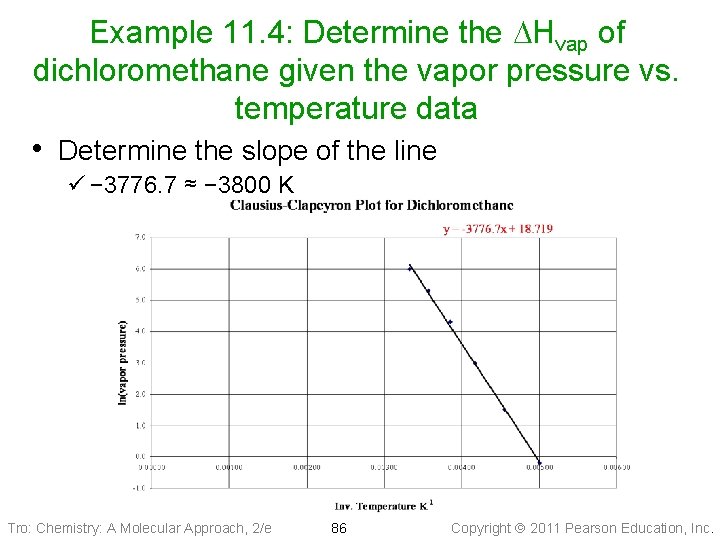
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Determine the slope of the line ü − 3776. 7 ≈ − 3800 K Tro: Chemistry: A Molecular Approach, 2/e 86 Copyright 2011 Pearson Education, Inc.

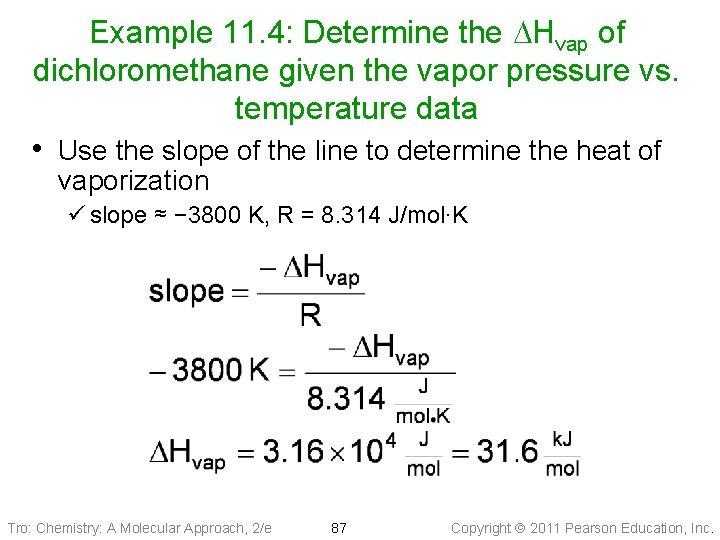
Example 11. 4: Determine the DHvap of dichloromethane given the vapor pressure vs. temperature data • Use the slope of the line to determine the heat of vaporization ü slope ≈ − 3800 K, R = 8. 314 J/mol∙K Tro: Chemistry: A Molecular Approach, 2/e 87 Copyright 2011 Pearson Education, Inc.

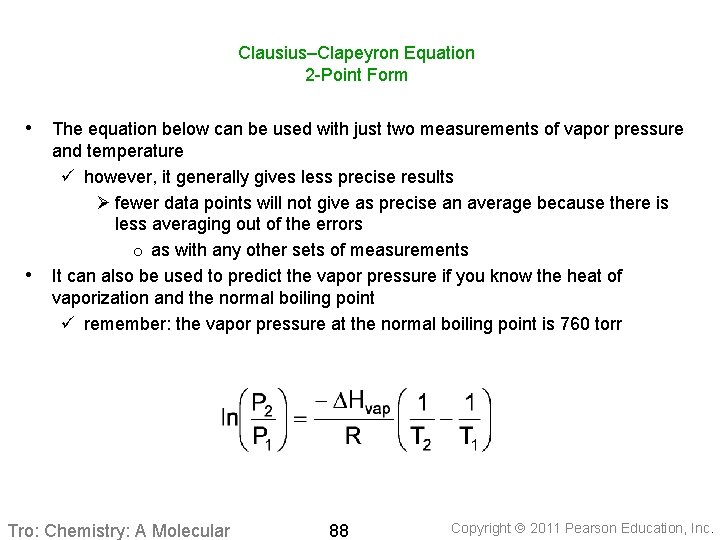
Clausius–Clapeyron Equation 2 -Point Form • The equation below can be used with just two measurements of vapor pressure • and temperature ü however, it generally gives less precise results Ø fewer data points will not give as precise an average because there is less averaging out of the errors o as with any other sets of measurements It can also be used to predict the vapor pressure if you know the heat of vaporization and the normal boiling point ü remember: the vapor pressure at the normal boiling point is 760 torr Tro: Chemistry: A Molecular 88 Copyright 2011 Pearson Education, Inc.

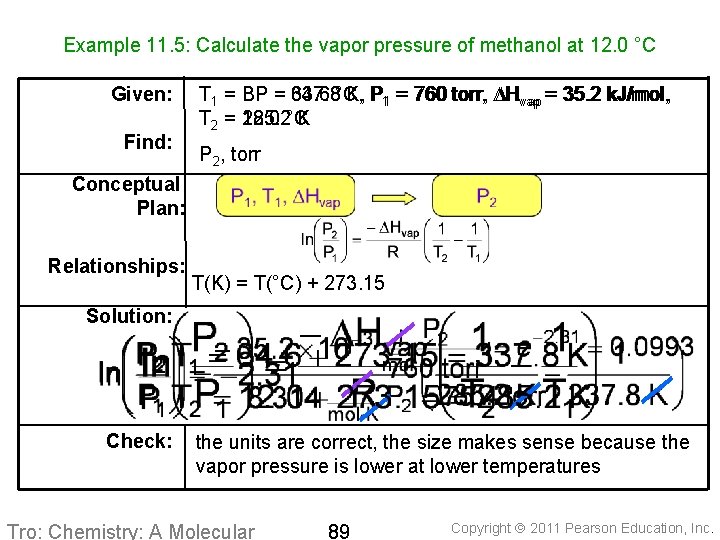
Example 11. 5: Calculate the vapor pressure of methanol at 12. 0 °C Given: Find: T 1 = BP = 337. 8 64. 6 °C, K, P P 11 == 760 torr, DH DHvap = 35. 2 k. J/mol, vap = T 2 = 285. 2 12. 0 °C K P 2, torr Conceptual Plan: Relationships: T(K) = T(°C) + 273. 15 Solution: Check: the units are correct, the size makes sense because the vapor pressure is lower at lower temperatures Tro: Chemistry: A Molecular 89 Copyright 2011 Pearson Education, Inc.

Practice – Determine the vapor pressure of water at 25 C (normal BP = 100. 0 C, DHvap= 40. 7 k. J/mol) Tro: Chemistry: A Molecular Approach, 2/e 90 Copyright 2011 Pearson Education, Inc.

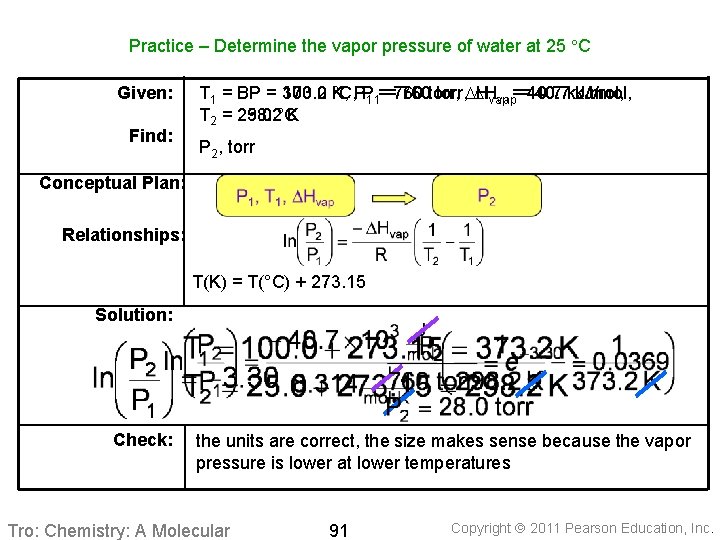
Practice – Determine the vapor pressure of water at 25 C Given: Find: T 1 = BP = 373. 2 100. 0 K, °C, PP 1 1==760 760 torr, DH DHvap 40. 7 k. J/mol, vap==40. 7 T 2 = 298. 2 25. 0 °C K P 2, torr Conceptual Plan: Relationships: T(K) = T(°C) + 273. 15 Solution: Check: the units are correct, the size makes sense because the vapor pressure is lower at lower temperatures Tro: Chemistry: A Molecular 91 Copyright 2011 Pearson Education, Inc.

