Chemistry A Molecular Approach 2 nd Ed Nivaldo














- Slides: 84

Chemistry: A Molecular Approach, 2 nd Ed. Nivaldo Tro Chapter 17 Free Energy and Thermodynamics Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA Copyright 2011 Pearson Education, Inc.

First Law of Thermodynamics • You can’t win! • First Law of Thermodynamics: Energy cannot be created or destroyed ü the total energy of the universe cannot change ü though you can transfer it from one place to another · DEuniverse = 0 = DEsystem + DEsurroundings Tro: Chemistry: A Molecular Approach, 2/e 2 Copyright 2011 Pearson Education, Inc.

First Law of Thermodynamics • Conservation of Energy • For an exothermic reaction, “lost” heat from the • system goes into the surroundings Two ways energy is “lost” from a system ü converted to heat, q ü used to do work, w • Energy conservation requires that the energy change in the system equal the heat released + work done ü DE = q + w ü DE = DH + PDV • DE is a state function ü internal energy change independent of how done Tro: Chemistry: A Molecular Approach, 2/e 3 Copyright 2011 Pearson Education, Inc.

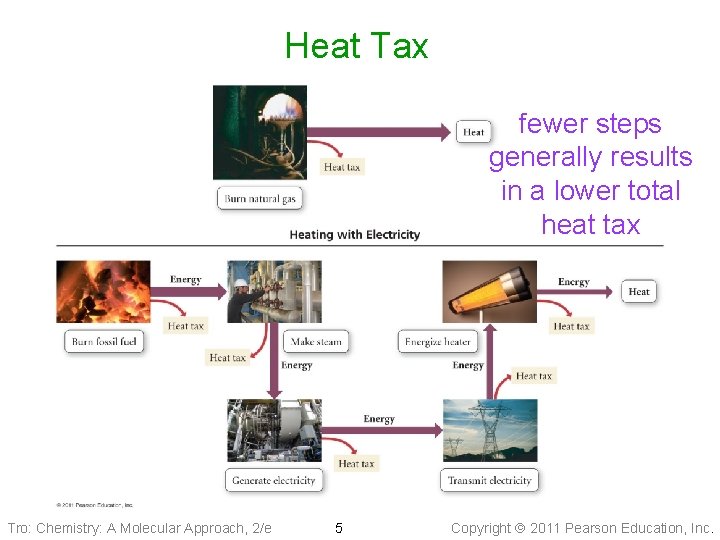
The Energy Tax • You can’t break even! • To recharge a battery with 100 k. J of useful energy will require more than 100 k. J ü because of the Second Law of Thermodynamics • Every energy transition results in a “loss” of energy ü an “Energy Tax” demanded by nature ü and conversion of energy to heat which is “lost” by heating up the surroundings Tro: Chemistry: A Molecular Approach, 2/e 4 Copyright 2011 Pearson Education, Inc.

Heat Tax fewer steps generally results in a lower total heat tax Tro: Chemistry: A Molecular Approach, 2/e 5 Copyright 2011 Pearson Education, Inc.

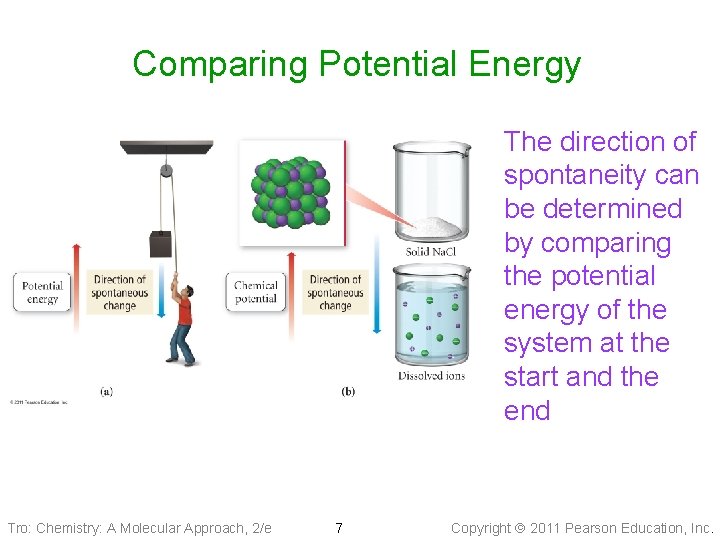
Thermodynamics and Spontaneity • Thermodynamics predicts whether a process will occur under the given conditions ü processes that will occur are called spontaneous Ønonspontaneous processes require energy input to go • Spontaneity is determined by comparing the chemical potential energy of the system before the reaction with the free energy of the system after the reaction ü if the system after reaction has less potential energy than before the reaction, the reaction is thermodynamically favorable. • Spontaneity ≠ fast or slow Tro: Chemistry: A Molecular Approach, 2/e 6 Copyright 2011 Pearson Education, Inc.

Comparing Potential Energy The direction of spontaneity can be determined by comparing the potential energy of the system at the start and the end Tro: Chemistry: A Molecular Approach, 2/e 7 Copyright 2011 Pearson Education, Inc.

Reversibility of Process • Any spontaneous process is irreversible because there is a net release of energy when it proceeds in that direction ü it will proceed in only one direction • A reversible process will proceed back and forth between the two end conditions ü any reversible process is at equilibrium ü results in no change in free energy • If a process is spontaneous in one direction, it must be nonspontaneous in the opposite direction Tro: Chemistry: A Molecular Approach, 2/e 8 Copyright 2011 Pearson Education, Inc.

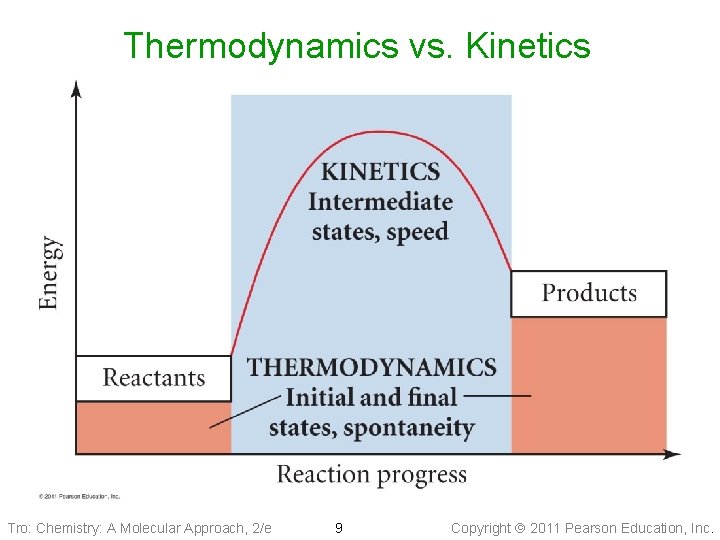
Thermodynamics vs. Kinetics Tro: Chemistry: A Molecular Approach, 2/e 9 Copyright 2011 Pearson Education, Inc.

Diamond → Graphite is more stable than diamond, so the conversion of diamond into graphite is spontaneous – but don’t worry, it’s so slow that your ring won’t turn into pencil lead in your lifetime (or through many of your generations) Tro: Chemistry: A Molecular Approach, 2/e 10 Copyright 2011 Pearson Education, Inc.

Spontaneous Processes • Spontaneous processes occur because they • release energy from the system Most spontaneous processes proceed from a system of higher potential energy to a system at lower potential energy ü exothermic • But there are some spontaneous processes that proceed from a system of lower potential energy to a system at higher potential energy ü endothermic • How can something absorb potential energy, yet have a net release of energy? Tro: Chemistry: A Molecular Approach, 2/e 11 Copyright 2011 Pearson Education, Inc.

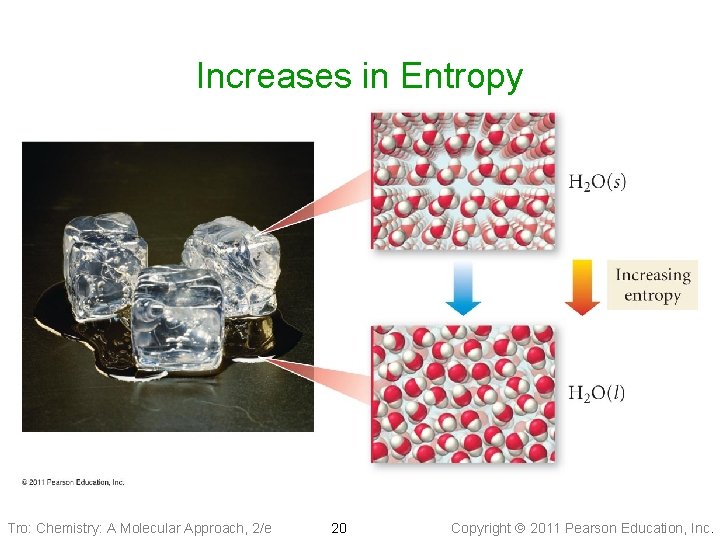
Melting Ice Tro: Chemistry: A Molecular Approach, 2/e 12 Melting is an When a solid process, melts, the Endothermic particles have more yet ice will freedom of movement. spontaneously melt above 0 °C. More freedom of motion increases the randomness of the system. When systems become more random, energy is released. We call this energy, entropy Copyright 2011 Pearson Education, Inc.

Factors Affecting Whether a Reaction Is Spontaneous • There are two factors that determine whether a • • reaction is spontaneous. They are the enthalpy change and the entropy change of the system The enthalpy change, DH, is the difference in the sum of the internal energy and PV work energy of the reactants to the products The entropy change, DS, is the difference in randomness of the reactants compared to the products Tro: Chemistry: A Molecular Approach, 2/e 13 Copyright 2011 Pearson Education, Inc.

Enthalpy Change • DH generally measured in k. J/mol • Stronger bonds = more stable molecules • A reaction is generally exothermic if the bonds in the products are stronger than the bonds in the reactants ü exothermic = energy released, DH is negative • A reaction is generally endothermic if the bonds in the products are weaker than the bonds in the reactants ü endothermic = energy absorbed, DH is positive • The enthalpy change is favorable for exothermic reactions and unfavorable for endothermic reactions Tro: Chemistry: A Molecular Approach, 2/e 14 Copyright 2011 Pearson Education, Inc.

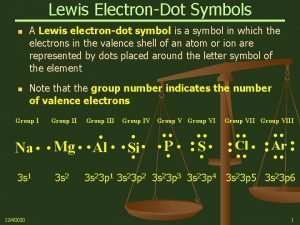
Entropy • Entropy is a thermodynamic function that increases as the number of energetically equivalent ways of arranging the components increases, S ü S generally J/mol • S = k ln W ü k = Boltzmann Constant = 1. 38 x 10− 23 J/K ü W is the number of energetically equivalent ways a system can exist Ø unitless • Random systems require less energy than ordered systems Tro: Chemistry: A Molecular Approach, 2/e 15 Copyright 2011 Pearson Education, Inc.

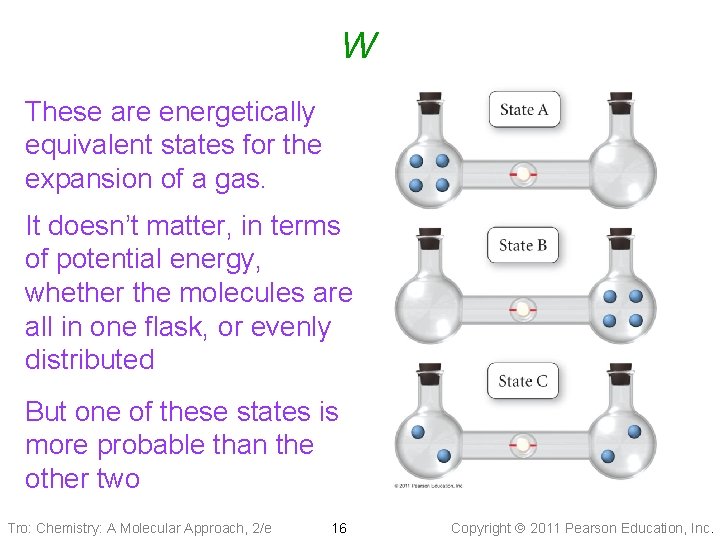
W These are energetically equivalent states for the expansion of a gas. It doesn’t matter, in terms of potential energy, whether the molecules are all in one flask, or evenly distributed But one of these states is more probable than the other two Tro: Chemistry: A Molecular Approach, 2/e 16 Copyright 2011 Pearson Education, Inc.

