Chemistry A Molecular Approach 1 st Ed Nivaldo









- Slides: 90

Chemistry: A Molecular Approach, 1 st Ed. Nivaldo Tro Chapter 9 Chemical Bonding I: Lewis Theory Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA Tro, Chemistry: A Molecular Approach 2008, Prentice Hall

Bonding Theories • explain how and why atoms attach together • explain why some combinations of atoms are stable and others are not ü why is water H 2 O, not HO or H 3 O • one of the simplest bonding theories was developed by • • G. N. Lewis and is called Lewis Theory emphasizes valence electrons to explain bonding using Lewis Theory, we can draw models – called Lewis structures – that allow us to predict many properties of molecules ü aka Electron Dot Structures ü such as molecular shape, size, polarity Tro, Chemistry: A Molecular Approach 2

Why Do Atoms Bond? • processes are spontaneous if they result in a system • • • with lower potential energy chemical bonds form because they lower the potential energy between the charged particles that compose atoms the potential energy between charged particles is directly proportional to the product of the charges the potential energy between charged particles is inversely proportional to the distance between the charges Tro, Chemistry: A Molecular Approach 3

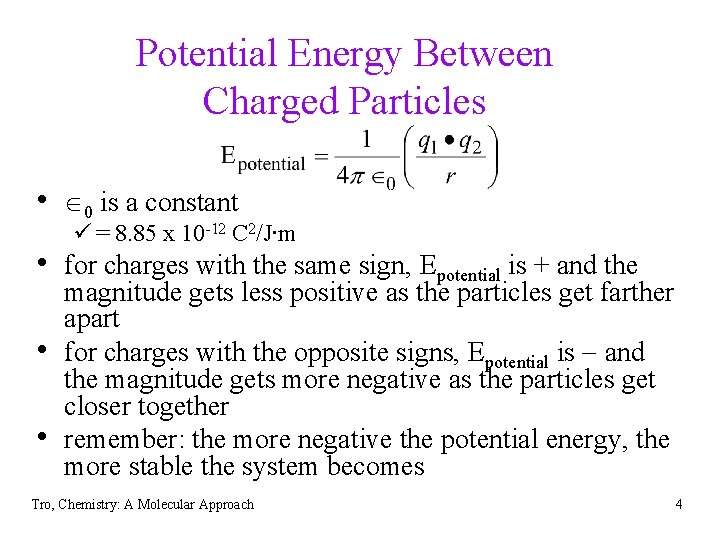
Potential Energy Between Charged Particles • 0 is a constant ü = 8. 85 x 10 -12 C 2/J∙m • for charges with the same sign, Epotential is + and the • • magnitude gets less positive as the particles get farther apart for charges with the opposite signs, Epotential is and the magnitude gets more negative as the particles get closer together remember: the more negative the potential energy, the more stable the system becomes Tro, Chemistry: A Molecular Approach 4

Potential Energy Between Charged Particles The attraction repulsion between like-charged particles opposite-charged increases particles as the as particles the particles get closer together. Bringing To bring them closer lowers requiresthe addition potential energy of more of the energy. system. Tro, Chemistry: A Molecular Approach 5

Bonding • a chemical bond forms when the potential • energy of the bonded atoms is less than the potential energy of the separate atoms have to consider following interactions: ünucleus-to-nucleus repulsion üelectron-to-electron repulsion ünucleus-to-electron attraction Tro, Chemistry: A Molecular Approach 6

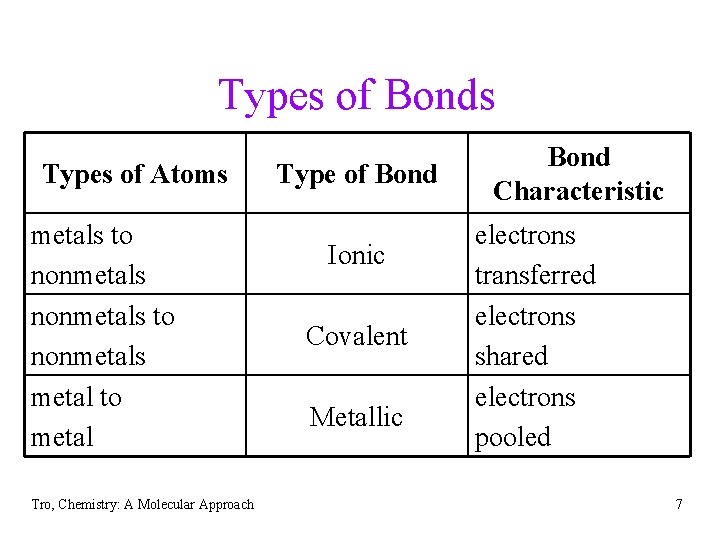
Types of Bonds Types of Atoms metals to nonmetals metal to metal Tro, Chemistry: A Molecular Approach Type of Bond Ionic Covalent Metallic Bond Characteristic electrons transferred electrons shared electrons pooled 7