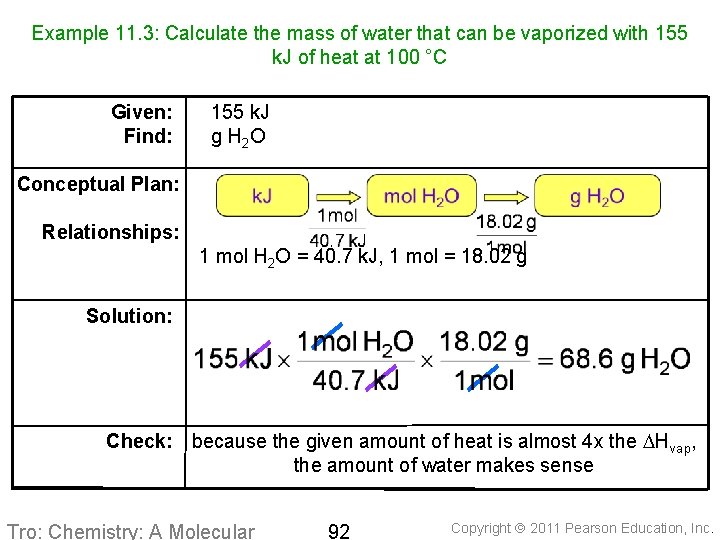
Example 11. 3: Calculate the mass of water that can be vaporized with 155 k. J of heat at 100 °C Given: Find: 155 k. J g H 2 O Conceptual Plan: Relationships: 1 mol H 2 O = 40. 7 k. J, 1 mol = 18. 02 g Solution: Check: because the given amount of heat is almost 4 x the DHvap, the amount of water makes sense Tro: Chemistry: A Molecular 92 Copyright 2011 Pearson Education, Inc.

Practice – Calculate the amount of heat needed to vaporize 90. 0 g of C 3 H 7 OH at its boiling point (DHvap = 39. 9 k. J/mol) Tro: Chemistry: A Molecular Approach, 2/e 93 Copyright 2011 Pearson Education, Inc.

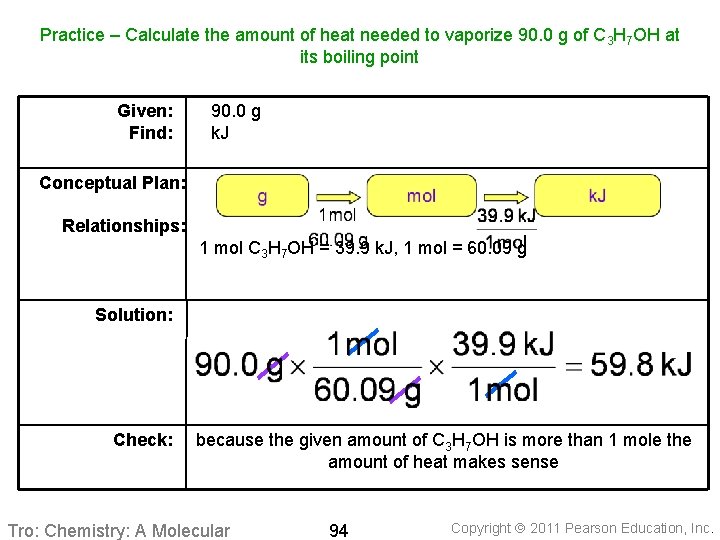
Practice – Calculate the amount of heat needed to vaporize 90. 0 g of C 3 H 7 OH at its boiling point Given: Find: 90. 0 g k. J Conceptual Plan: Relationships: 1 mol C 3 H 7 OH = 39. 9 k. J, 1 mol = 60. 09 g Solution: Check: because the given amount of C 3 H 7 OH is more than 1 mole the amount of heat makes sense Tro: Chemistry: A Molecular 94 Copyright 2011 Pearson Education, Inc.

Melting = Fusion n as a solid is heated, its temperature rises and the molecules vibrate more vigorously once the temperature reaches the melting point, the molecules have sufficient energy to overcome some of the attractions that hold them in position and the solid melts (or fuses) the opposite of melting is freezing Copyright 2011 Pearson Education, Inc.

Energetics of Melting n n when the high energy molecules are lost from the solid, it lowers the average kinetic energy if energy is not drawn back into the solid its temperature will decrease q and freezing is an exothermic process melting requires input of energy to overcome the attractions between molecules the amount of heat energy required to melt one mole of the solid is called the heat of fusion, DHfus sometimes called the enthalpy of fusion Copyright 2011 Pearson Education, Inc.

Sublimation and Deposition n Similar to the process in a liquid, molecules in a solid can break free from the surface and become a gas – this process is called sublimation the capturing of vapor molecules into a solid is called deposition the solid and vapor phases exist in dynamic equilibrium in a closed container q at temperatures below the melting point q therefore, molecular solids have a vapor pressure Copyright 2011 Pearson Education, Inc.

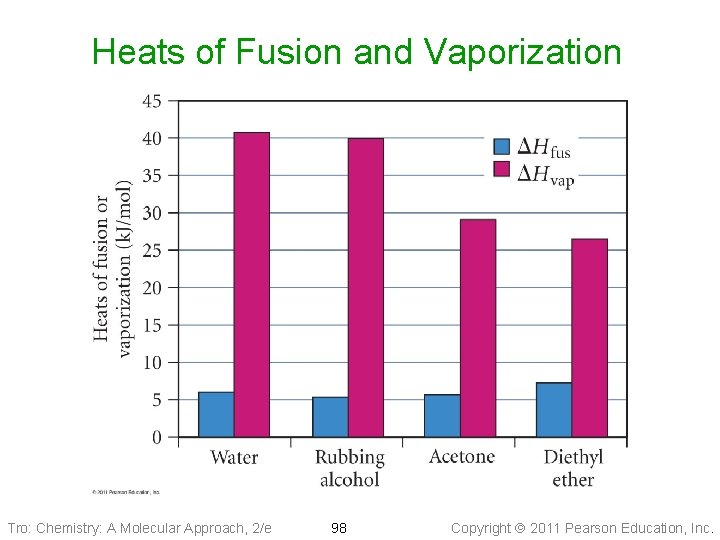
Heats of Fusion and Vaporization Tro: Chemistry: A Molecular Approach, 2/e 98 Copyright 2011 Pearson Education, Inc.

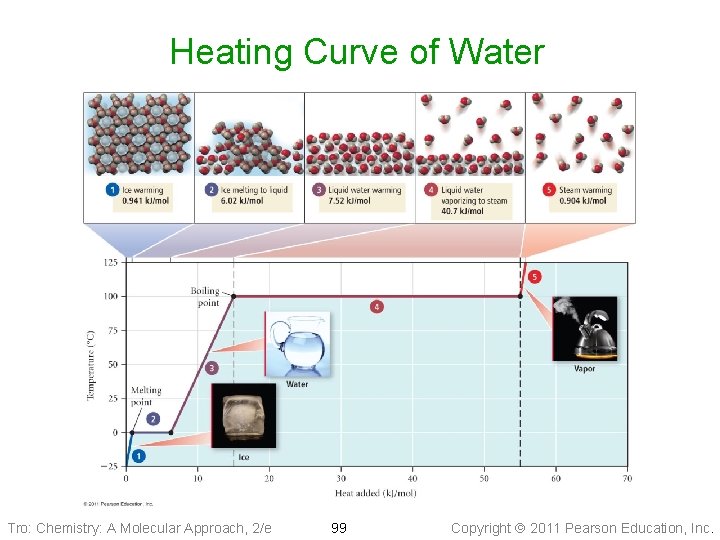
Heating Curve of Water Tro: Chemistry: A Molecular Approach, 2/e 99 Copyright 2011 Pearson Education, Inc.

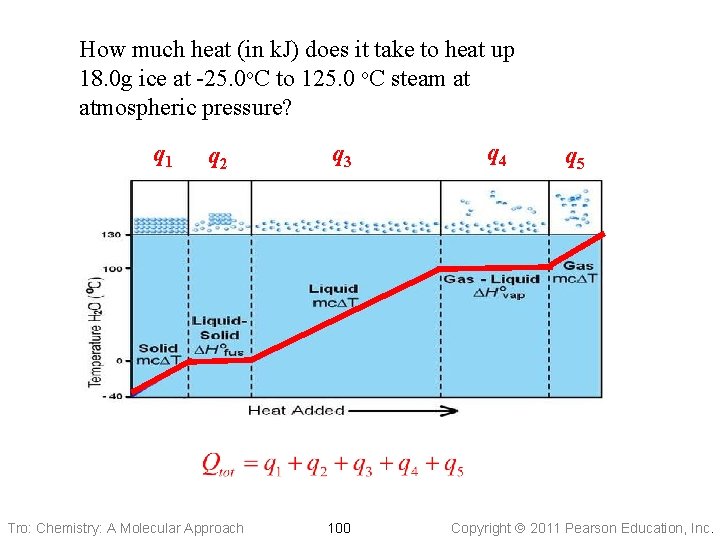
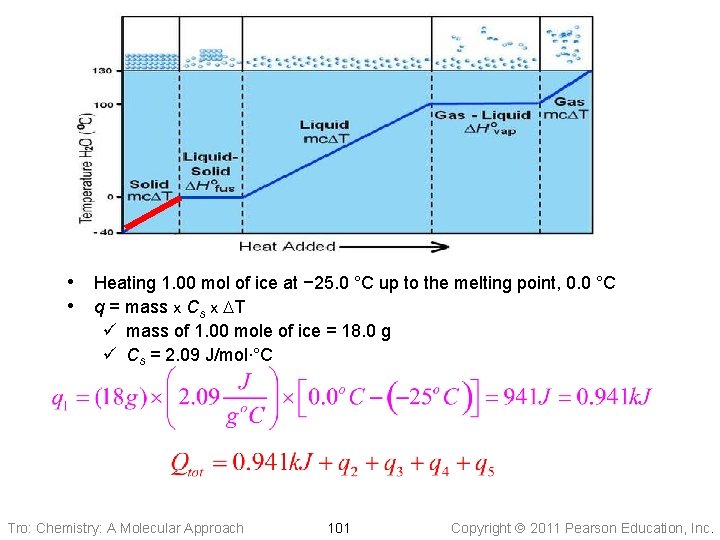
How much heat (in k. J) does it take to heat up 18. 0 g ice at -25. 0 o. C to 125. 0 o. C steam at atmospheric pressure? q 1 q 2 Tro: Chemistry: A Molecular Approach q 3 100 q 4 q 5 Copyright 2011 Pearson Education, Inc.