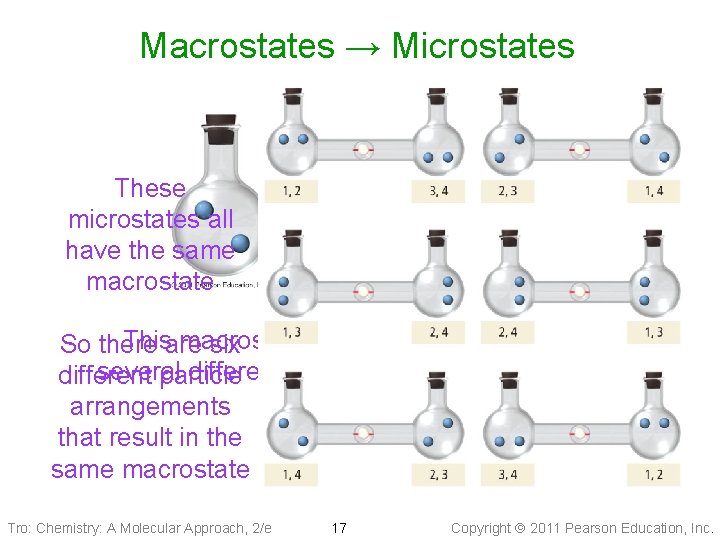
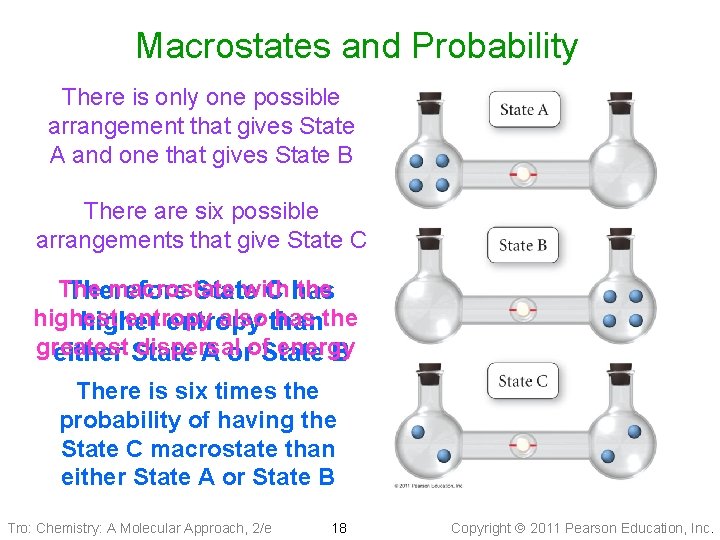
Macrostates → Microstates These microstates all have the same macrostate Thisare macrostate can be achieved through So there six several different arrangements of the particles different particle arrangements that result in the same macrostate Tro: Chemistry: A Molecular Approach, 2/e 17 Copyright 2011 Pearson Education, Inc.

Macrostates and Probability There is only one possible arrangement that gives State A and one that gives State B There are six possible arrangements that give State C The macrostate with the Therefore State C has highest entropy also than has the higher entropy greatest dispersal energy either State A orof. State B There is six times the probability of having the State C macrostate than either State A or State B Tro: Chemistry: A Molecular Approach, 2/e 18 Copyright 2011 Pearson Education, Inc.

Changes in Entropy, DS • DS = Sfinal − Sinitial • Entropy change is favorable when the result is a more random system ü DS is positive • Some changes that increase the entropy are ü reactions whose products are in a more random state Øsolid more ordered than liquid more ordered than gas ü reactions that have larger numbers of product molecules than reactant molecules ü increase in temperature ü solids dissociating into ions upon dissolving Tro: Chemistry: A Molecular Approach, 2/e 19 Copyright 2011 Pearson Education, Inc.

Increases in Entropy Tro: Chemistry: A Molecular Approach, 2/e 20 Copyright 2011 Pearson Education, Inc.

DS • For a process where the final condition is more random than the initial condition, DSsystem is positive and the entropy change is favorable for the process to be spontaneous • For a process where the final condition is more orderly than the initial condition, DSsystem is negative and the entropy change is unfavorable for the process to be spontaneous · DSsystem = DSreaction = Sn(S°products) − Sn(S°reactants) Tro: Chemistry: A Molecular Approach, 2/e 21 Copyright 2011 Pearson Education, Inc.

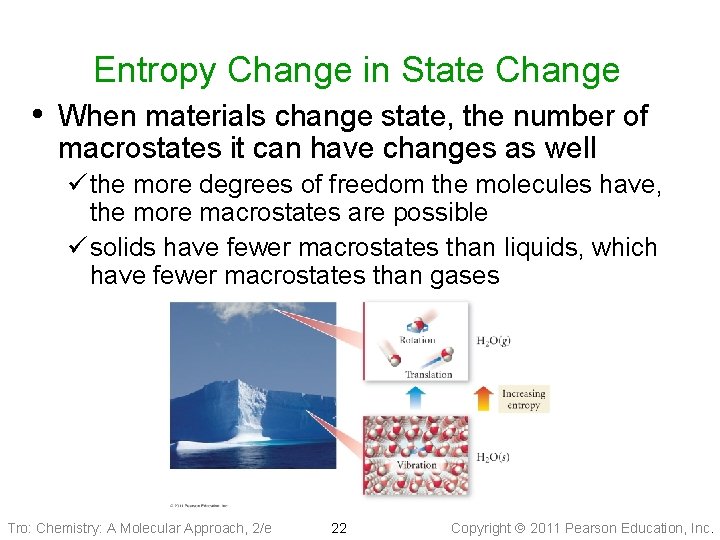
Entropy Change in State Change • When materials change state, the number of macrostates it can have changes as well ü the more degrees of freedom the molecules have, the more macrostates are possible ü solids have fewer macrostates than liquids, which have fewer macrostates than gases Tro: Chemistry: A Molecular Approach, 2/e 22 Copyright 2011 Pearson Education, Inc.

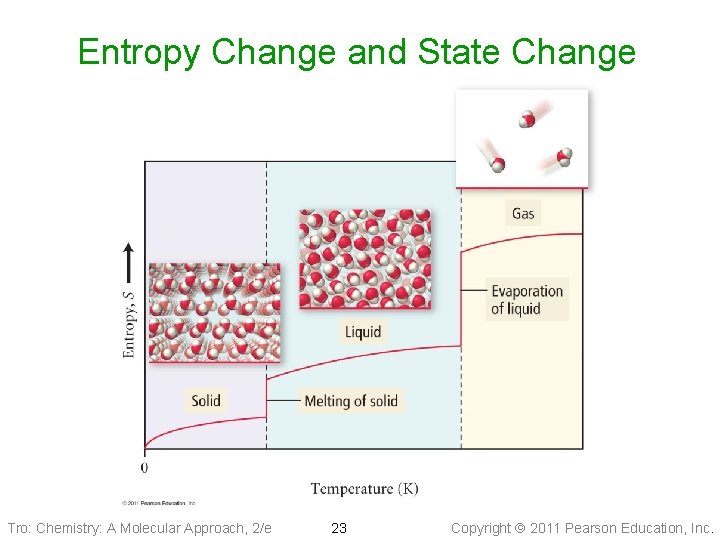
Entropy Change and State Change Tro: Chemistry: A Molecular Approach, 2/e 23 Copyright 2011 Pearson Education, Inc.

Practice – Predict whether DSsystem is + or − for each of the following • A hot beaker burning your fingers DS is + • Water vapor condensing DS is − • Separation of oil and vinegar salad dressing DS is − DS is + • Dissolving sugar in tea • 2 Pb. O 2(s) 2 Pb. O(s) + O 2(g) DS is + • 2 NH 3(g) N 2(g) + 3 H 2(g) DS is + • Ag+(aq) + Cl−(aq) Ag. Cl(s) DS is − Tro: Chemistry: A Molecular Approach, 2/e 24 Copyright 2011 Pearson Education, Inc.

The 2 nd Law of Thermodynamics • The 2 nd Law of Thermodynamics says that the total entropy change of the universe must be positive for a process to be spontaneous ü for reversible process DSuniv = 0 ü for irreversible (spontaneous) process DSuniv > 0 • DSuniverse = DSsystem + DSsurroundings • If the entropy of the system decreases, then the entropy of the surroundings must increase by a larger amount ü when DSsystem is negative, DSsurroundings must be positive and big for a spontaneous process Tro: Chemistry: A Molecular Approach, 2/e 25 Copyright 2011 Pearson Education, Inc.

Heat Flow, Entropy, and the 2 nd Law When ice is placed in water, heat flows from the water into the ice According to the 2 nd Law, heat must flow from water to ice because it results in more dispersal of heat. The entropy of the universe increases. Tro: Chemistry: A Molecular Approach, 2/e 26 Copyright 2011 Pearson Education, Inc.

Heat Transfer and Changes in Entropy of the Surroundings • The 2 nd Law demands that the entropy of the • • • universe increase for a spontaneous process Yet processes like water vapor condensing are spontaneous, even though the water vapor is more random than the liquid water If a process is spontaneous, yet the entropy change of the process is unfavorable, there must have been a large increase in the entropy of the surroundings The entropy increase must come from heat released by the system – the process must be exothermic! Tro: Chemistry: A Molecular Approach, 2/e 27 Copyright 2011 Pearson Education, Inc.

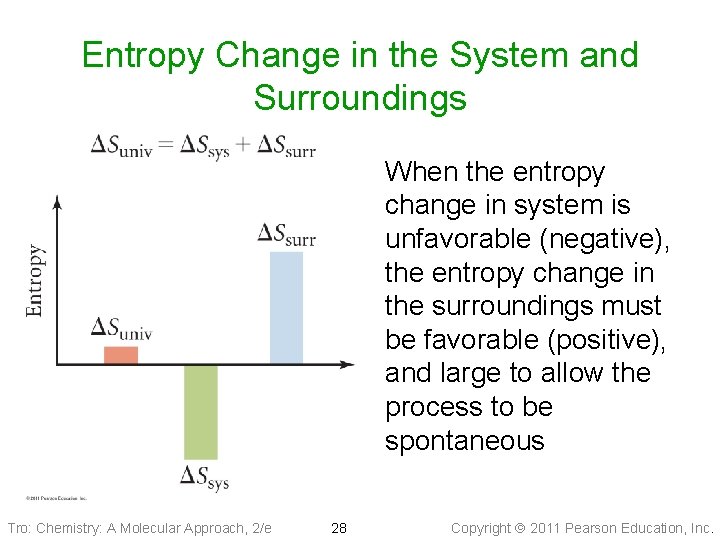
Entropy Change in the System and Surroundings When the entropy change in system is unfavorable (negative), the entropy change in the surroundings must be favorable (positive), and large to allow the process to be spontaneous Tro: Chemistry: A Molecular Approach, 2/e 28 Copyright 2011 Pearson Education, Inc.

Heat Exchange and DSsurroundings • When a system process is exothermic, it adds heat to • • the surroundings, increasing the entropy of the surroundings When a system process is endothermic, it takes heat from the surroundings, decreasing the entropy of the surroundings The amount the entropy of the surroundings changes depends on its original temperature ü the higher the original temperature, the less effect addition or removal of heat has Tro: Chemistry: A Molecular Approach, 2/e 29 Copyright 2011 Pearson Education, Inc.

Temperature Dependence of DSsurroundings • When heat is added to surroundings that are • cool it has more of an effect on the entropy than it would have if the surroundings were already hot Water freezes spontaneously below 0 °C because the heat released on freezing increases the entropy of the surroundings enough to make DS positive ü above 0 °C the increase in entropy of the surroundings is insufficient to make DS positive Tro: Chemistry: A Molecular Approach, 2/e 30 Copyright 2011 Pearson Education, Inc.

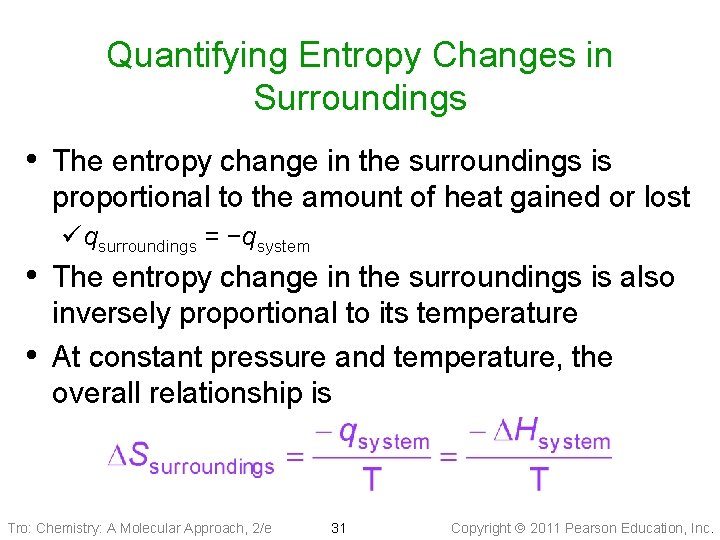
Quantifying Entropy Changes in Surroundings • The entropy change in the surroundings is proportional to the amount of heat gained or lost ü qsurroundings = −qsystem • The entropy change in the surroundings is also • inversely proportional to its temperature At constant pressure and temperature, the overall relationship is Tro: Chemistry: A Molecular Approach, 2/e 31 Copyright 2011 Pearson Education, Inc.

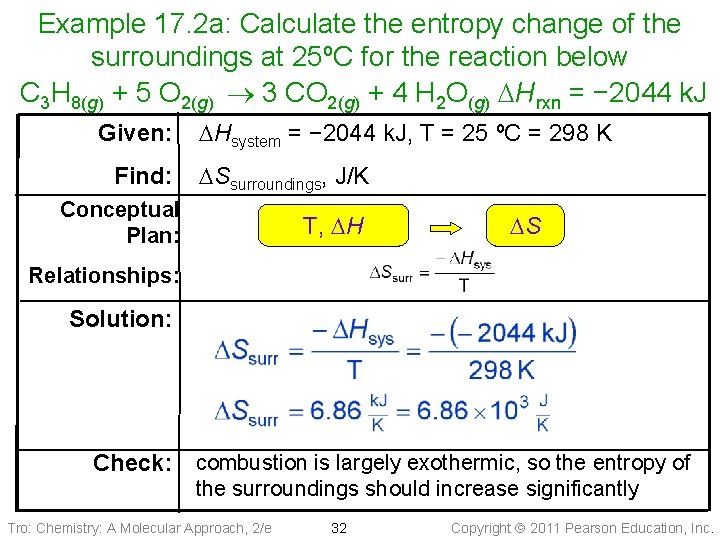
Example 17. 2 a: Calculate the entropy change of the surroundings at 25ºC for the reaction below C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) DHrxn = − 2044 k. J Given: Find: DHsystem = − 2044 k. J, T = 25 ºC = 298 K DSsurroundings, J/K Conceptual Plan: T, DH DS Relationships: Solution: Check: combustion is largely exothermic, so the entropy of the surroundings should increase significantly Tro: Chemistry: A Molecular Approach, 2/e 32 Copyright 2011 Pearson Education, Inc.