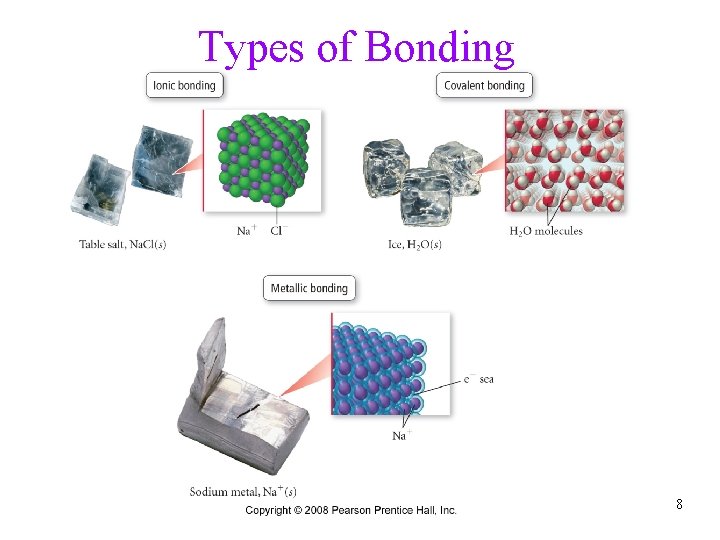
Types of Bonding 8

Ionic Bonds • when metals bond to nonmetals, some electrons from the metal atoms are transferred to the nonmetal atoms ümetals have low ionization energy, relatively easy to remove an electron from ünonmetals have high electron affinities, relatively good to add electrons to Tro, Chemistry: A Molecular Approach 9

Covalent Bonds • nonmetals have relatively high ionization energies, so it • is difficult to remove electrons from them when nonmetals bond together, it is better in terms of potential energy for the atoms to share valence electrons ü potential energy lowest when the electrons are between the nuclei • shared electrons hold the atoms together by attracting nuclei of both atoms Tro, Chemistry: A Molecular Approach 10

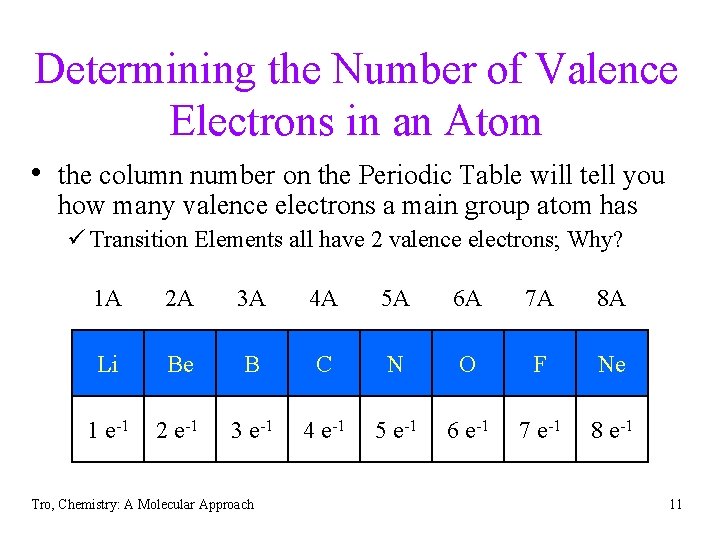
Determining the Number of Valence Electrons in an Atom • the column number on the Periodic Table will tell you how many valence electrons a main group atom has ü Transition Elements all have 2 valence electrons; Why? 1 A 2 A 3 A 4 A 5 A 6 A 7 A 8 A Li Be B C N O F Ne 1 e-1 2 e-1 3 e-1 4 e-1 5 e-1 6 e-1 7 e-1 8 e-1 Tro, Chemistry: A Molecular Approach 11