• Heating 1. 00 mol of ice at − 25. 0 °C up to the melting point, 0. 0 °C • q = mass x Cs x DT ü mass of 1. 00 mole of ice = 18. 0 g ü Cs = 2. 09 J/mol∙°C Tro: Chemistry: A Molecular Approach 101 Copyright 2011 Pearson Education, Inc.

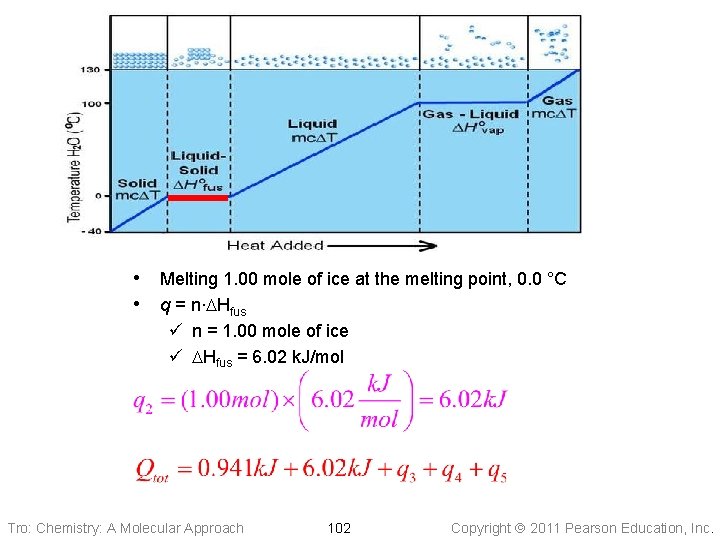
• Melting 1. 00 mole of ice at the melting point, 0. 0 °C • q = n∙DHfus ü n = 1. 00 mole of ice ü DHfus = 6. 02 k. J/mol Tro: Chemistry: A Molecular Approach 102 Copyright 2011 Pearson Education, Inc.

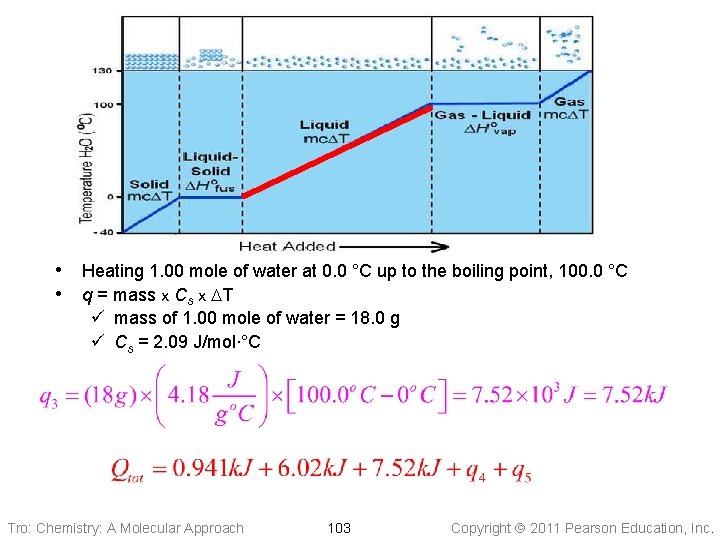
• Heating 1. 00 mole of water at 0. 0 °C up to the boiling point, 100. 0 °C • q = mass x Cs x DT ü mass of 1. 00 mole of water = 18. 0 g ü Cs = 2. 09 J/mol∙°C Tro: Chemistry: A Molecular Approach 103 Copyright 2011 Pearson Education, Inc.

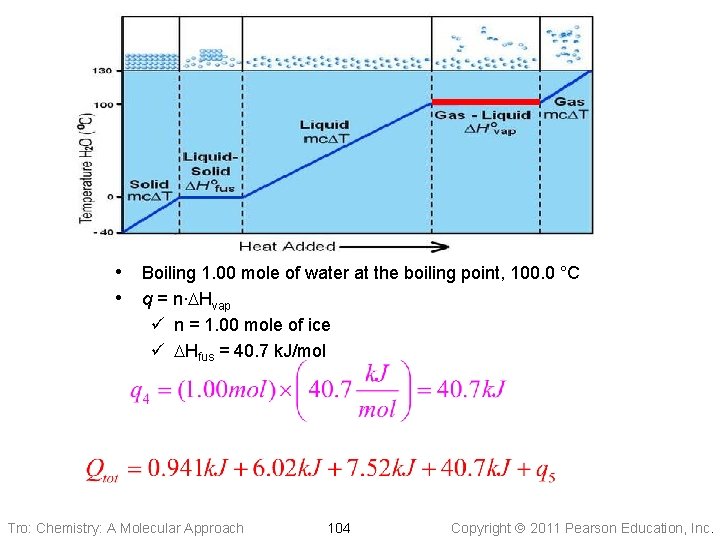
• Boiling 1. 00 mole of water at the boiling point, 100. 0 °C • q = n∙DHvap ü n = 1. 00 mole of ice ü DHfus = 40. 7 k. J/mol Tro: Chemistry: A Molecular Approach 104 Copyright 2011 Pearson Education, Inc.

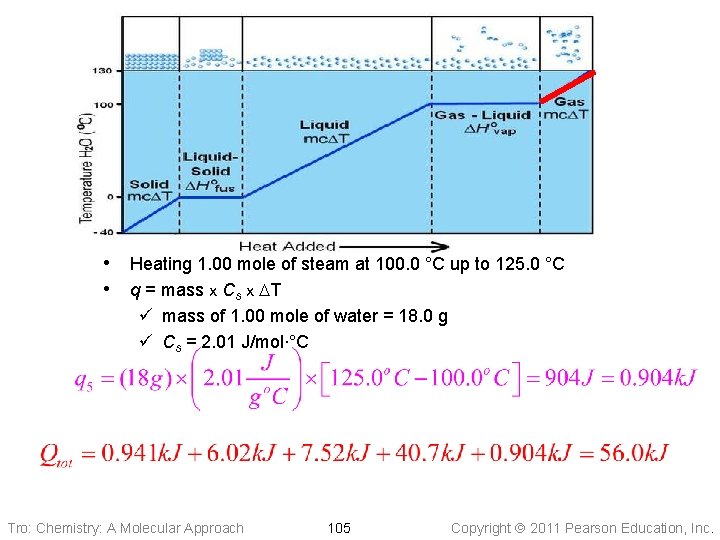
• Heating 1. 00 mole of steam at 100. 0 °C up to 125. 0 °C • q = mass x Cs x DT ü mass of 1. 00 mole of water = 18. 0 g ü Cs = 2. 01 J/mol∙°C Tro: Chemistry: A Molecular Approach 105 Copyright 2011 Pearson Education, Inc.

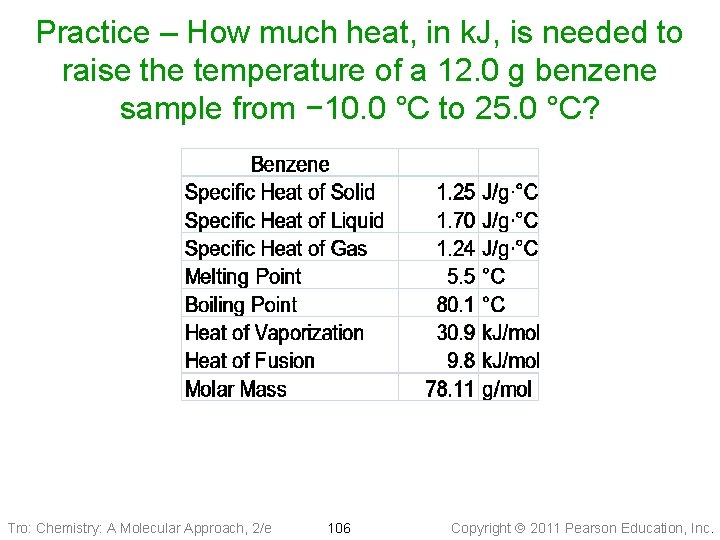
Practice – How much heat, in k. J, is needed to raise the temperature of a 12. 0 g benzene sample from − 10. 0 °C to 25. 0 °C? Tro: Chemistry: A Molecular Approach, 2/e 106 Copyright 2011 Pearson Education, Inc.