Practice – The reaction below has DHrxn = +66. 4 k. J at 25 °C. (a) Determine the Dssurroundings, (b) the sign of DSsystem, and (c) whether the process is spontaneous 2 O 2(g) + N 2(g) 2 NO 2(g) Tro: Chemistry: A Molecular Approach, 2/e 33 Copyright 2011 Pearson Education, Inc.

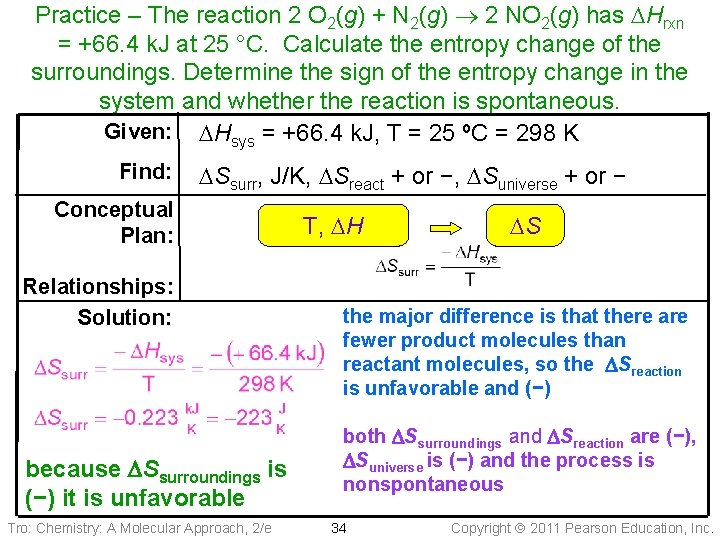
Practice – The reaction 2 O 2(g) + N 2(g) 2 NO 2(g) has DHrxn = +66. 4 k. J at 25 °C. Calculate the entropy change of the surroundings. Determine the sign of the entropy change in the system and whether the reaction is spontaneous. Given: DHsys = +66. 4 k. J, T = 25 ºC = 298 K Find: DSsurr, J/K, DSreact + or −, DSuniverse + or − Conceptual Plan: Relationships: Solution: because DSsurroundings is (−) it is unfavorable Tro: Chemistry: A Molecular Approach, 2/e T, DH DS the major difference is that there are fewer product molecules than reactant molecules, so the DSreaction is unfavorable and (−) both DSsurroundings and DSreaction are (−), DSuniverse is (−) and the process is nonspontaneous 34 Copyright 2011 Pearson Education, Inc.

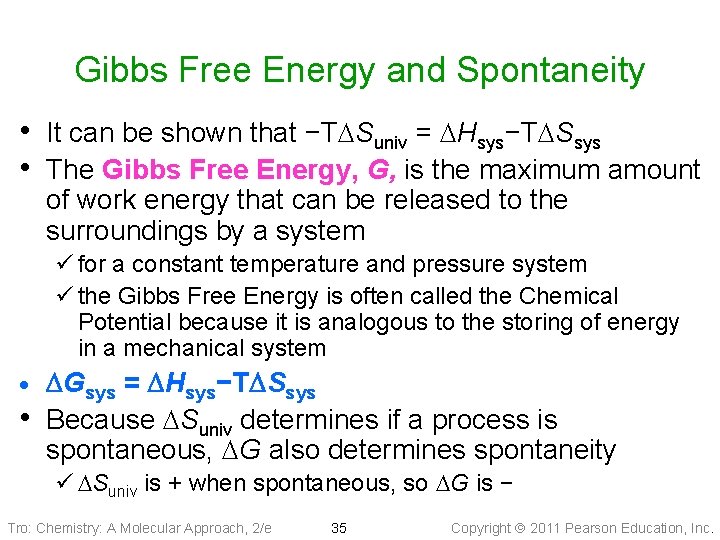
Gibbs Free Energy and Spontaneity • It can be shown that −TDSuniv = DHsys−TDSsys • The Gibbs Free Energy, G, is the maximum amount of work energy that can be released to the surroundings by a system ü for a constant temperature and pressure system ü the Gibbs Free Energy is often called the Chemical Potential because it is analogous to the storing of energy in a mechanical system · • DGsys = DHsys−TDSsys Because DSuniv determines if a process is spontaneous, DG also determines spontaneity ü DSuniv is + when spontaneous, so DG is − Tro: Chemistry: A Molecular Approach, 2/e 35 Copyright 2011 Pearson Education, Inc.

Gibbs Free Energy, DG • A process will be spontaneous when DG is negative · DG will be negative when ü DH is negative and DS is positive Øexothermic and more random ü DH is negative and large and DS is negative but small ü DH is positive but small and DS is positive and large Øor high temperature • DG will be positive when DH is + and DS is − ü never spontaneous at any temperature • When DG = 0 the reaction is at equilibrium Tro: Chemistry: A Molecular Approach, 2/e 36 Copyright 2011 Pearson Education, Inc.

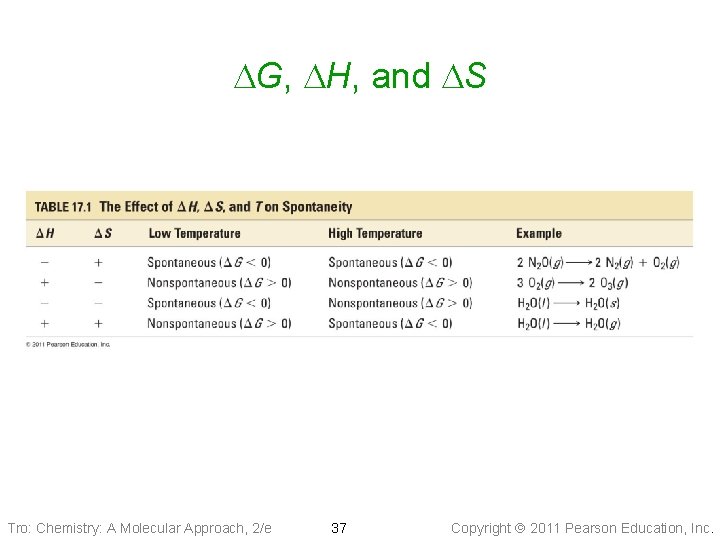
DG, DH, and DS Tro: Chemistry: A Molecular Approach, 2/e 37 Copyright 2011 Pearson Education, Inc.

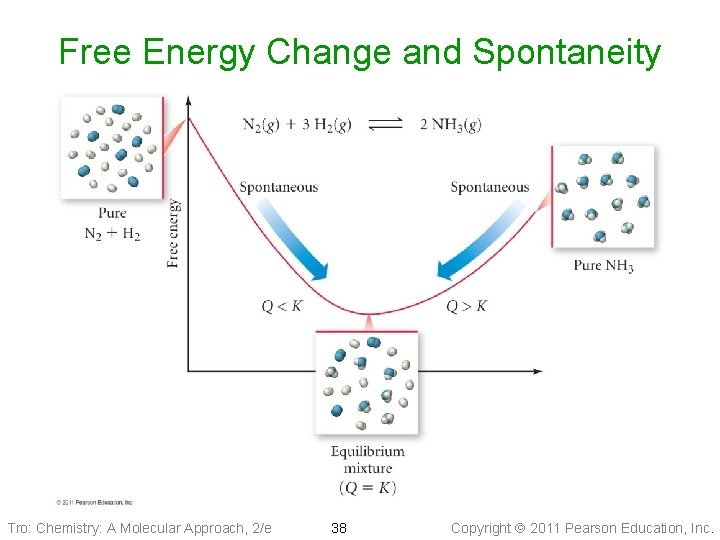
Free Energy Change and Spontaneity Tro: Chemistry: A Molecular Approach, 2/e 38 Copyright 2011 Pearson Education, Inc.

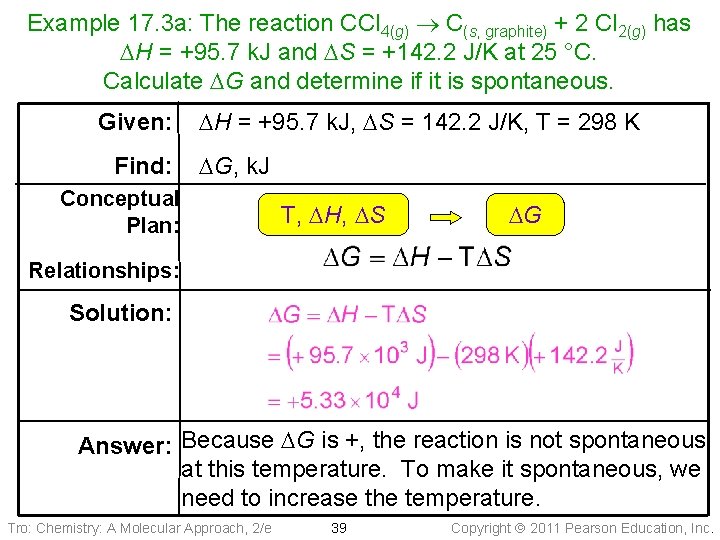
Example 17. 3 a: The reaction CCl 4(g) C(s, graphite) + 2 Cl 2(g) has DH = +95. 7 k. J and DS = +142. 2 J/K at 25 °C. Calculate DG and determine if it is spontaneous. Given: Find: DH = +95. 7 k. J, DS = 142. 2 J/K, T = 298 K DG, k. J Conceptual Plan: T, DH, DS DG Relationships: Solution: Answer: Because DG is +, the reaction is not spontaneous at this temperature. To make it spontaneous, we need to increase the temperature. Tro: Chemistry: A Molecular Approach, 2/e 39 Copyright 2011 Pearson Education, Inc.

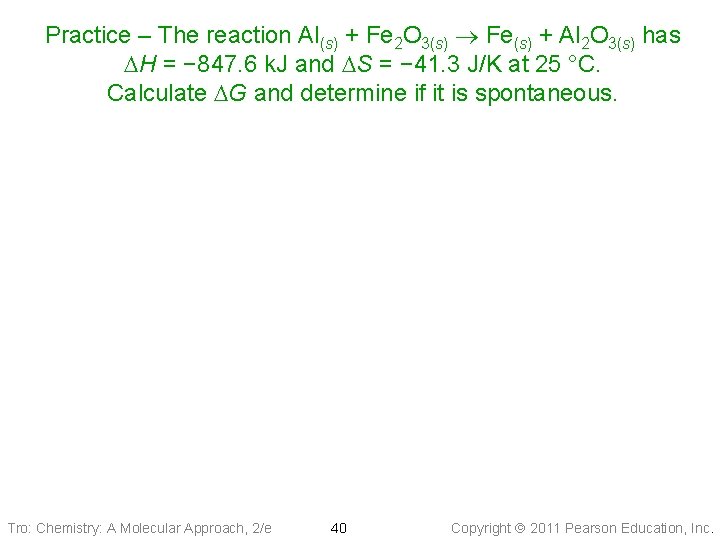
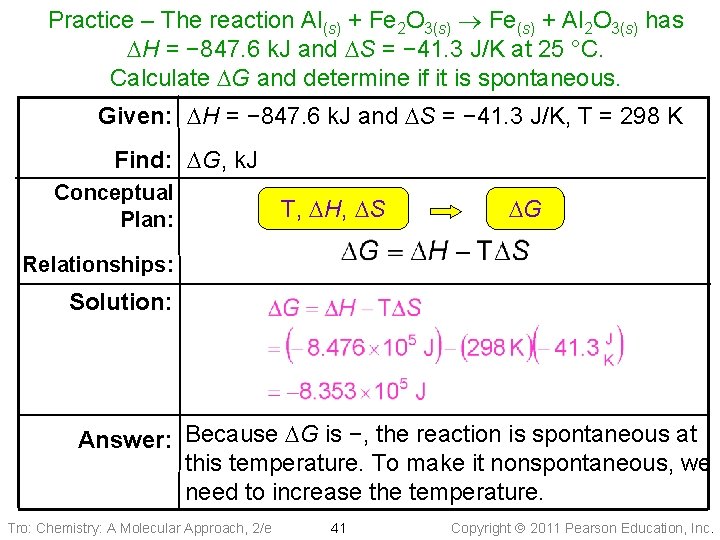
Practice – The reaction Al(s) + Fe 2 O 3(s) Fe(s) + Al 2 O 3(s) has DH = − 847. 6 k. J and DS = − 41. 3 J/K at 25 °C. Calculate DG and determine if it is spontaneous. Tro: Chemistry: A Molecular Approach, 2/e 40 Copyright 2011 Pearson Education, Inc.

Practice – The reaction Al(s) + Fe 2 O 3(s) Fe(s) + Al 2 O 3(s) has DH = − 847. 6 k. J and DS = − 41. 3 J/K at 25 °C. Calculate DG and determine if it is spontaneous. Given: DH = − 847. 6 k. J and DS = − 41. 3 J/K, T = 298 K Find: DG, k. J Conceptual Plan: T, DH, DS DG Relationships: Solution: Answer: Because DG is −, the reaction is spontaneous at this temperature. To make it nonspontaneous, we need to increase the temperature. Tro: Chemistry: A Molecular Approach, 2/e 41 Copyright 2011 Pearson Education, Inc.