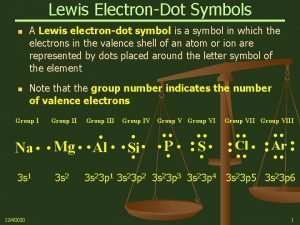
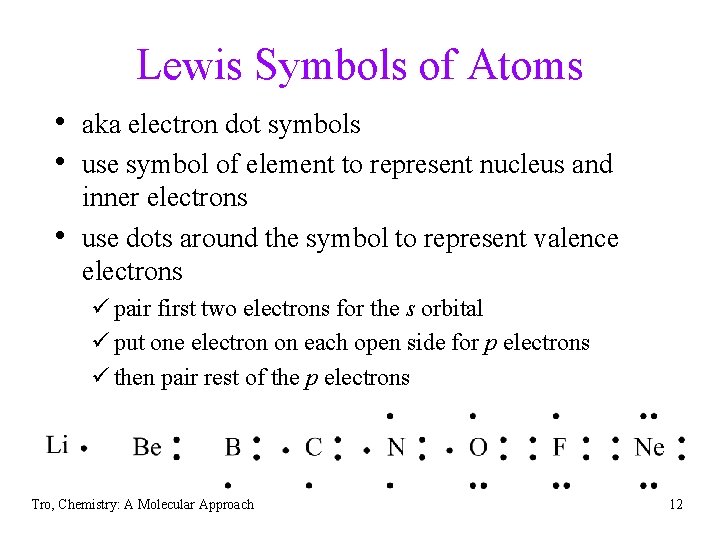
Lewis Symbols of Atoms • aka electron dot symbols • use symbol of element to represent nucleus and • inner electrons use dots around the symbol to represent valence electrons ü pair first two electrons for the s orbital ü put one electron on each open side for p electrons ü then pair rest of the p electrons Tro, Chemistry: A Molecular Approach 12

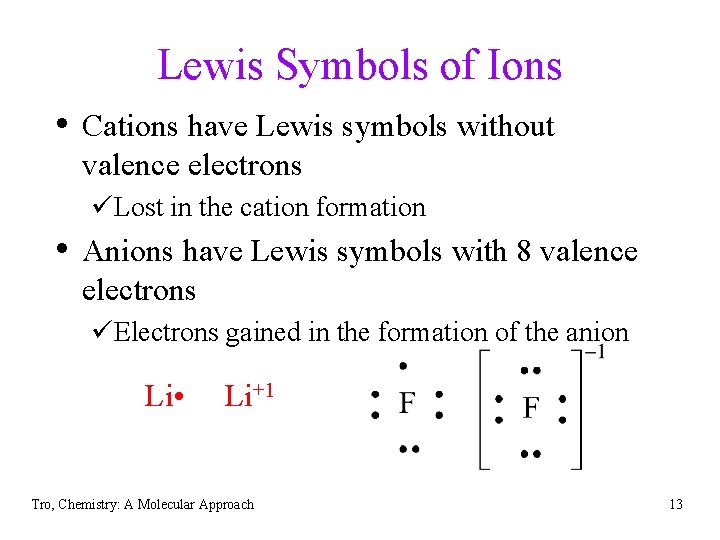
Lewis Symbols of Ions • Cations have Lewis symbols without valence electrons üLost in the cation formation • Anions have Lewis symbols with 8 valence electrons üElectrons gained in the formation of the anion Li • Li+1 Tro, Chemistry: A Molecular Approach 13

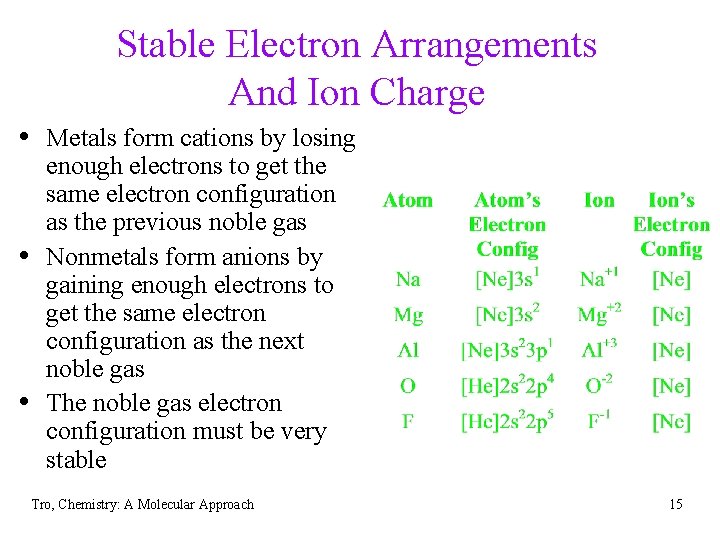
Stable Electron Arrangements And Ion Charge • Metals form cations by losing • • enough electrons to get the same electron configuration as the previous noble gas Nonmetals form anions by gaining enough electrons to get the same electron configuration as the next noble gas The noble gas electron configuration must be very stable Tro, Chemistry: A Molecular Approach 15