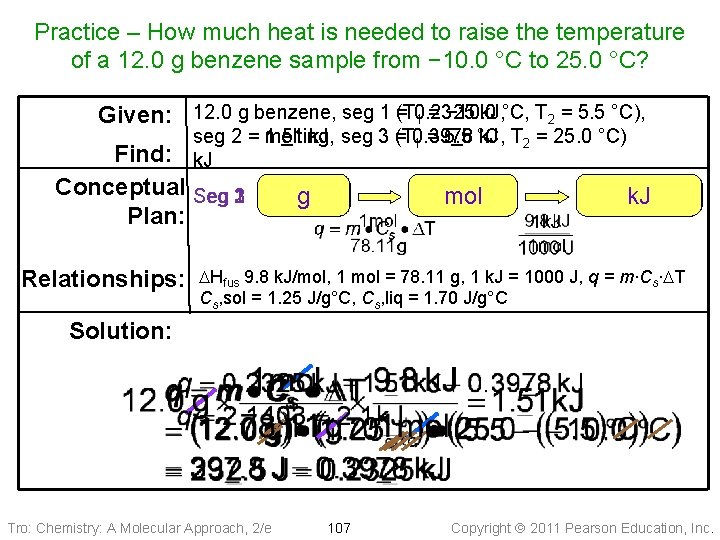
Practice – How much heat is needed to raise the temperature of a 12. 0 g benzene sample from − 10. 0 °C to 25. 0 °C? k. J, °C, T 2 = 5. 5 °C), Given: 12. 0 g benzene, seg 1 =(T 0. 2325 1 = − 10. 0 seg 2 = 1. 51 melting, k. J, seg 3 (T = 0. 3978 k. J T 2 = 25. 0 °C) 1 = 5. 5 °C, k. J Find: Conceptual Seg 231 Plan: Relationships: g mol J k. J DHfus 9. 8 k. J/mol, 1 mol = 78. 11 g, 1 k. J = 1000 J, q = m∙Cs∙DT Cs, sol = 1. 25 J/g°C, Cs, liq = 1. 70 J/g°C Solution: Tro: Chemistry: A Molecular Approach, 2/e 107 Copyright 2011 Pearson Education, Inc.

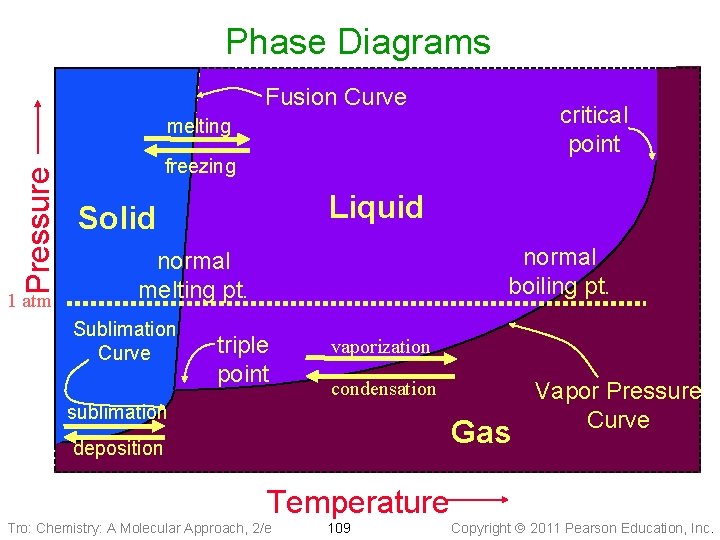
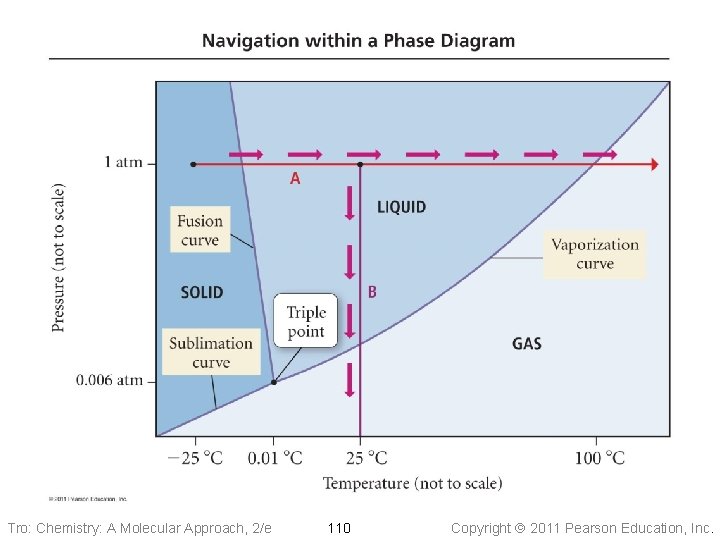
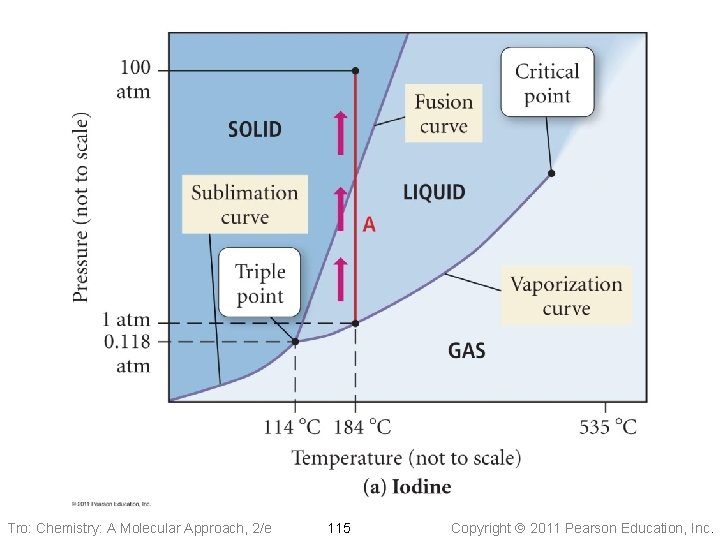
Phase Diagrams • Phase diagrams describe the different states and state changes that occur at • • various temperature/pressure conditions Regions represent states Lines represent state changes ü liquid/gas line is vapor pressure curve ü both states exist simultaneously ü critical point is the furthest point on the vapor pressure curve Triple point is the temperature/pressure condition where all three states exist simultaneously For most substances, freezing point increases as pressure increases Tro: Chemistry: A Molecular Approach, 2/e 108 Copyright 2011 Pearson Education, Inc.

Phase Diagrams Fusion Curve critical point Pressure melting 1 atm freezing Liquid Solid normal boiling pt. normal melting pt. Sublimation Curve triple point vaporization condensation sublimation Gas deposition Temperature Tro: Chemistry: A Molecular Approach, 2/e 109 Vapor Pressure Curve Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 110 Copyright 2011 Pearson Education, Inc.

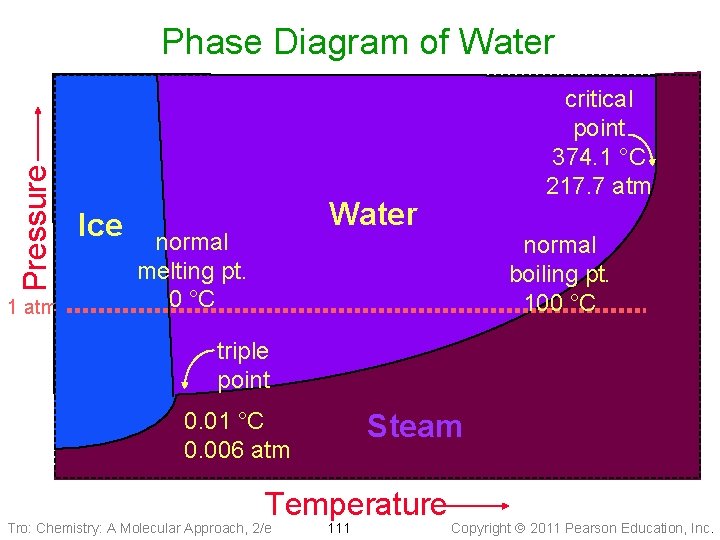
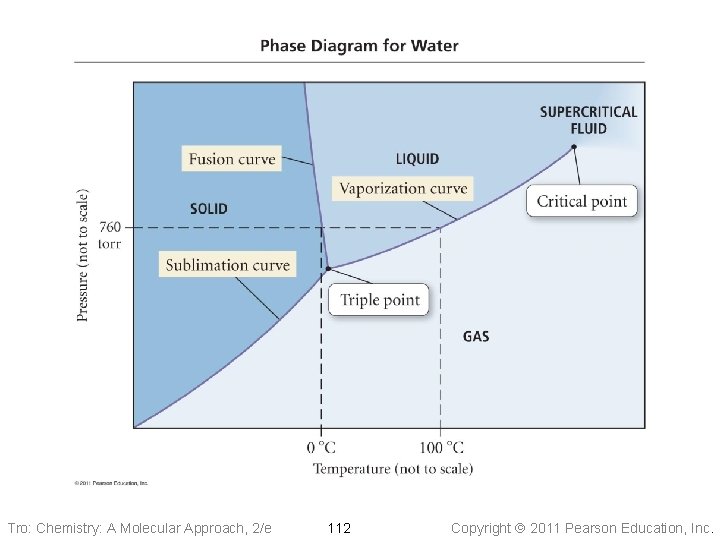
Pressure Phase Diagram of Water 1 atm Ice critical point 374. 1 °C 217. 7 atm Water normal melting pt. 0 °C normal boiling pt. 100 °C triple point 0. 01 °C 0. 006 atm Steam Temperature Tro: Chemistry: A Molecular Approach, 2/e 111 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 112 Copyright 2011 Pearson Education, Inc.