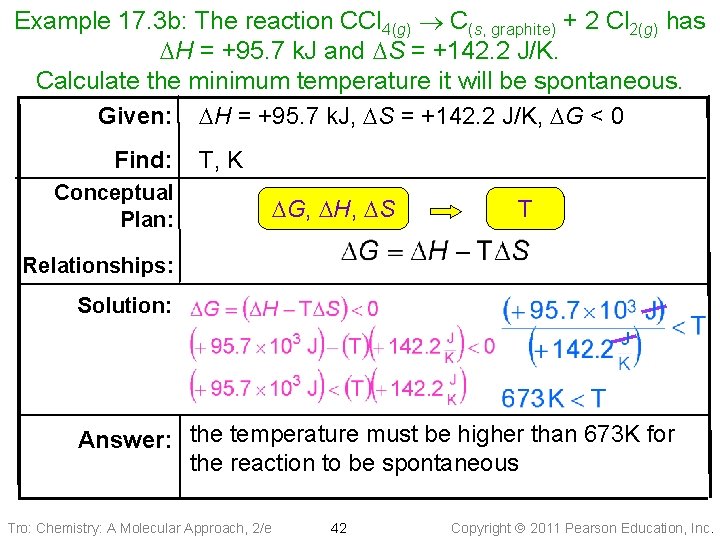
Example 17. 3 b: The reaction CCl 4(g) C(s, graphite) + 2 Cl 2(g) has DH = +95. 7 k. J and DS = +142. 2 J/K. Calculate the minimum temperature it will be spontaneous. Given: DH = +95. 7 k. J, DS = +142. 2 J/K, DG < 0 Find: T, K Conceptual Plan: DG, DH, DS T Relationships: Solution: Answer: the temperature must be higher than 673 K for the reaction to be spontaneous Tro: Chemistry: A Molecular Approach, 2/e 42 Copyright 2011 Pearson Education, Inc.

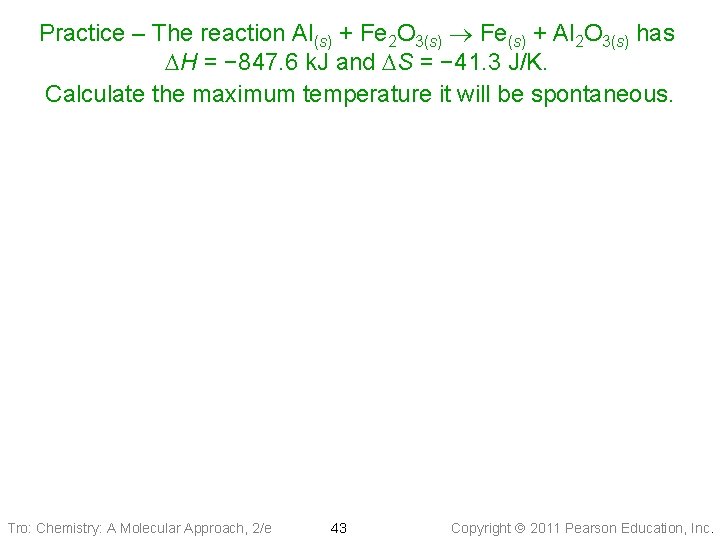
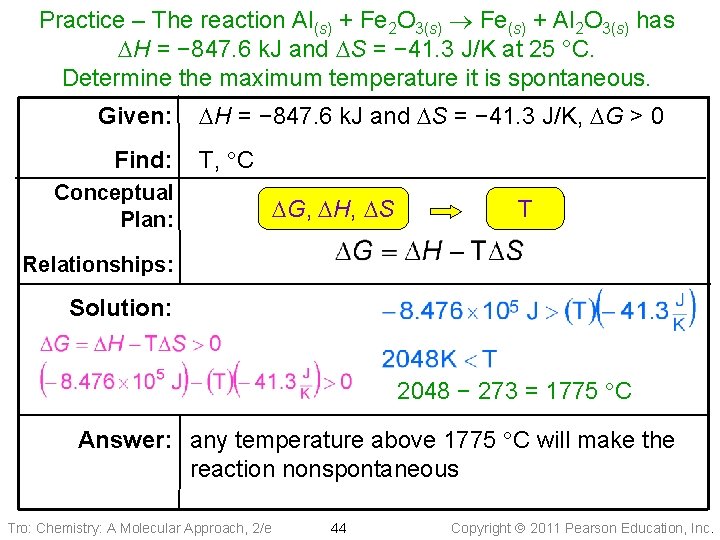
Practice – The reaction Al(s) + Fe 2 O 3(s) Fe(s) + Al 2 O 3(s) has DH = − 847. 6 k. J and DS = − 41. 3 J/K. Calculate the maximum temperature it will be spontaneous. Tro: Chemistry: A Molecular Approach, 2/e 43 Copyright 2011 Pearson Education, Inc.

Practice – The reaction Al(s) + Fe 2 O 3(s) Fe(s) + Al 2 O 3(s) has DH = − 847. 6 k. J and DS = − 41. 3 J/K at 25 °C. Determine the maximum temperature it is spontaneous. Given: Find: DH = − 847. 6 k. J and DS = − 41. 3 J/K, DG > 0 T, C Conceptual Plan: DG, DH, DS T Relationships: Solution: 2048 − 273 = 1775 C Answer: any temperature above 1775 C will make the reaction nonspontaneous Tro: Chemistry: A Molecular Approach, 2/e 44 Copyright 2011 Pearson Education, Inc.

Standard Conditions • The standard state is the state of a • • material at a defined set of conditions Gas = pure gas at exactly 1 atm pressure Solid or Liquid = pure solid or liquid in its most stable form at exactly 1 atm pressure and temperature of interest üusually 25 °C • Solution = substance in a solution with concentration 1 M Tro: Chemistry: A Molecular Approach, 2/e 45 Copyright 2011 Pearson Education, Inc.

The 3 rd Law of Thermodynamics: Absolute Entropy • The absolute entropy of a • substance is the amount of energy it has due to dispersion of energy through its particles The 3 rd Law states that for a perfect crystal at absolute zero, the absolute entropy = 0 J/mol∙K ü therefore, every substance that is not a perfect crystal at absolute zero has some energy from entropy ü therefore, the absolute entropy of substances is always + Tro: Chemistry: A Molecular Approach, 2/e 46 Copyright 2011 Pearson Education, Inc.

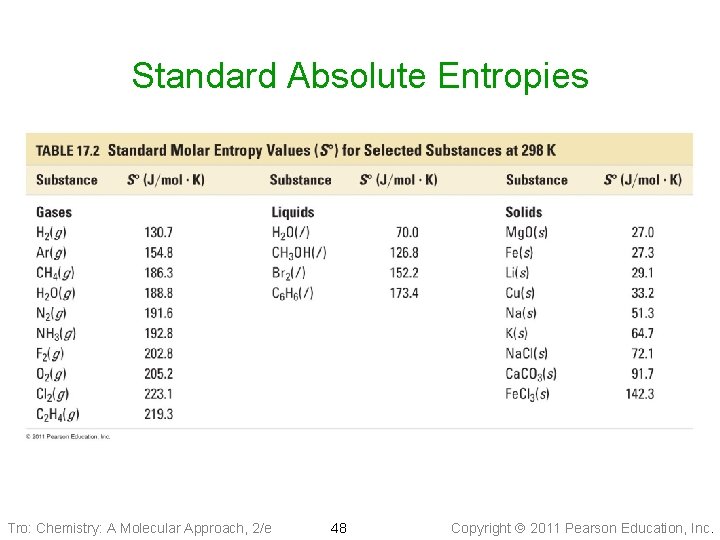
Standard Absolute Entropies • S° • Extensive • Entropies for 1 mole of a substance at 298 K for a particular state, a particular allotrope, particular molecular complexity, a particular molar mass, and a particular degree of dissolution Tro: Chemistry: A Molecular Approach, 2/e 47 Copyright 2011 Pearson Education, Inc.

Standard Absolute Entropies Tro: Chemistry: A Molecular Approach, 2/e 48 Copyright 2011 Pearson Education, Inc.

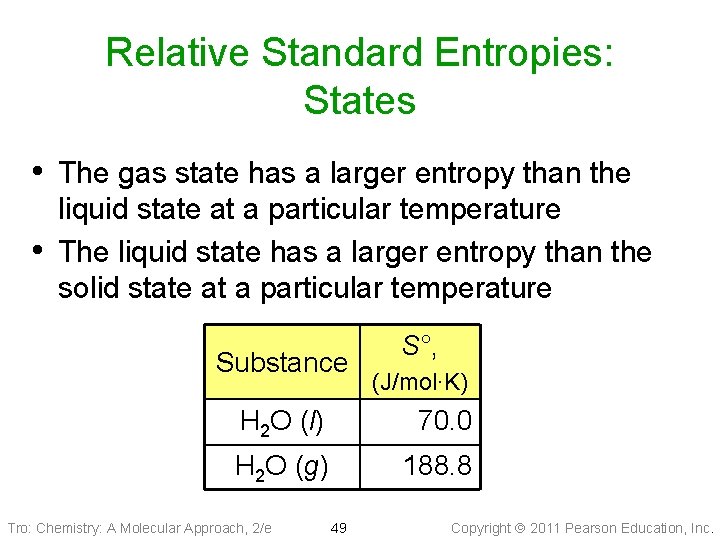
Relative Standard Entropies: States • The gas state has a larger entropy than the • liquid state at a particular temperature The liquid state has a larger entropy than the solid state at a particular temperature Substance S°, (J/mol∙K) H 2 O (l) 70. 0 H 2 O (g) 188. 8 Tro: Chemistry: A Molecular Approach, 2/e 49 Copyright 2011 Pearson Education, Inc.

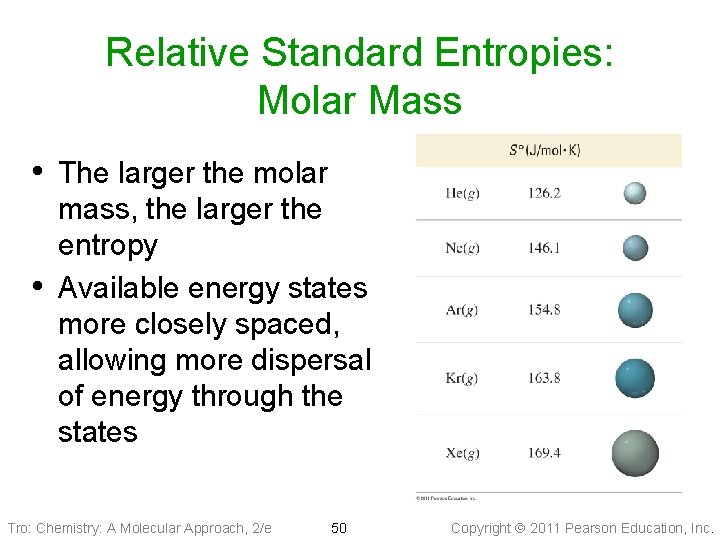
Relative Standard Entropies: Molar Mass • The larger the molar • mass, the larger the entropy Available energy states more closely spaced, allowing more dispersal of energy through the states Tro: Chemistry: A Molecular Approach, 2/e 50 Copyright 2011 Pearson Education, Inc.

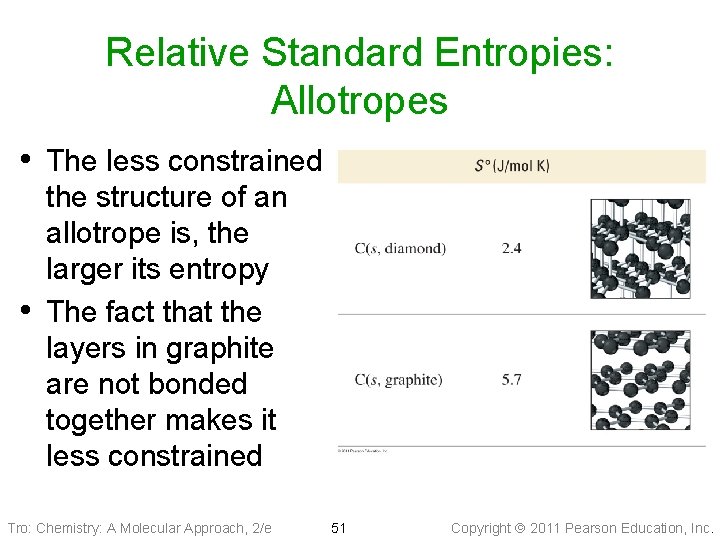
Relative Standard Entropies: Allotropes • The less constrained • the structure of an allotrope is, the larger its entropy The fact that the layers in graphite are not bonded together makes it less constrained Tro: Chemistry: A Molecular Approach, 2/e 51 Copyright 2011 Pearson Education, Inc.