Octet Rule • when atoms bond, they tend to gain, lose, or share electrons to • result in 8 valence electrons ns 2 np 6 ü noble gas configuration • many exceptions ü H, Li, Be, B attain an electron configuration like He Ø He = 2 valence electrons Ø Li loses its one valence electron Ø H shares or gains one electron v though it commonly loses its one electron to become H+ Ø Be loses 2 electrons to become Be 2+ v though it commonly shares its two electrons in covalent bonds, resulting in 4 valence electrons Ø B loses 3 electrons to become B 3+ v though it commonly shares its three electrons in covalent bonds, resulting in 6 valence electrons ü expanded octets for elements in Period 3 or below Ø using empty valence d orbitals Tro, Chemistry: A Molecular Approach 16

Lewis Theory • the basis of Lewis Theory is that there are certain electron arrangements in the atom that are more stable üoctet rule • bonding occurs so atoms attain a more stable electron configuration ümore stable = lower potential energy üno attempt to quantify the energy as the calculation is extremely complex Tro, Chemistry: A Molecular Approach 17

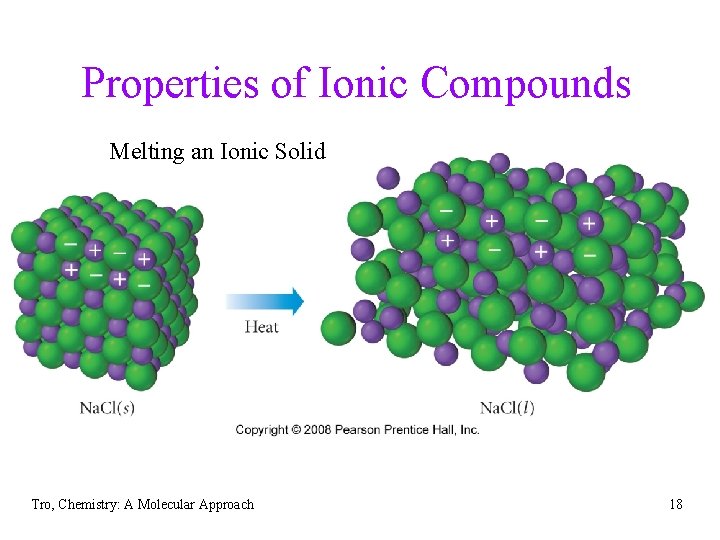
Properties of Ionic Compounds Melting an Ionic Solid • hard and brittle crystalline solids üall are solids at room temperature • melting points generally > 300 C • the liquid state conducts electricity üthe solid state does not conduct electricity • many are soluble in water üthe solution conducts electricity well Tro, Chemistry: A Molecular Approach 18

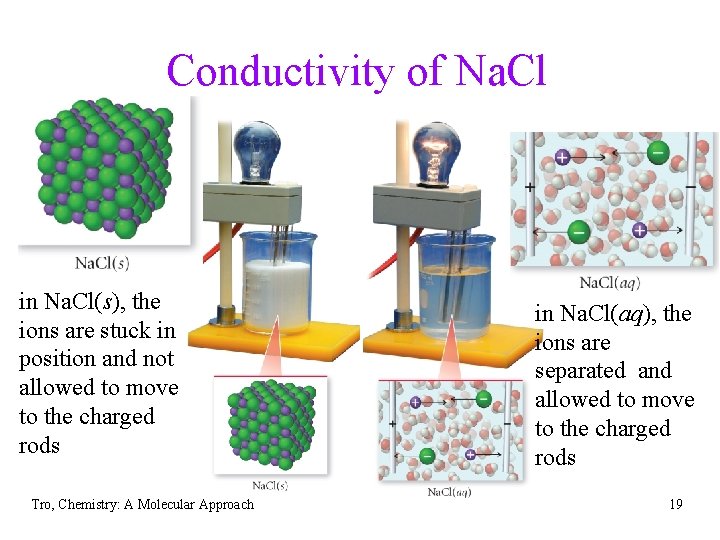
Conductivity of Na. Cl in Na. Cl(s), the ions are stuck in position and not allowed to move to the charged rods Tro, Chemistry: A Molecular Approach in Na. Cl(aq), the ions are separated and allowed to move to the charged rods 19