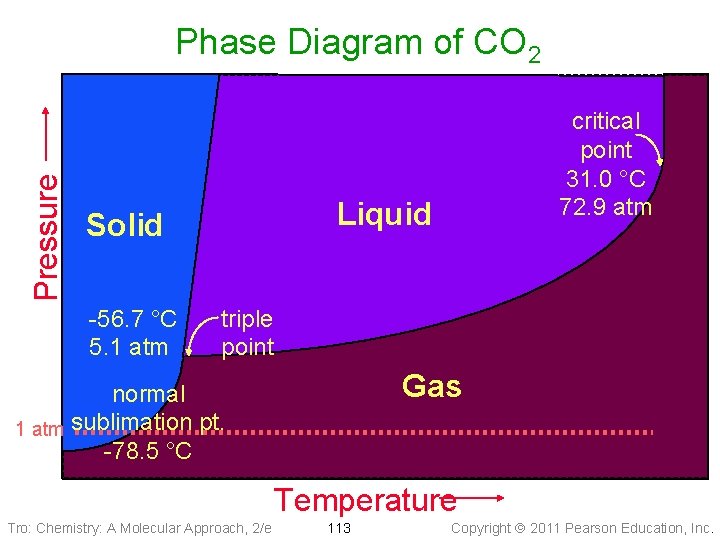
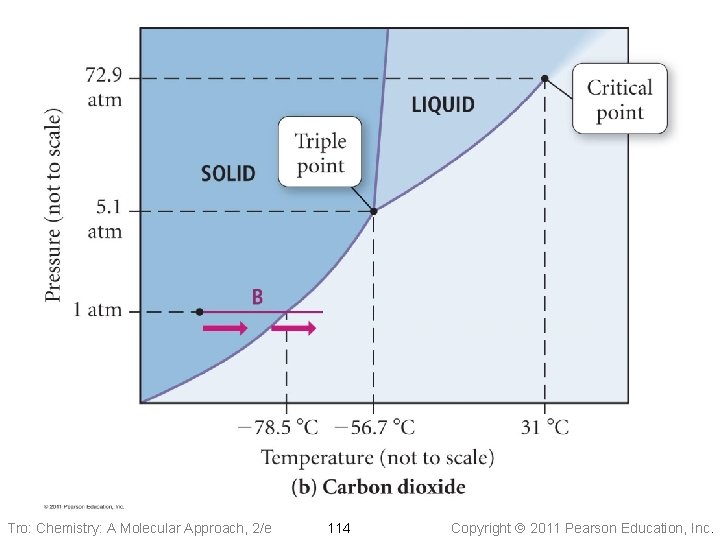
Pressure Phase Diagram of CO 2 Liquid Solid -56. 7 °C 5. 1 atm critical point 31. 0 °C 72. 9 atm triple point Gas normal 1 atm sublimation pt. -78. 5 °C Temperature Tro: Chemistry: A Molecular Approach, 2/e 113 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 114 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 115 Copyright 2011 Pearson Education, Inc.

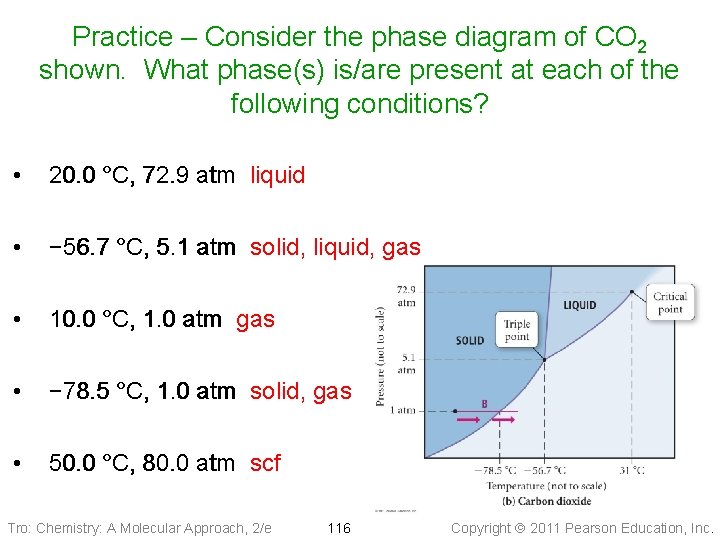
Practice – Consider the phase diagram of CO 2 shown. What phase(s) is/are present at each of the following conditions? • 20. 0 °C, 72. 9 atm liquid • − 56. 7 °C, 5. 1 atm solid, liquid, gas • 10. 0 °C, 1. 0 atm gas • − 78. 5 °C, 1. 0 atm solid, gas • 50. 0 °C, 80. 0 atm scf Tro: Chemistry: A Molecular Approach, 2/e 116 Copyright 2011 Pearson Education, Inc.

Water – An Extraordinary Substance • Water is a liquid at room temperature ü most molecular substances with similar molar masses are gases at room temperature Ø e. g. NH 3, CH 4 ü due to H-bonding between molecules • Water is an excellent solvent – dissolving many ionic and polar molecular substances ü because of its large dipole moment ü even many small nonpolar molecules have some solubility in water Ø e. g. O 2, CO 2 • Water has a very high specific heat for a molecular substance ü moderating effect on coastal climates • Water expands when it freezes Ø at a pressure of 1 atm ü about 9% ü making ice less dense than liquid water Tro: Chemistry: A Molecular Approach, 2/e 117 Copyright 2011 Pearson Education, Inc.

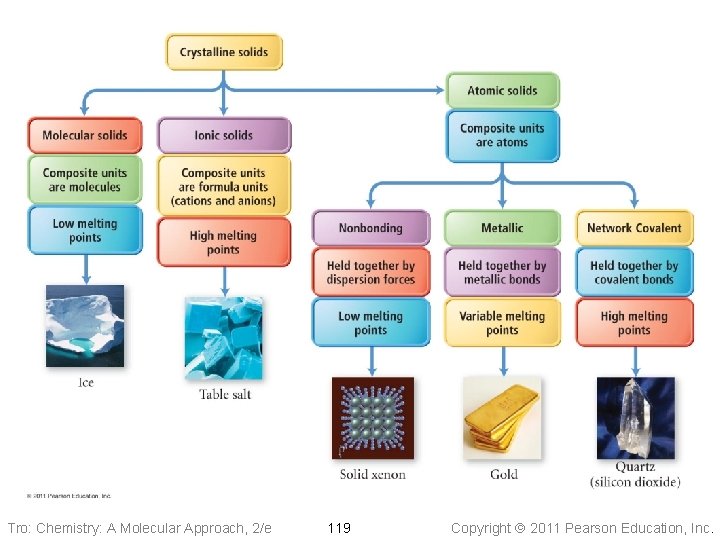
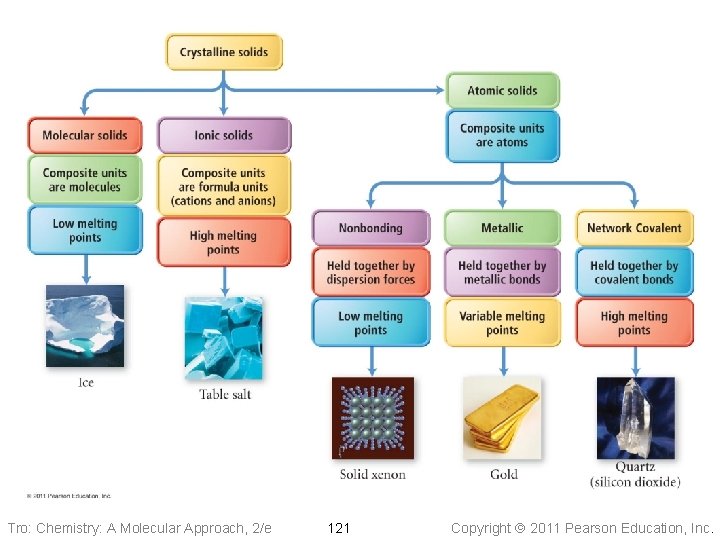
Solids Properties & Structure Tro: Chemistry: A Molecular Approach, 2/e Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 119 Copyright 2011 Pearson Education, Inc.