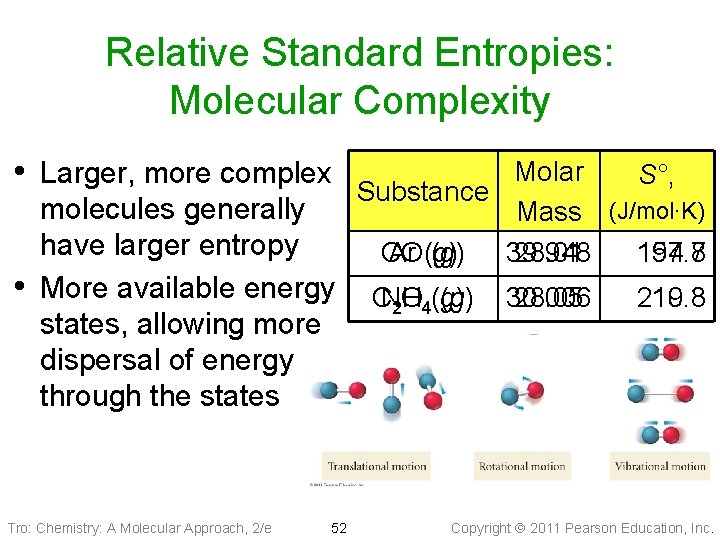
Relative Standard Entropies: Molecular Complexity • Larger, more complex • molecules generally have larger entropy More available energy states, allowing more dispersal of energy through the states Tro: Chemistry: A Molecular Approach, 2/e 52 Molar S°, Substance Mass (J/mol∙K) CO Ar (g) 39. 948 28. 01 154. 8 197. 7 CNO (g) 2 H 4(g) 30. 006 28. 05 210. 8 219. 3 Copyright 2011 Pearson Education, Inc.

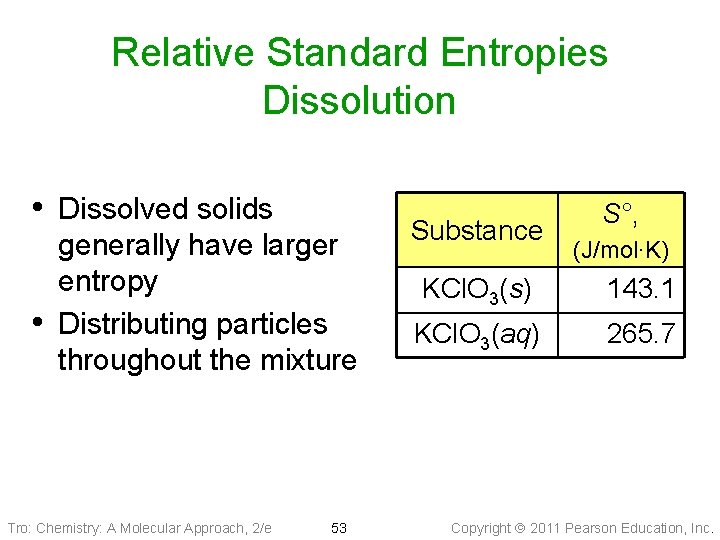
Relative Standard Entropies Dissolution • Dissolved solids • generally have larger entropy Distributing particles throughout the mixture Tro: Chemistry: A Molecular Approach, 2/e 53 Substance S°, (J/mol∙K) KCl. O 3(s) 143. 1 KCl. O 3(aq) 265. 7 Copyright 2011 Pearson Education, Inc.

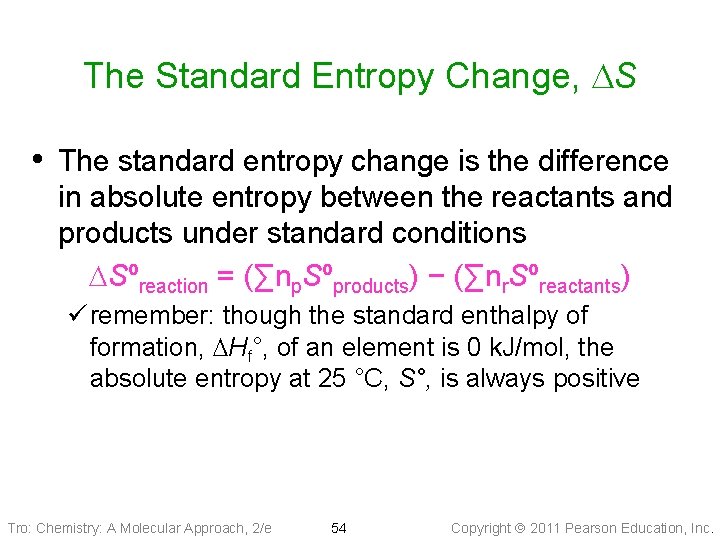
The Standard Entropy Change, DS • The standard entropy change is the difference in absolute entropy between the reactants and products under standard conditions DSºreaction = (∑np. Sºproducts) − (∑nr. Sºreactants) ü remember: though the standard enthalpy of formation, DHf°, of an element is 0 k. J/mol, the absolute entropy at 25 °C, S°, is always positive Tro: Chemistry: A Molecular Approach, 2/e 54 Copyright 2011 Pearson Education, Inc.

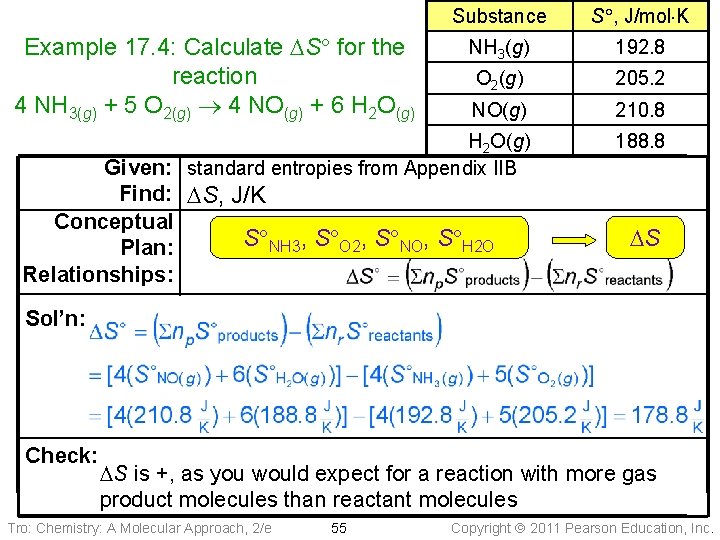
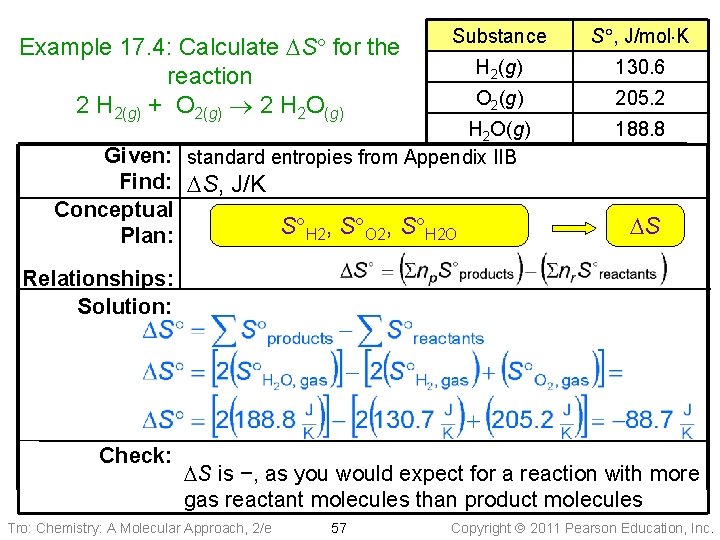
Substance S , J/mol K NH 3(g) 192. 8 O 2(g) 205. 2 NO(g) 210. 8 H 2 O(g) Given: standard entropies from Appendix IIB 188. 8 Example 17. 4: Calculate DS for the reaction 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g) Find: DS, J/K Conceptual S NH 3, S O 2, S NO, S H 2 O Plan: Relationships: DS Sol’n: Check: DS is +, as you would expect for a reaction with more gas product molecules than reactant molecules Tro: Chemistry: A Molecular Approach, 2/e 55 Copyright 2011 Pearson Education, Inc.

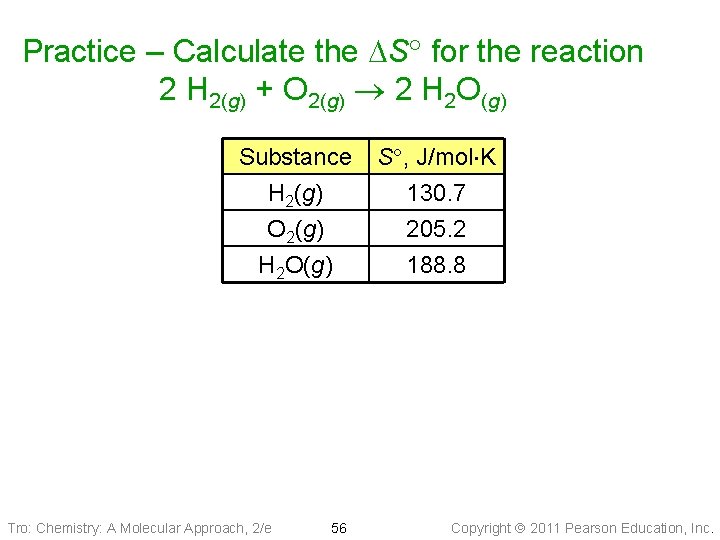
Practice – Calculate the DS for the reaction 2 H 2(g) + O 2(g) 2 H 2 O(g) Substance H 2(g) O 2(g) H 2 O(g) Tro: Chemistry: A Molecular Approach, 2/e 56 S , J/mol K 130. 7 205. 2 188. 8 Copyright 2011 Pearson Education, Inc.

Example 17. 4: Calculate DS for the reaction 2 H 2(g) + O 2(g) 2 H 2 O(g) Substance S , J/mol K H 2(g) 130. 6 O 2(g) 205. 2 H 2 O(g) Given: standard entropies from Appendix IIB Find: DS, J/K Conceptual S H 2, S O 2, S H 2 O Plan: 188. 8 DS Relationships: Solution: Check: DS is −, as you would expect for a reaction with more gas reactant molecules than product molecules Tro: Chemistry: A Molecular Approach, 2/e 57 Copyright 2011 Pearson Education, Inc.

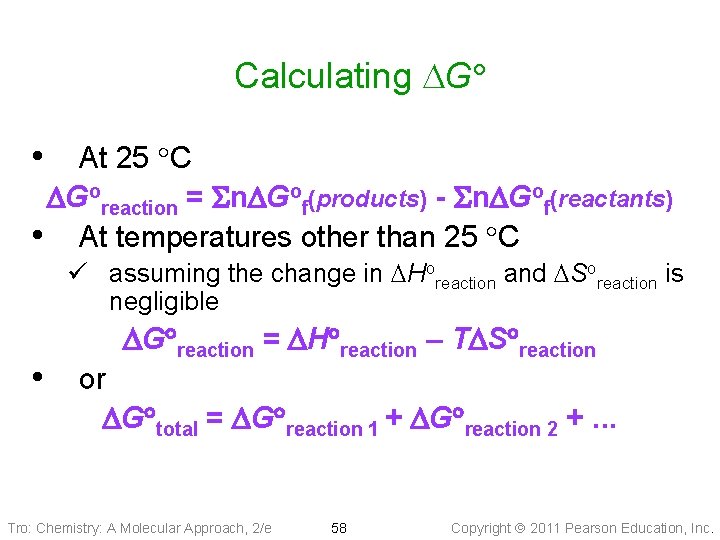
Calculating DG • At 25 C DGoreaction = Sn. DGof(products) - Sn. DGof(reactants) • At temperatures other than 25 C ü assuming the change in DHoreaction and DSoreaction is negligible • or DG reaction = DH reaction – TDS reaction DG total = DG reaction 1 + DG reaction 2 +. . . Tro: Chemistry: A Molecular Approach, 2/e 58 Copyright 2011 Pearson Education, Inc.

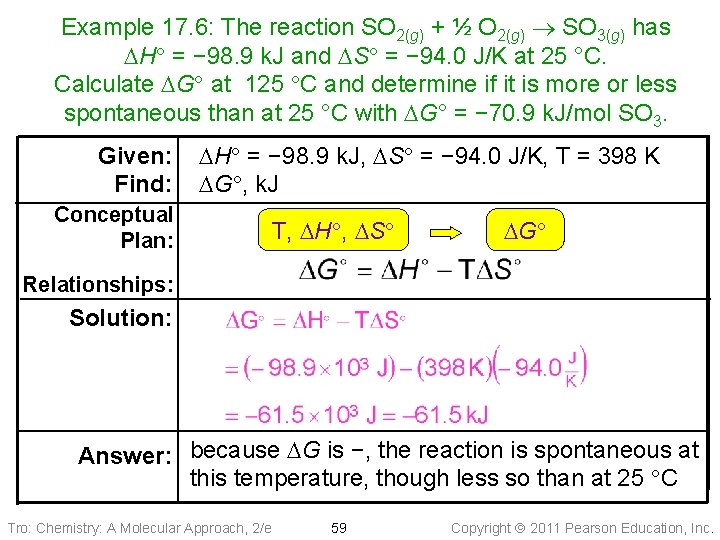
Example 17. 6: The reaction SO 2(g) + ½ O 2(g) SO 3(g) has DH = − 98. 9 k. J and DS = − 94. 0 J/K at 25 °C. Calculate DG at 125 C and determine if it is more or less spontaneous than at 25 °C with DG° = − 70. 9 k. J/mol SO 3. Given: Find: DH = − 98. 9 k. J, DS = − 94. 0 J/K, T = 398 K DG , k. J Conceptual Plan: T, DH , DS DG Relationships: Solution: Answer: because DG is −, the reaction is spontaneous at this temperature, though less so than at 25 C Tro: Chemistry: A Molecular Approach, 2/e 59 Copyright 2011 Pearson Education, Inc.