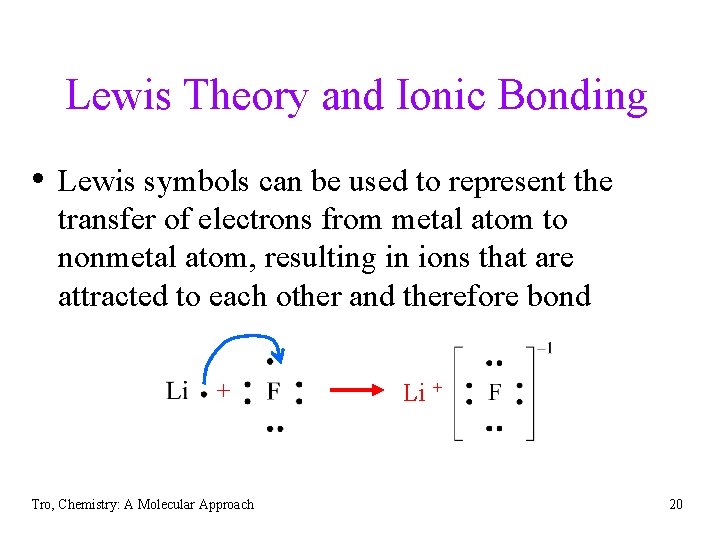
Lewis Theory and Ionic Bonding • Lewis symbols can be used to represent the transfer of electrons from metal atom to nonmetal atom, resulting in ions that are attracted to each other and therefore bond + Tro, Chemistry: A Molecular Approach Li + 20

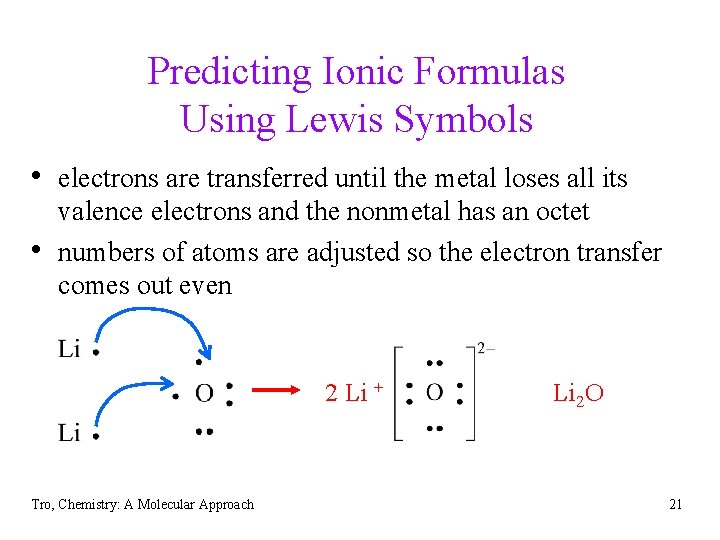
Predicting Ionic Formulas Using Lewis Symbols • electrons are transferred until the metal loses all its • valence electrons and the nonmetal has an octet numbers of atoms are adjusted so the electron transfer comes out even 2 Li + Tro, Chemistry: A Molecular Approach Li 2 O 21

Energetics of Ionic Bond Formation • the ionization energy of the metal is endothermic ü Na(s) → Na+(g) + 1 e ─ DH° = +603 k. J/mol • the electron affinity of the nonmetal is exothermic ü ½Cl 2(g) + 1 e ─ → Cl─(g) DH° = ─ 227 k. J/mol • generally, the ionization energy of the metal is larger • than the electron affinity of the nonmetal, therefore the formation of the ionic compound should be endothermic but the heat of formation of most ionic compounds is exothermic and generally large; Why? ü Na(s) + ½Cl 2(g) → Na. Cl(s) Tro, Chemistry: A Molecular Approach DH°f = -410 k. J/mol 22