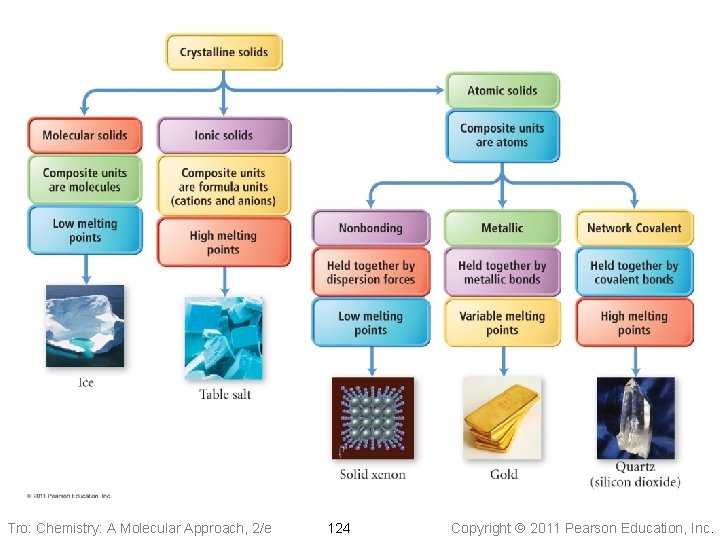
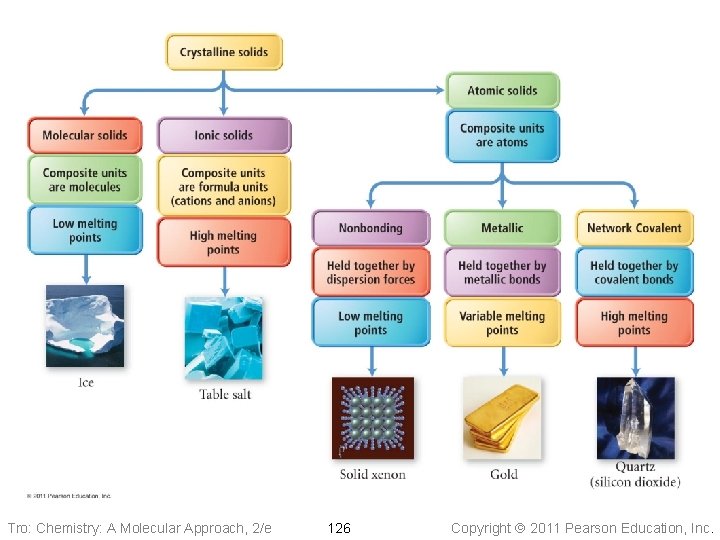
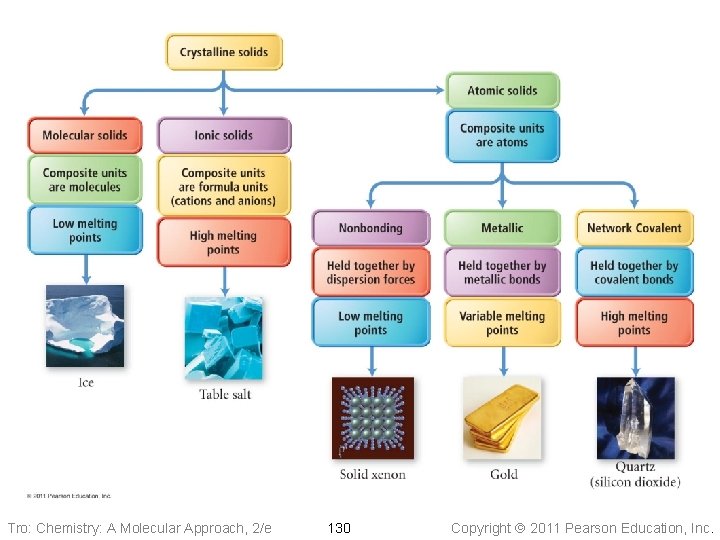
Molecular Solids • The lattice sites are occupied by molecules ü CO 2, H 2 O, C 12 H 22 O 11 • The molecules are held together by intermolecular attractive forces ü dispersion forces, dipole–dipole attractions, and H-bonds • Because the attractive forces are weak, they tend to have low melting points ü generally < 300 °C Tro: Chemistry: A Molecular Approach, 2/e 120 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 121 Copyright 2011 Pearson Education, Inc.

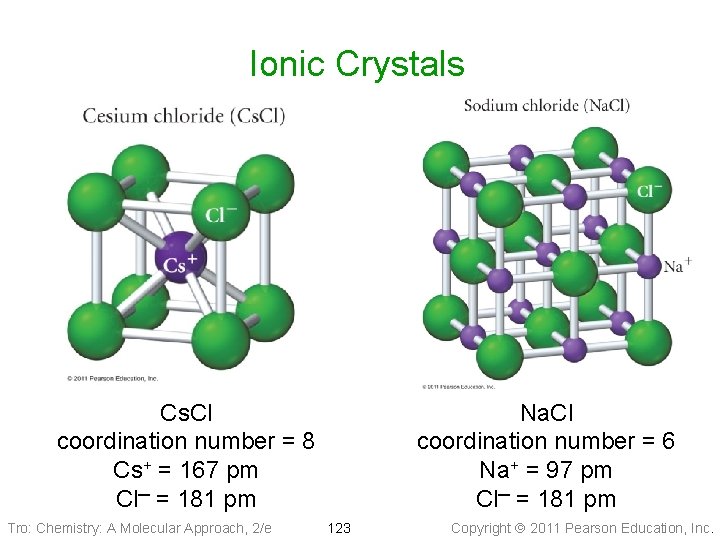
Ionic Solids • Lattice sites occupied by ions • Held together by attractions between oppositely charged ions ü nondirectional ü therefore every cation attracts all anions around it, and vice-versa • The coordination number represents the number of close cation–anion interactions in the crystal • The higher the coordination number, the more stable the solid ü lowers the potential energy of the solid • The coordination number depends on the relative sizes of the cations and anions that maintains charge balance ü generally, anions are larger than cations ü the number of anions that can surround the cation is limited by the size of the cation ü the closer in size the ions are, the higher the coordination number is Tro: Chemistry: A Molecular Approach, 2/e 122 Copyright 2011 Pearson Education, Inc.

Ionic Crystals Cs. Cl coordination number = 8 Cs+ = 167 pm Cl─ = 181 pm Tro: Chemistry: A Molecular Approach, 2/e Na. Cl coordination number = 6 Na+ = 97 pm Cl─ = 181 pm 123 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 124 Copyright 2011 Pearson Education, Inc.

Nonbonding Atomic Solids • Noble gases in solid form • Solid held together by weak dispersion forces ü very low melting • Tend to arrange atoms in closest-packed structure ü either hexagonal cp or cubic cp ü maximizes attractive forces and minimizes energy Tro: Chemistry: A Molecular Approach, 2/e 125 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 126 Copyright 2011 Pearson Education, Inc.

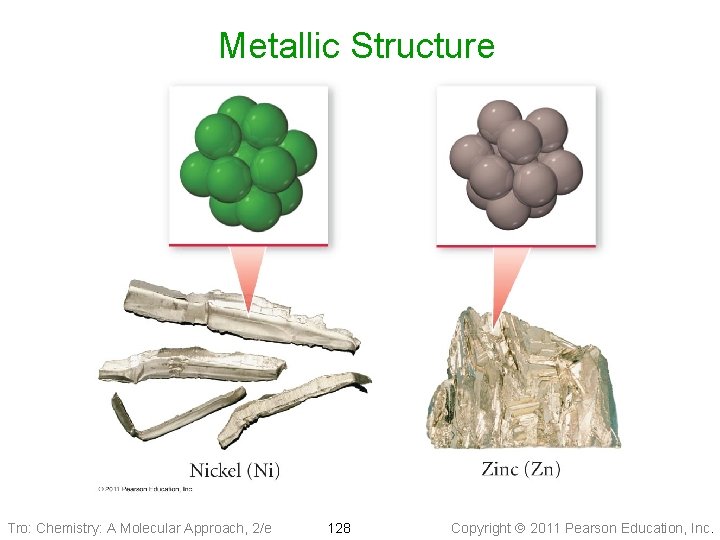
Metallic Atomic Solids • Solid held together by metallic bonds ü strength varies with sizes and charges of cations Ø coulombic attractions • Melting point varies • Mostly closest-packed arrangements of the lattice points ü cations Tro: Chemistry: A Molecular Approach, 2/e 127 Copyright 2011 Pearson Education, Inc.

Metallic Structure Tro: Chemistry: A Molecular Approach, 2/e 128 Copyright 2011 Pearson Education, Inc.

Metallic Bonding • Metal atoms release their valence • + + + ee- electrons Metal cation “islands” fixed in a “sea” of mobile electrons + + ee- + + + ee- Tro: Chemistry: A Molecular Approach, 2/e + + + ee- 129 + + + ee- + + Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 130 Copyright 2011 Pearson Education, Inc.

Network Covalent Solids • Atoms attached to their nearest neighbors by covalent bonds • Because of the directionality of the covalent bonds, these do not tend • • to form closest-packed arrangements in the crystal Because of the strength of the covalent bonds, these have very high melting points ü generally > 1000 °C Dimensionality of the network affects other physical properties Tro: Chemistry: A Molecular Approach, 2/e 131 Copyright 2011 Pearson Education, Inc.