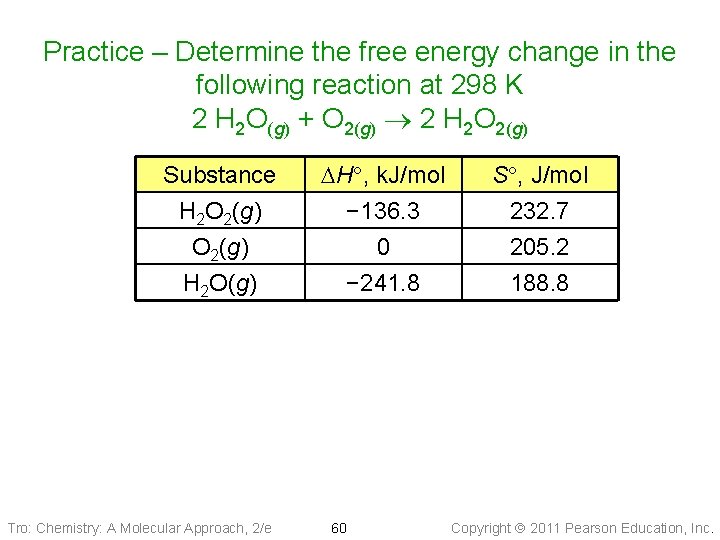
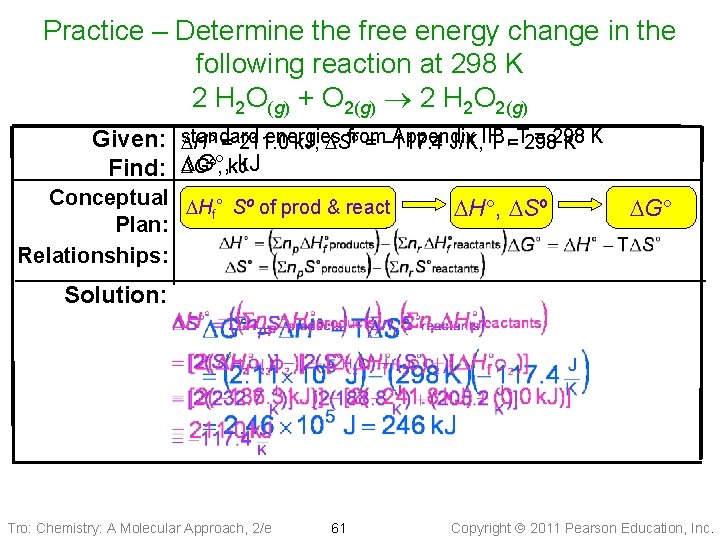
Practice – Determine the free energy change in the following reaction at 298 K 2 H 2 O(g) + O 2(g) 2 H 2 O 2(g) Substance H 2 O 2(g) H 2 O(g) Tro: Chemistry: A Molecular Approach, 2/e DH , k. J/mol − 136. 3 0 − 241. 8 60 S , J/mol 232. 7 205. 2 188. 8 Copyright 2011 Pearson Education, Inc.

Practice – Determine the free energy change in the following reaction at 298 K 2 H 2 O(g) + O 2(g) 2 H 2 O 2(g) Given: Find: standard energies from Appendix = 298 DH = 211. 0 k. J, DS = − 117. 4 J/K, IIB, T =T 298 K K DG , k. J Conceptual DH ° Sº of prod & react f Plan: Relationships: DH , DSº DG Solution: Tro: Chemistry: A Molecular Approach, 2/e 61 Copyright 2011 Pearson Education, Inc.

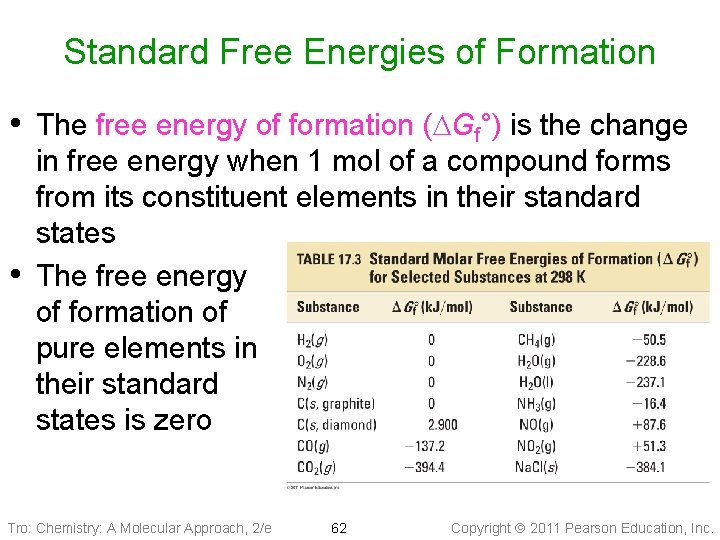
Standard Free Energies of Formation • The free energy of formation (DGf°) is the change • in free energy when 1 mol of a compound forms from its constituent elements in their standard states The free energy of formation of pure elements in their standard states is zero Tro: Chemistry: A Molecular Approach, 2/e 62 Copyright 2011 Pearson Education, Inc.

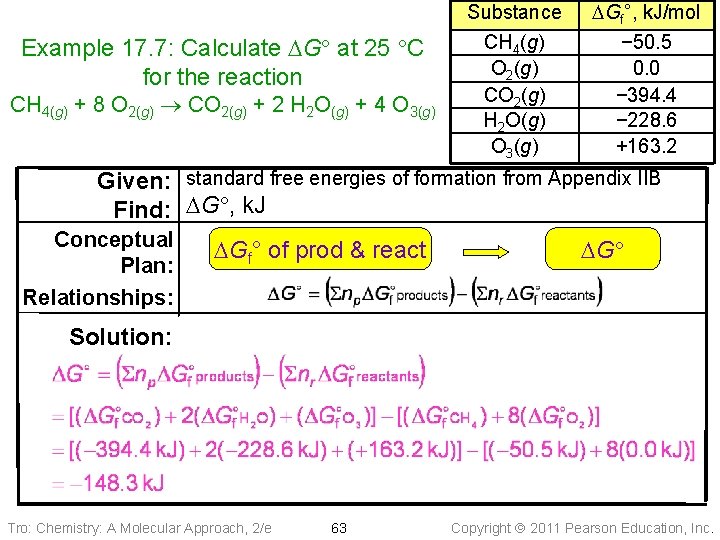
Example 17. 7: Calculate DG at 25 C for the reaction CH 4(g) + 8 O 2(g) CO 2(g) + 2 H 2 O(g) + 4 O 3(g) Substance CH 4(g) O 2(g) CO 2(g) H 2 O(g) O 3(g) DGf°, k. J/mol − 50. 5 0. 0 − 394. 4 − 228. 6 +163. 2 Given: standard free energies of formation from Appendix IIB Find: DG , k. J Conceptual Plan: Relationships: DGf° of prod & react DG Solution: Tro: Chemistry: A Molecular Approach, 2/e 63 Copyright 2011 Pearson Education, Inc.

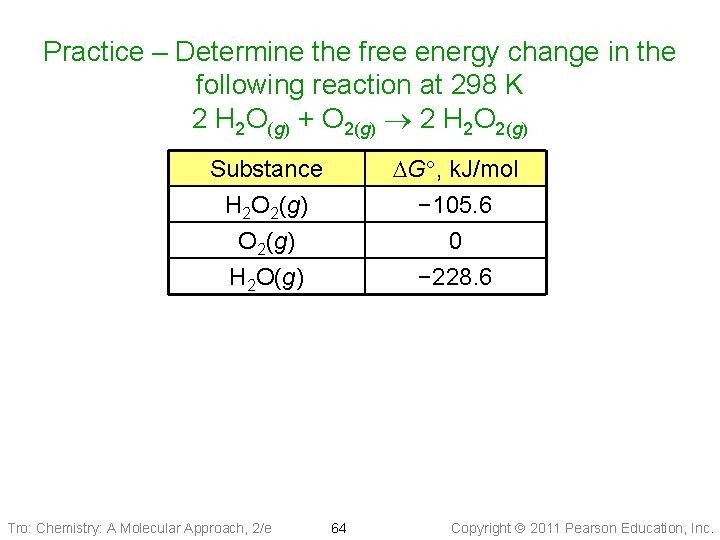
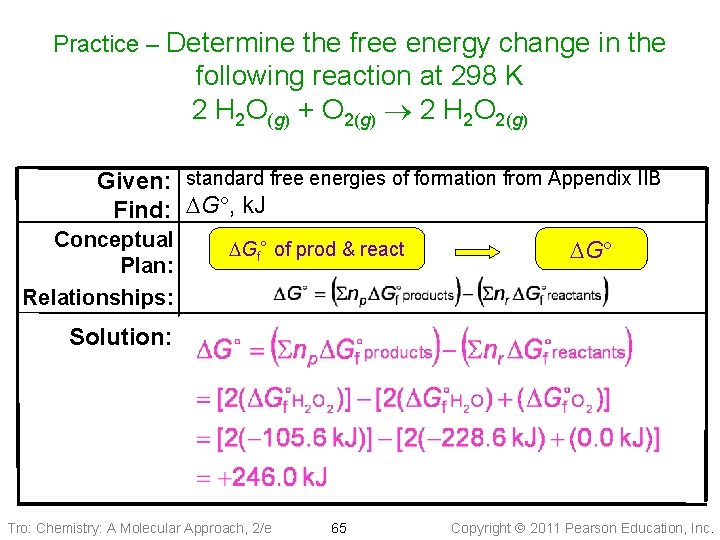
Practice – Determine the free energy change in the following reaction at 298 K 2 H 2 O(g) + O 2(g) 2 H 2 O 2(g) DG , k. J/mol − 105. 6 0 − 228. 6 Substance H 2 O 2(g) H 2 O(g) Tro: Chemistry: A Molecular Approach, 2/e 64 Copyright 2011 Pearson Education, Inc.

Practice – Determine the free energy change in the following reaction at 298 K 2 H 2 O(g) + O 2(g) 2 H 2 O 2(g) Given: standard free energies of formation from Appendix IIB Find: DG , k. J Conceptual Plan: Relationships: DGf° of prod & react DG Solution: Tro: Chemistry: A Molecular Approach, 2/e 65 Copyright 2011 Pearson Education, Inc.

DG Relationships • If a reaction can be expressed as a series of reactions, the sum of the DG values of the individual reaction is the DG of the total reaction ü DG is a state function • If a reaction is reversed, the sign of its DG value • reverses If the amount of materials is multiplied by a factor, the value of the DG is multiplied by the same factor ü the value of DG of a reaction is extensive Tro: Chemistry: A Molecular Approach, 2/e 66 Copyright 2011 Pearson Education, Inc.

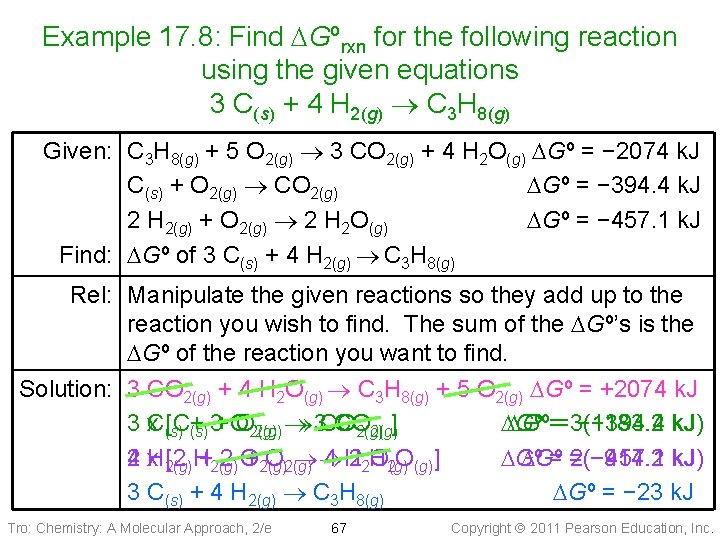
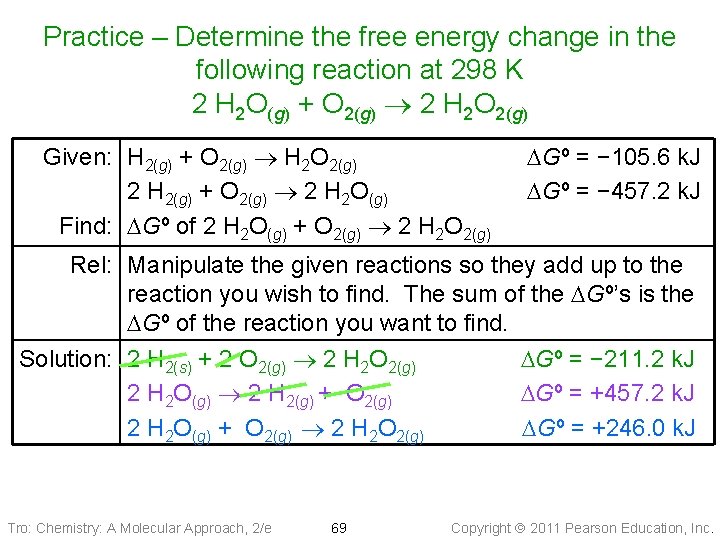
Example 17. 8: Find DGºrxn for the following reaction using the given equations 3 C(s) + 4 H 2(g) C 3 H 8(g) Given: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) DGº = − 2074 k. J C(s) + O 2(g) CO 2(g) DGº = − 394. 4 k. J 2 H 2(g) + O 2(g) 2 H 2 O(g) DGº = − 457. 1 k. J Find: DGº of 3 C(s) + 4 H 2(g) C 3 H 8(g) Rel: Manipulate the given reactions so they add up to the reaction you wish to find. The sum of the DGº’s is the DGº of the reaction you want to find. Solution: 3 CO 2(g) + 4 H 2 O(g) C 3 H 8(g) + 5 O 2(g) DGº = +2074 k. J 3 x. C(s) [C+ +O O 2(g) 3 CO CO ] DGº==3(− 394. 4 − 1183. 2 k. J) (s) 3 2(g) 2 H 4 x 2(g) [2 H+2(g) 2 O +2(g) O 2(g) 4 H 22 H O 2(g) O(g)] DGº == 2(− 457. 1 − 914. 2 k. J) 3 C(s) + 4 H 2(g) C 3 H 8(g) DGº = − 23 k. J Tro: Chemistry: A Molecular Approach, 2/e 67 Copyright 2011 Pearson Education, Inc.