Ionic Bonds • electrostatic attraction is nondirectional!! üno direct anion-cation pair • no ionic molecule üchemical formula is an empirical formula, simply giving the ratio of ions based on charge balance • ions arranged in a pattern called a crystal lattice üevery cation surrounded by anions; and every anion surrounded by cations ümaximizes attractions between + and - ions Tro, Chemistry: A Molecular Approach 23

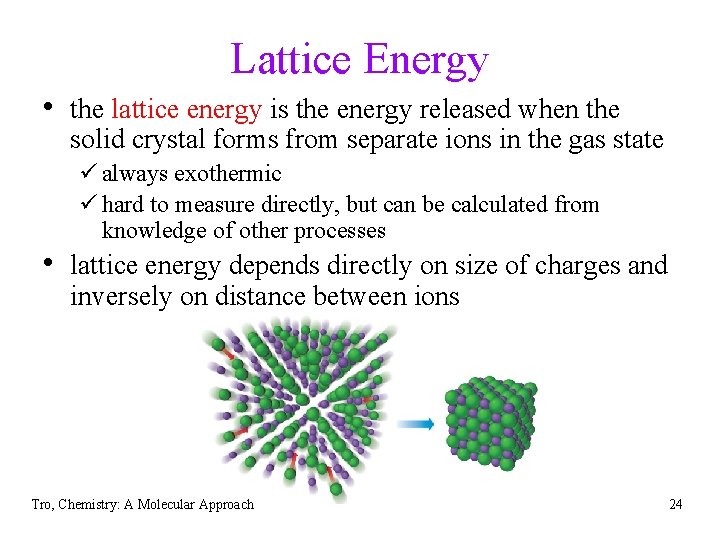
Lattice Energy • the lattice energy is the energy released when the solid crystal forms from separate ions in the gas state ü always exothermic ü hard to measure directly, but can be calculated from knowledge of other processes • lattice energy depends directly on size of charges and inversely on distance between ions Tro, Chemistry: A Molecular Approach 24

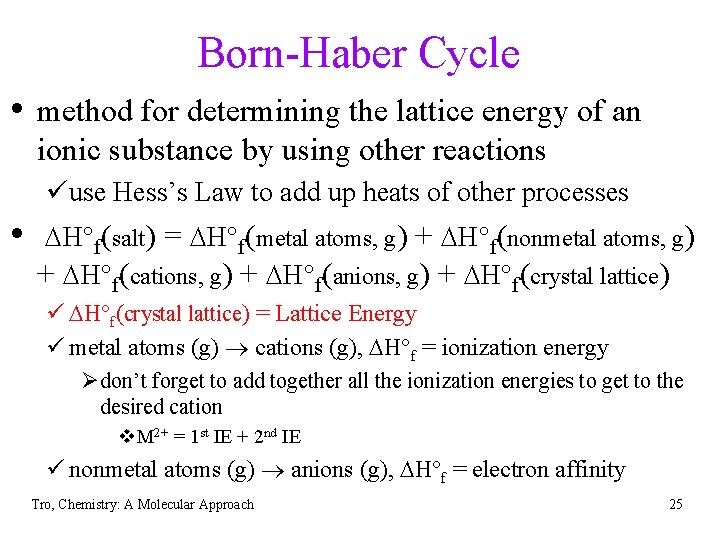
Born-Haber Cycle • method for determining the lattice energy of an ionic substance by using other reactions üuse Hess’s Law to add up heats of other processes • DH°f(salt) = DH°f(metal atoms, g) + DH°f(nonmetal atoms, g) + DH°f(cations, g) + DH°f(anions, g) + DH°f(crystal lattice) ü DH°f(crystal lattice) = Lattice Energy ü metal atoms (g) cations (g), DH°f = ionization energy Ødon’t forget to add together all the ionization energies to get to the desired cation v. M 2+ = 1 st IE + 2 nd IE ü nonmetal atoms (g) anions (g), DH°f = electron affinity Tro, Chemistry: A Molecular Approach 25