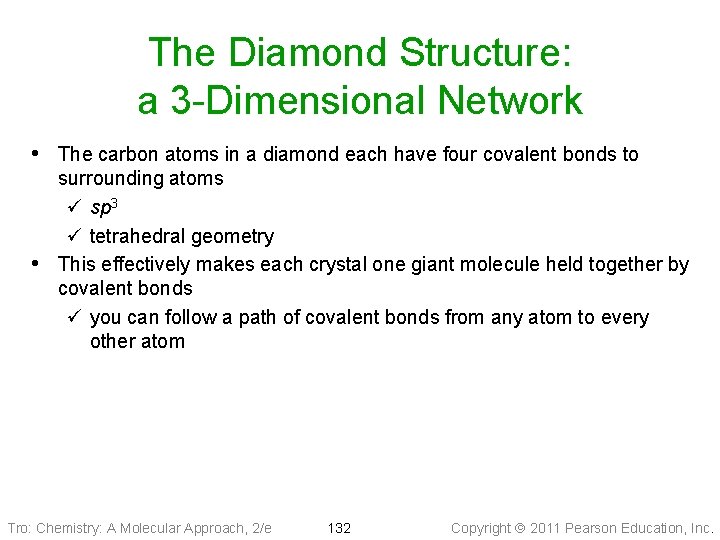
The Diamond Structure: a 3 -Dimensional Network • The carbon atoms in a diamond each have four covalent bonds to • surrounding atoms ü sp 3 ü tetrahedral geometry This effectively makes each crystal one giant molecule held together by covalent bonds ü you can follow a path of covalent bonds from any atom to every other atom Tro: Chemistry: A Molecular Approach, 2/e 132 Copyright 2011 Pearson Education, Inc.

Properties of Diamond • Very high melting point, ~3800 °C • • • ü need to overcome some covalent bonds Very rigid ü due to the directionality of the covalent bonds Very hard ü due to the strong covalent bonds holding the atoms in position ü used as abrasives Electrical insulator Thermal conductor ü best known Chemically very nonreactive Tro: Chemistry: A Molecular Approach, 2/e 133 Copyright 2011 Pearson Education, Inc.

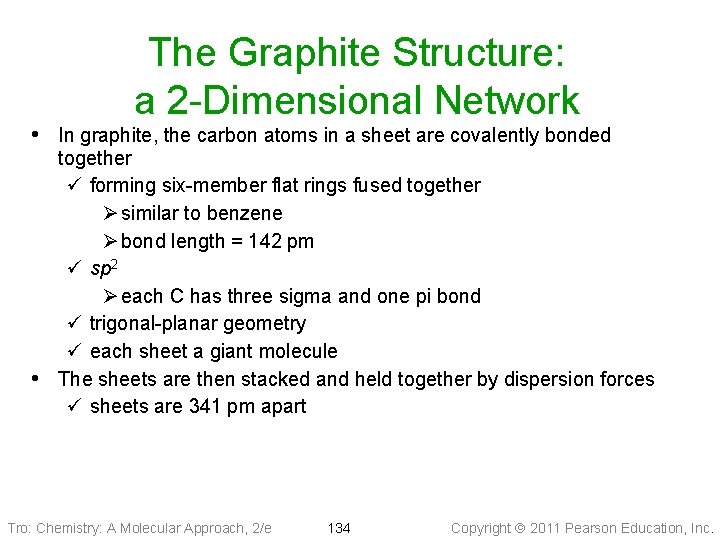
The Graphite Structure: a 2 -Dimensional Network • In graphite, the carbon atoms in a sheet are covalently bonded • together ü forming six-member flat rings fused together Ø similar to benzene Ø bond length = 142 pm ü sp 2 Ø each C has three sigma and one pi bond ü trigonal-planar geometry ü each sheet a giant molecule The sheets are then stacked and held together by dispersion forces ü sheets are 341 pm apart Tro: Chemistry: A Molecular Approach, 2/e 134 Copyright 2011 Pearson Education, Inc.

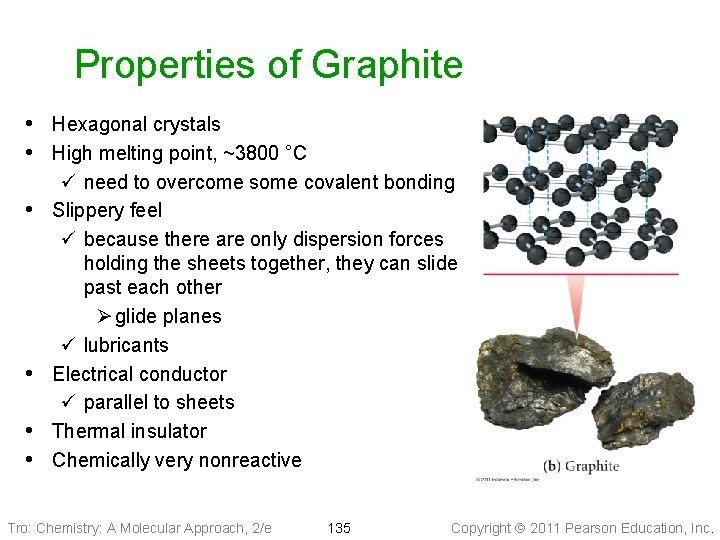
Properties of Graphite • Hexagonal crystals • High melting point, ~3800 °C • • ü need to overcome some covalent bonding Slippery feel ü because there are only dispersion forces holding the sheets together, they can slide past each other Ø glide planes ü lubricants Electrical conductor ü parallel to sheets Thermal insulator Chemically very nonreactive Tro: Chemistry: A Molecular Approach, 2/e 135 Copyright 2011 Pearson Education, Inc.

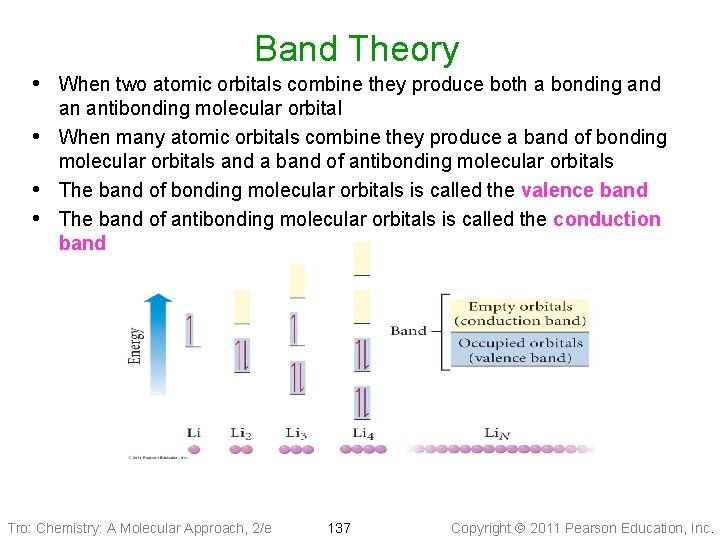
Band Theory • The structures of metals and covalent network solids result in every • atom’s orbitals being shared by the entire structure For large numbers of atoms, this results in a large number of molecular orbitals that have approximately the same energy; we call this an energy band Tro: Chemistry: A Molecular Approach, 2/e 136 Copyright 2011 Pearson Education, Inc.

Band Theory • When two atomic orbitals combine they produce both a bonding and • • • an antibonding molecular orbital When many atomic orbitals combine they produce a band of bonding molecular orbitals and a band of antibonding molecular orbitals The band of bonding molecular orbitals is called the valence band The band of antibonding molecular orbitals is called the conduction band Tro: Chemistry: A Molecular Approach, 2/e 137 Copyright 2011 Pearson Education, Inc.

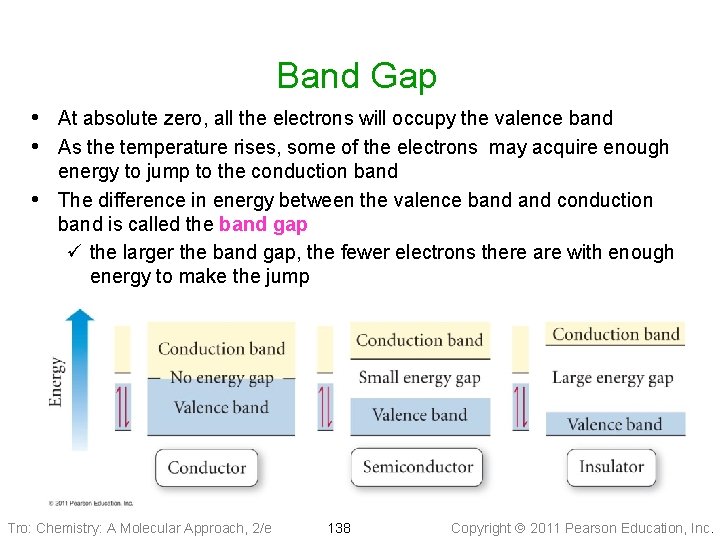
Band Gap • At absolute zero, all the electrons will occupy the valence band • As the temperature rises, some of the electrons may acquire enough • energy to jump to the conduction band The difference in energy between the valence band conduction band is called the band gap ü the larger the band gap, the fewer electrons there are with enough energy to make the jump Tro: Chemistry: A Molecular Approach, 2/e 138 Copyright 2011 Pearson Education, Inc.