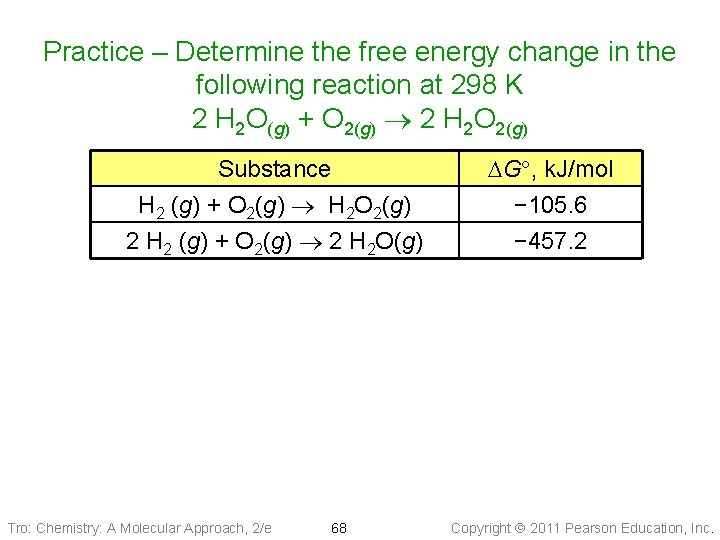
Practice – Determine the free energy change in the following reaction at 298 K 2 H 2 O(g) + O 2(g) 2 H 2 O 2(g) Substance H 2 (g) + O 2(g) H 2 O 2(g) 2 H 2 (g) + O 2(g) 2 H 2 O(g) Tro: Chemistry: A Molecular Approach, 2/e 68 DG , k. J/mol − 105. 6 − 457. 2 Copyright 2011 Pearson Education, Inc.

Practice – Determine the free energy change in the following reaction at 298 K 2 H 2 O(g) + O 2(g) 2 H 2 O 2(g) Given: H 2(g) + O 2(g) H 2 O 2(g) 2 H 2(g) + O 2(g) 2 H 2 O(g) Find: DGº of 2 H 2 O(g) + O 2(g) 2 H 2 O 2(g) DGº = − 105. 6 k. J DGº = − 457. 2 k. J Rel: Manipulate the given reactions so they add up to the reaction you wish to find. The sum of the DGº’s is the DGº of the reaction you want to find. Solution: 2 H 2(s) + 2 O 2(g) 2 H 2 O 2(g) 2 H 2 O(g) 2 H 2(g) + O 2(g) 2 H 2 O(g) + O 2(g) 2 H 2 O 2(g) Tro: Chemistry: A Molecular Approach, 2/e 69 DGº = − 211. 2 k. J DGº = +457. 2 k. J DGº = +246. 0 k. J Copyright 2011 Pearson Education, Inc.

What’s “Free” About Free Energy? • The free energy is the maximum amount of • • energy released from a system that is available to do work on the surroundings For many exothermic reactions, some of the heat released due to the enthalpy change goes into increasing the entropy of the surroundings, so it is not available to do work And even some of this free energy is generally lost to heating up the surroundings Tro: Chemistry: A Molecular Approach, 2/e 70 Copyright 2011 Pearson Education, Inc.

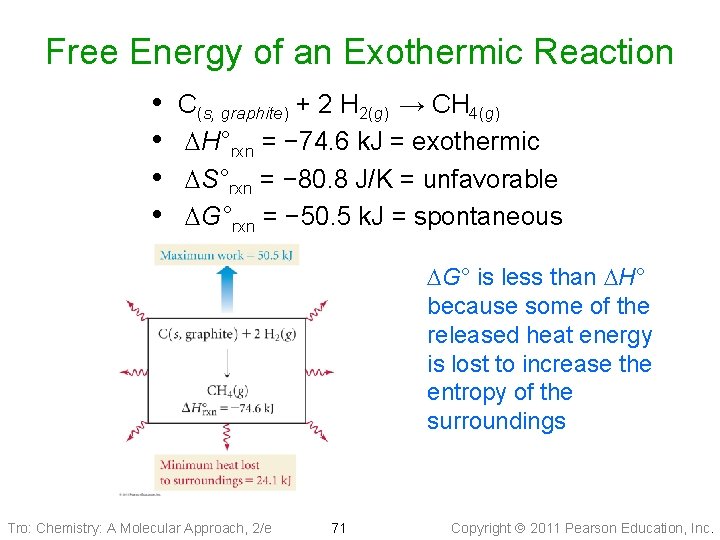
Free Energy of an Exothermic Reaction • • C(s, graphite) + 2 H 2(g) → CH 4(g) DH°rxn = − 74. 6 k. J = exothermic DS°rxn = − 80. 8 J/K = unfavorable DG°rxn = − 50. 5 k. J = spontaneous DG° is less than DH° because some of the released heat energy is lost to increase the entropy of the surroundings Tro: Chemistry: A Molecular Approach, 2/e 71 Copyright 2011 Pearson Education, Inc.

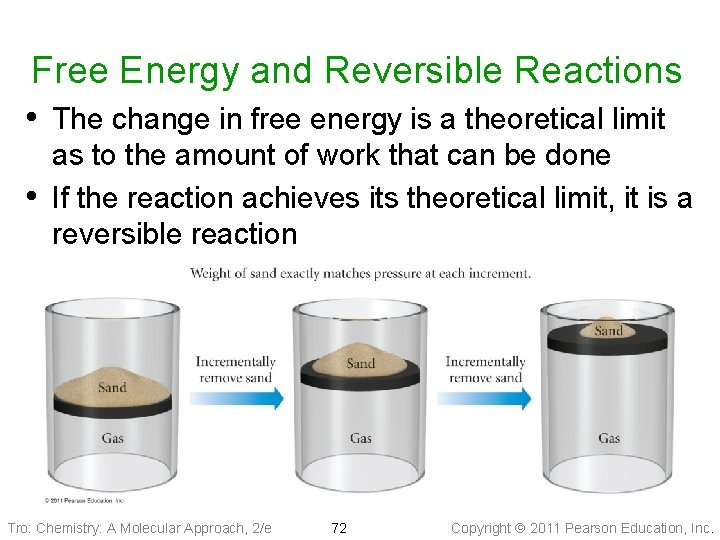
Free Energy and Reversible Reactions • The change in free energy is a theoretical limit • as to the amount of work that can be done If the reaction achieves its theoretical limit, it is a reversible reaction Tro: Chemistry: A Molecular Approach, 2/e 72 Copyright 2011 Pearson Education, Inc.

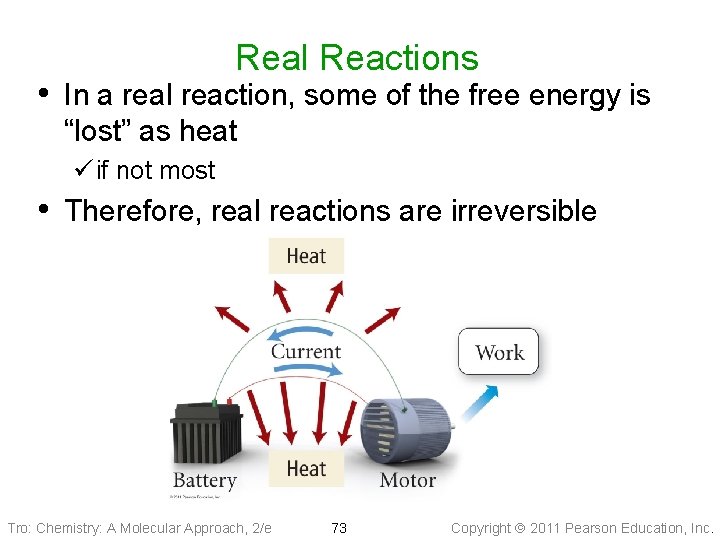
Real Reactions • In a real reaction, some of the free energy is “lost” as heat ü if not most • Therefore, real reactions are irreversible Tro: Chemistry: A Molecular Approach, 2/e 73 Copyright 2011 Pearson Education, Inc.

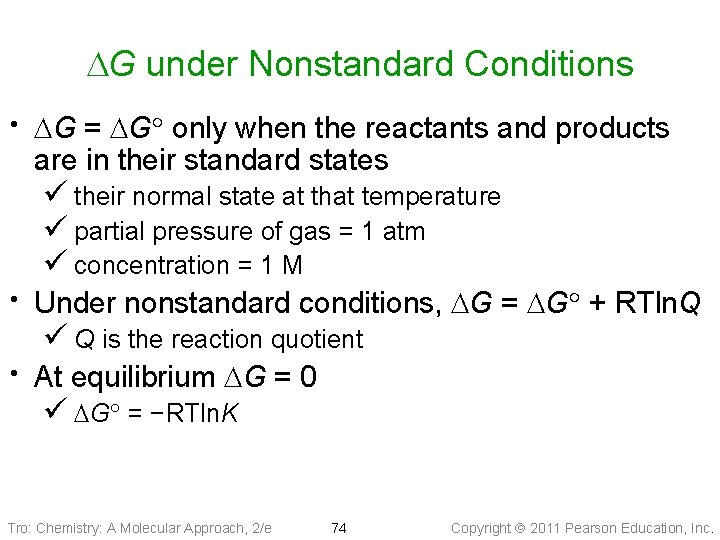
DG under Nonstandard Conditions DG = DG only when the reactants and products are in their standard states ü their normal state at that temperature ü partial pressure of gas = 1 atm ü concentration = 1 M Under nonstandard conditions, DG = DG + RTln. Q ü Q is the reaction quotient At equilibrium DG = 0 ü DG = −RTln. K Tro: Chemistry: A Molecular Approach, 2/e 74 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 75 Copyright 2011 Pearson Education, Inc.

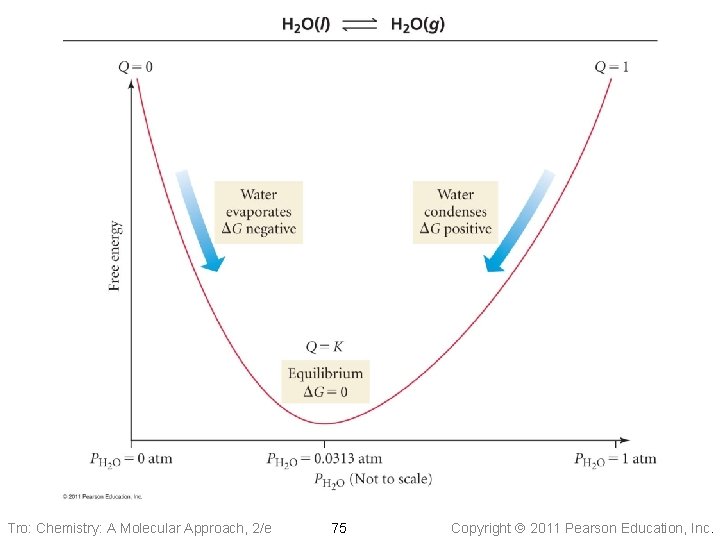
Example 17. 9: Calculate DG at 298 K for the reaction under the given conditions 2 NO(g) + O 2(g) 2 NO 2(g) DGº = − 71. 2 k. J Substance P, atm NO(g) O 2(g) NO 2(g) 0. 100 2. 00 Given: non-standard conditions, DGº, T Find: DG, k. J Conceptual Plan: Relationships: DG , PNO, PO 2, PNO 2 DG Solution: Tro: Chemistry: A Molecular Approach, 2/e 76 Copyright 2011 Pearson Education, Inc.