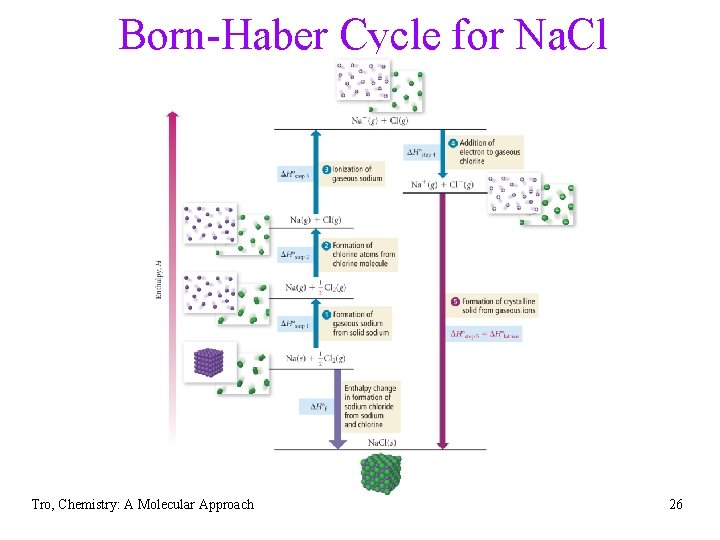
Born-Haber Cycle for Na. Cl Tro, Chemistry: A Molecular Approach 26

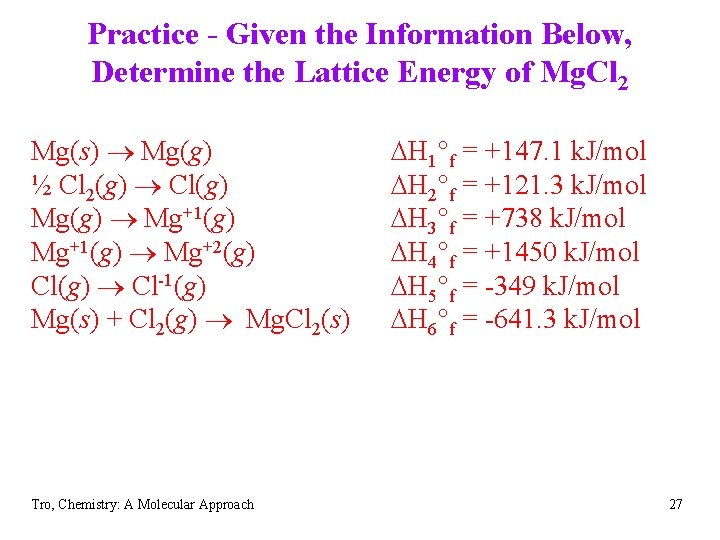
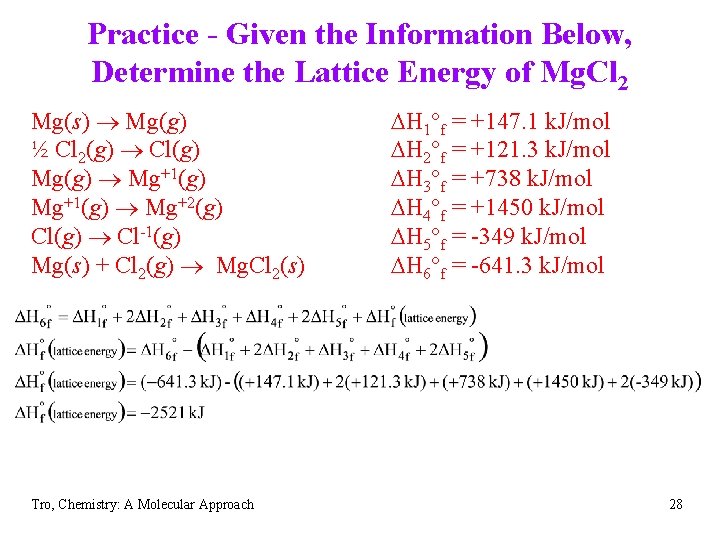
Practice - Given the Information Below, Determine the Lattice Energy of Mg. Cl 2 Mg(s) Mg(g) ½ Cl 2(g) Cl(g) Mg(g) Mg+1(g) Mg+2(g) Cl(g) Cl-1(g) Mg(s) + Cl 2(g) Mg. Cl 2(s) Tro, Chemistry: A Molecular Approach DH 1°f = +147. 1 k. J/mol DH 2°f = +121. 3 k. J/mol DH 3°f = +738 k. J/mol DH 4°f = +1450 k. J/mol DH 5°f = -349 k. J/mol DH 6°f = -641. 3 k. J/mol 27