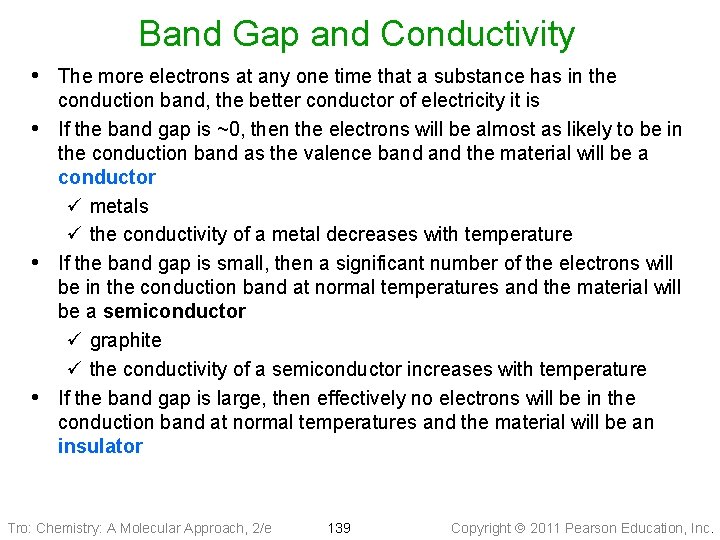
Band Gap and Conductivity • The more electrons at any one time that a substance has in the • • • conduction band, the better conductor of electricity it is If the band gap is ~0, then the electrons will be almost as likely to be in the conduction band as the valence band the material will be a conductor ü metals ü the conductivity of a metal decreases with temperature If the band gap is small, then a significant number of the electrons will be in the conduction band at normal temperatures and the material will be a semiconductor ü graphite ü the conductivity of a semiconductor increases with temperature If the band gap is large, then effectively no electrons will be in the conduction band at normal temperatures and the material will be an insulator Tro: Chemistry: A Molecular Approach, 2/e 139 Copyright 2011 Pearson Education, Inc.

Doping Semiconductors • Doping is adding impurities to the semiconductor’s crystal to increase its • • • conductivity Goal is to increase the number of electrons in the conduction band n-type semiconductors do not have enough electrons themselves to add to the conduction band, so they are doped by adding electron-rich impurities p-type semiconductors are doped with an electron-deficient impurity, resulting in electron “holes” in the valence band. Electrons can jump between these holes in the valence band, allowing conduction of electricity. Tro: Chemistry: A Molecular Approach, 2/e 140 Copyright 2011 Pearson Education, Inc.
 Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Democritus atomic model diagram
Democritus atomic model diagram Covalently bonded substances
Covalently bonded substances Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Ap chemistry molecular geometry
Ap chemistry molecular geometry Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Approach chemistry chalk chapter
Approach chemistry chalk chapter Virtual circuit vs datagram
Virtual circuit vs datagram Theoretical models of counseling
Theoretical models of counseling Waterfall and sprinkler strategy
Waterfall and sprinkler strategy Multiple approach-avoidance
Multiple approach-avoidance Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach What is a research
What is a research Traditional approach vs object oriented approach
Traditional approach vs object oriented approach Tony wagner's seven survival skills
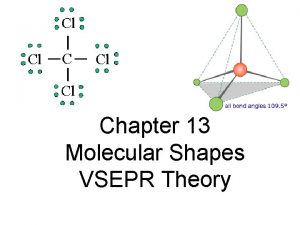
Tony wagner's seven survival skills Vsepr theory
Vsepr theory Molecular shapes
Molecular shapes Pf3 number of vsepr electron groups
Pf3 number of vsepr electron groups Molecular biology crash course
Molecular biology crash course Molecular level vs cellular level
Molecular level vs cellular level Molecular absorption
Molecular absorption Ab6 molecular geometry
Ab6 molecular geometry Ideal gas law examples
Ideal gas law examples Properties of network covalent solids
Properties of network covalent solids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids What is the significance of a chemical formula
What is the significance of a chemical formula Criterios de amsterdam cancer de colon
Criterios de amsterdam cancer de colon Molecular geometry chart
Molecular geometry chart 5 examples of palindromic dna sequences
5 examples of palindromic dna sequences Molecular rebar design
Molecular rebar design Properties of covalent molecular substances
Properties of covalent molecular substances Empirical formula of haemoglobin
Empirical formula of haemoglobin Number molecular weight
Number molecular weight Dicots
Dicots How to calculate empirical formula with percentages
How to calculate empirical formula with percentages Atomic mass of kmno4
Atomic mass of kmno4 Water percentage composition
Water percentage composition Empirical formula from percentages
Empirical formula from percentages Molecular modelling laboratory
Molecular modelling laboratory Molecular replacement method
Molecular replacement method Shortened structural formula
Shortened structural formula Naming and writing formulas for molecular compounds
Naming and writing formulas for molecular compounds Binary molecules
Binary molecules Molecular biophysics unit
Molecular biophysics unit Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Number-average molecular weight
Number-average molecular weight Cl4 lewis structure
Cl4 lewis structure Molecular geometry quiz
Molecular geometry quiz Molecular shapes and polarity
Molecular shapes and polarity Patterson function
Patterson function Molecular replacement method
Molecular replacement method Px py pz orbitals
Px py pz orbitals Heteronuclear diatomic molecules molecular orbital diagram
Heteronuclear diatomic molecules molecular orbital diagram Li2 molecular orbital diagram
Li2 molecular orbital diagram Standardised cml monitoring
Standardised cml monitoring What is an unshared pair of electrons
What is an unshared pair of electrons Molecular microbiology definition
Molecular microbiology definition Cmp shorthand
Cmp shorthand Is bent polar
Is bent polar Lewis structure for pf3
Lewis structure for pf3 Scl molecular geometry
Scl molecular geometry Molecular geometry and bonding theories
Molecular geometry and bonding theories What is a bond order in chemistry
What is a bond order in chemistry Molecular gastronomy restaurants
Molecular gastronomy restaurants Define molecular formula
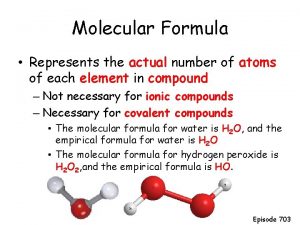
Define molecular formula Molecular pharming definition
Molecular pharming definition Concln
Concln How to convert moles to grams
How to convert moles to grams Is molarity and concentration the same
Is molarity and concentration the same Cintico
Cintico Alfredo clemente
Alfredo clemente Cohesion molecular del agua
Cohesion molecular del agua Molecular dynamics limitations
Molecular dynamics limitations Cyanate ion formula
Cyanate ion formula Bent lewis dot structure
Bent lewis dot structure Gluten molecular formula
Gluten molecular formula Kinetic molecular theory
Kinetic molecular theory Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory volume
Kinetic molecular theory volume Bond angle of nocl
Bond angle of nocl Introduction to molecular spectroscopy
Introduction to molecular spectroscopy Molecular replacement method
Molecular replacement method Covalent molecular and covalent network
Covalent molecular and covalent network Bio molecular systems ltd
Bio molecular systems ltd Square planer
Square planer 14-3 human molecular genetics
14-3 human molecular genetics Molecular gastronomy history
Molecular gastronomy history Lichefiaza
Lichefiaza Convergent evolution definition
Convergent evolution definition Molar mass table
Molar mass table Geometria molecular gangorra
Geometria molecular gangorra Polaridade das moléculas
Polaridade das moléculas Piramide trigonal
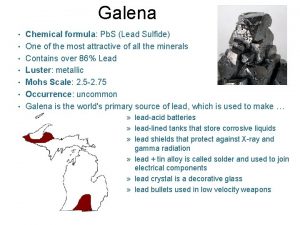
Piramide trigonal Chemical name of galena
Chemical name of galena Molecular mass
Molecular mass Formula mass vs molecular mass
Formula mass vs molecular mass Science
Science What is the percent of iodine in ointment
What is the percent of iodine in ointment Etilbenceno masa molar
Etilbenceno masa molar Silicon carbide covalent network
Silicon carbide covalent network Silicon carbide covalent network
Silicon carbide covalent network Eric savory
Eric savory Empirical formula of c5h12
Empirical formula of c5h12 Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Molecular formula from empirical formula
Molecular formula from empirical formula Determine the empirical formula
Determine the empirical formula Empirical formula poem
Empirical formula poem Empirical formula chemistry
Empirical formula chemistry Molecular formula example
Molecular formula example Cho molecular formula
Cho molecular formula Zone distillation
Zone distillation Electronegativity and dipole moment
Electronegativity and dipole moment Netmegs
Netmegs Methods for determining molecular weight of polymers
Methods for determining molecular weight of polymers Combustion products
Combustion products Nh4 + molecular geometry
Nh4 + molecular geometry Chemical formula vs molecular formula
Chemical formula vs molecular formula Electronegativity bond type chart
Electronegativity bond type chart Molecular vibration animation
Molecular vibration animation Cooh molecular geometry
Cooh molecular geometry Periodic table with molar masses
Periodic table with molar masses What electronegativity difference is polar
What electronegativity difference is polar Charles darwin
Charles darwin Electron domain geometry vs molecular geometry
Electron domain geometry vs molecular geometry Hbr electron geometry
Hbr electron geometry Ibuprofen percent composition
Ibuprofen percent composition Neutral theory of molecular evolution notes
Neutral theory of molecular evolution notes Hydrosulfuric acid
Hydrosulfuric acid Molecular substance
Molecular substance Hcn hybridization diagram
Hcn hybridization diagram Co2 relative molecular mass
Co2 relative molecular mass Sp3d2 bond angle
Sp3d2 bond angle Chapter 16 the molecular basis of inheritance
Chapter 16 the molecular basis of inheritance Structure of polymers
Structure of polymers 13 molecular shapes
13 molecular shapes Chapter 12 section 1: dna: the genetic material
Chapter 12 section 1: dna: the genetic material Chapter 12 section 1 molecular genetics answer key
Chapter 12 section 1 molecular genetics answer key