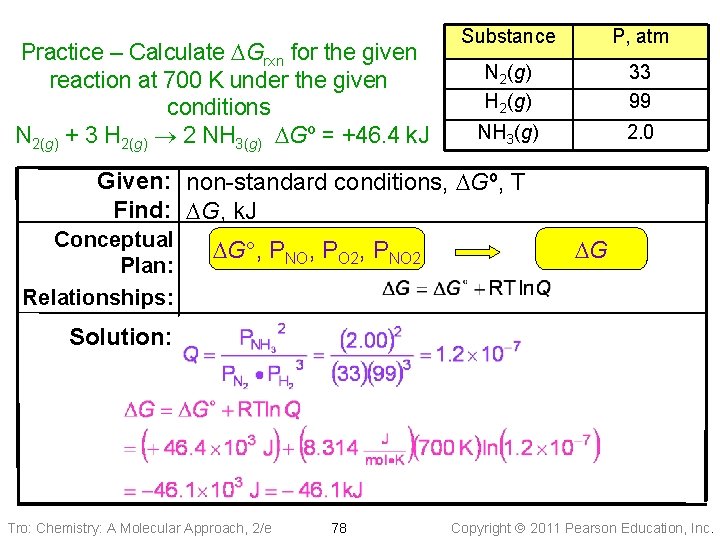
Practice – Calculate DGrxn for the given reaction at 700 K under the given conditions N 2(g) + 3 H 2(g) 2 NH 3(g) DGº = +46. 4 k. J Substance N 2(g) H 2(g) NH 3(g) Tro: Chemistry: A Molecular Approach, 2/e P, atm 33 99 2. 0 77 Copyright 2011 Pearson Education, Inc.

Practice – Calculate DGrxn for the given reaction at 700 K under the given conditions N 2(g) + 3 H 2(g) 2 NH 3(g) DGº = +46. 4 k. J Substance P, atm N 2(g) H 2(g) 33 99 NH 3(g) 2. 0 Given: non-standard conditions, DGº, T Find: DG, k. J Conceptual Plan: Relationships: DG , PNO, PO 2, PNO 2 DG Solution: Tro: Chemistry: A Molecular Approach, 2/e 78 Copyright 2011 Pearson Education, Inc.

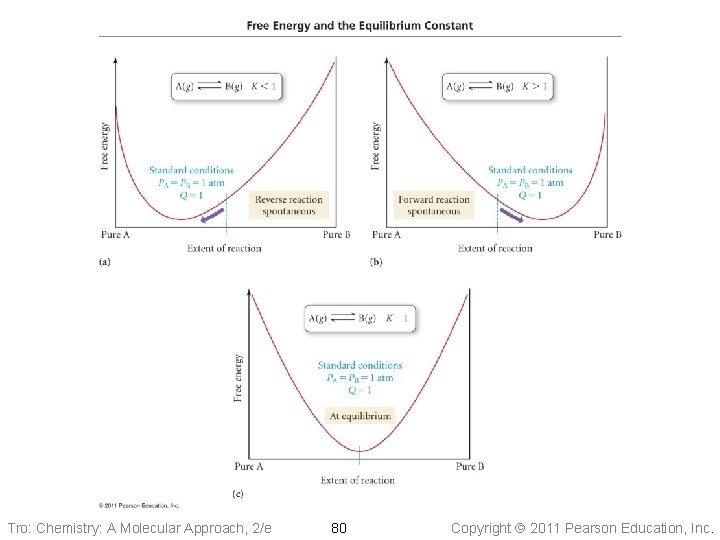
DGº and K • Because DGrxn = 0 at equilibrium, then • DGº = −RTln(K) When K < 1, DGº is + and the reaction is spontaneous in the reverse direction under standard conditions ü nothing will happen if there are no products yet! • When K > 1, DGº is − and the reaction is • spontaneous in the forward direction under standard conditions When K = 1, DGº is 0 and the reaction is at equilibrium under standard conditions Tro: Chemistry: A Molecular Approach, 2/e 79 Copyright 2011 Pearson Education, Inc.

Tro: Chemistry: A Molecular Approach, 2/e 80 Copyright 2011 Pearson Education, Inc.

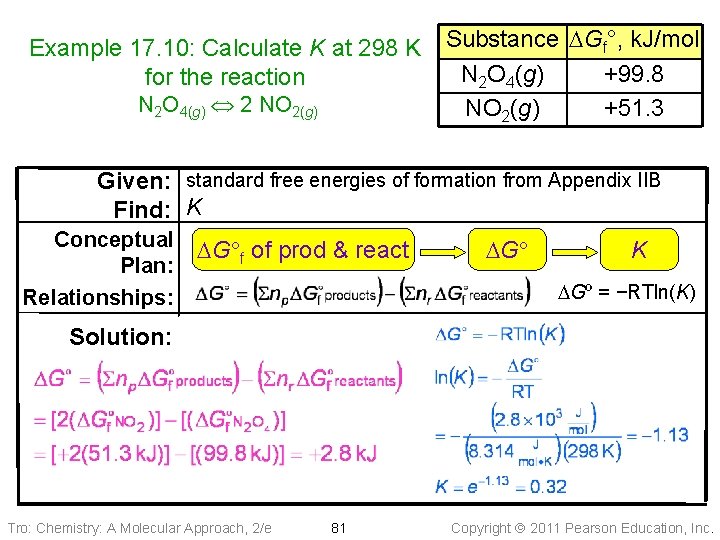
Example 17. 10: Calculate K at 298 K for the reaction N 2 O 4(g) 2 NO 2(g) Substance DGf°, k. J/mol N 2 O 4(g) +99. 8 NO 2(g) +51. 3 Given: standard free energies of formation from Appendix IIB Find: K Conceptual Plan: Relationships: DG f of prod & react DG K DGº = −RTln(K) Solution: Tro: Chemistry: A Molecular Approach, 2/e 81 Copyright 2011 Pearson Education, Inc.

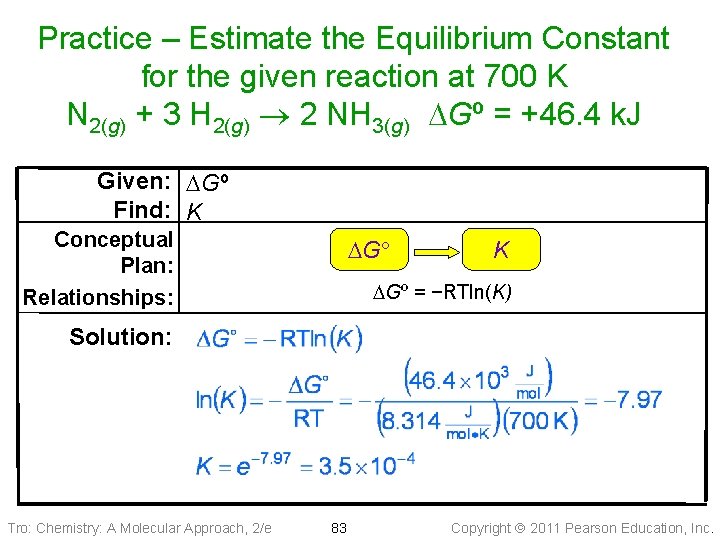
Practice – Estimate the equilibrium constant for the given reaction at 700 K N 2(g) + 3 H 2(g) 2 NH 3(g) DGº = +46. 4 k. J Tro: Chemistry: A Molecular Approach, 2/e 82 Copyright 2011 Pearson Education, Inc.

Practice – Estimate the Equilibrium Constant for the given reaction at 700 K N 2(g) + 3 H 2(g) 2 NH 3(g) DGº = +46. 4 k. J Given: DGº Find: K Conceptual Plan: Relationships: DG K DGº = −RTln(K) Solution: Tro: Chemistry: A Molecular Approach, 2/e 83 Copyright 2011 Pearson Education, Inc.

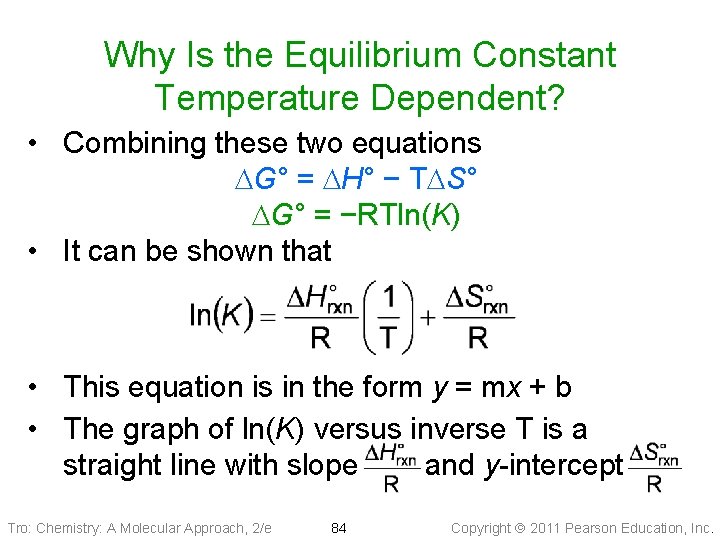
Why Is the Equilibrium Constant Temperature Dependent? • Combining these two equations DG° = DH° − TDS° DG° = −RTln(K) • It can be shown that • This equation is in the form y = mx + b • The graph of ln(K) versus inverse T is a straight line with slope and y-intercept Tro: Chemistry: A Molecular Approach, 2/e 84 Copyright 2011 Pearson Education, Inc.
 Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition answers
Introductory chemistry 5th edition answers Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Democritus atomic model diagram
Democritus atomic model diagram Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Ap chemistry unit 9 notes
Ap chemistry unit 9 notes Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Approach chemistry chalk chapter
Approach chemistry chalk chapter Virtual circuit and datagram networks in computer networks
Virtual circuit and datagram networks in computer networks Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Fine grained screening
Fine grained screening Multiple approach avoidance
Multiple approach avoidance Bandura's reciprocal determinism
Bandura's reciprocal determinism What is research approach definition
What is research approach definition Traditional approach in system analysis and design
Traditional approach in system analysis and design Tony wagner's seven survival skills
Tony wagner's seven survival skills Vsepr formula
Vsepr formula Trigonal pyramidal
Trigonal pyramidal Pf3 number of vsepr electron groups
Pf3 number of vsepr electron groups Crash course molecular biology
Crash course molecular biology Molecular level vs cellular level
Molecular level vs cellular level Molecular absorption
Molecular absorption Ab6 molecular geometry
Ab6 molecular geometry Kinetic molecular theory
Kinetic molecular theory Covalent molecular and covalent network
Covalent molecular and covalent network Kinetic molecular theory of solids
Kinetic molecular theory of solids Chemical formula vs molecular formula
Chemical formula vs molecular formula Clasificacion molecular cancer de endometrio
Clasificacion molecular cancer de endometrio Unit chemical bonding molecular geometry
Unit chemical bonding molecular geometry 5 examples of palindromic dna sequences
5 examples of palindromic dna sequences Molecular rebar design
Molecular rebar design What is a covalent molecular substance
What is a covalent molecular substance Empirical formula of haemoglobin
Empirical formula of haemoglobin Number-average molecular weight
Number-average molecular weight Dicots
Dicots Percent composition of magnesium nitrate
Percent composition of magnesium nitrate Percent composition
Percent composition Potassium permanganate percent composition
Potassium permanganate percent composition Empirical formula from percentages
Empirical formula from percentages Molecular modelling laboratory
Molecular modelling laboratory Molecular replacement method
Molecular replacement method Alkane chemical formula
Alkane chemical formula Naming compounds and writing formulas
Naming compounds and writing formulas Binary molecules
Binary molecules N srinivasan iisc passed away
N srinivasan iisc passed away Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Number-average molecular weight
Number-average molecular weight Ch2o lewis structure molecular geometry
Ch2o lewis structure molecular geometry Molecular shapes quiz
Molecular shapes quiz Molecular shape for water
Molecular shape for water Patterson function
Patterson function Patterson
Patterson F-2
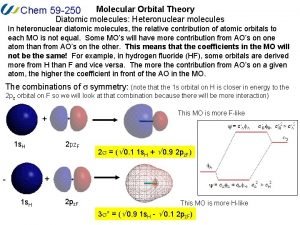
F-2 Molecular orbital diagram of heteronuclear diatomic
Molecular orbital diagram of heteronuclear diatomic B2 molecular orbital diagram
B2 molecular orbital diagram Standardised cml monitoring
Standardised cml monitoring Hydrogen monochloride
Hydrogen monochloride Microbiology definition
Microbiology definition C2h4 vsper
C2h4 vsper Polar molecule
Polar molecule Pf3 number of vsepr electron groups
Pf3 number of vsepr electron groups Molecular geometry of no3-
Molecular geometry of no3- Molecular geometry and bonding theories
Molecular geometry and bonding theories What is a bond order in chemistry
What is a bond order in chemistry Molecular gastronomy restaurants
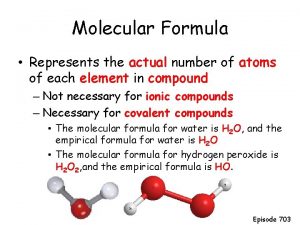
Molecular gastronomy restaurants Empirical formula vs molecular formula
Empirical formula vs molecular formula Molecular farming definition
Molecular farming definition Molecular ecological network analyses
Molecular ecological network analyses Mass to moles conversion
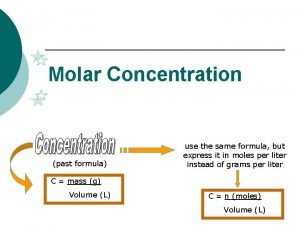
Mass to moles conversion How to find molecular concentration
How to find molecular concentration Modelo cinetico molecular
Modelo cinetico molecular Osometer
Osometer Elevada fuerza de adhesión
Elevada fuerza de adhesión Molecular dynamics limitations
Molecular dynamics limitations Cocl2 molecular geometry
Cocl2 molecular geometry Bent lewis dot structure
Bent lewis dot structure Gluten molecular structure
Gluten molecular structure Kinetic molecular theory of gases
Kinetic molecular theory of gases Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory volume
Kinetic molecular theory volume Nocl molecular shape
Nocl molecular shape Applications of uv visible spectroscopy
Applications of uv visible spectroscopy