Practice - Given the Information Below, Determine the Lattice Energy of Mg. Cl 2 Mg(s) Mg(g) ½ Cl 2(g) Cl(g) Mg(g) Mg+1(g) Mg+2(g) Cl(g) Cl-1(g) Mg(s) + Cl 2(g) Mg. Cl 2(s) Tro, Chemistry: A Molecular Approach DH 1°f = +147. 1 k. J/mol DH 2°f = +121. 3 k. J/mol DH 3°f = +738 k. J/mol DH 4°f = +1450 k. J/mol DH 5°f = -349 k. J/mol DH 6°f = -641. 3 k. J/mol 28

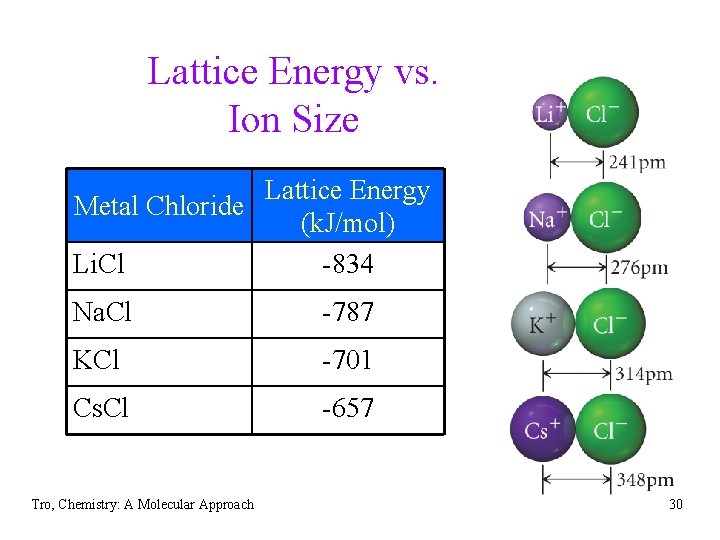
Trends in Lattice Energy Ion Size • the force of attraction between charged • particles is inversely proportional to the distance between them larger ions mean the center of positive charge (nucleus of the cation) is farther away from negative charge (electrons of the anion) ülarger ion = weaker attraction = smaller lattice energy Tro, Chemistry: A Molecular Approach 29

Lattice Energy vs. Ion Size Lattice Energy Metal Chloride (k. J/mol) Li. Cl -834 Na. Cl -787 KCl -701 Cs. Cl -657 Tro, Chemistry: A Molecular Approach 30

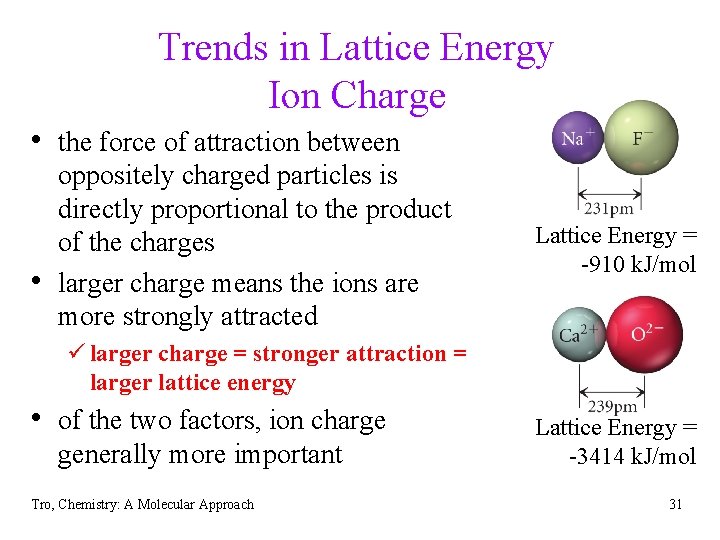
Trends in Lattice Energy Ion Charge • the force of attraction between • oppositely charged particles is directly proportional to the product of the charges larger charge means the ions are more strongly attracted Lattice Energy = -910 k. J/mol ü larger charge = stronger attraction = larger lattice energy • of the two factors, ion charge generally more important Tro, Chemistry: A Molecular Approach Lattice Energy = -3414 k. J/mol 31

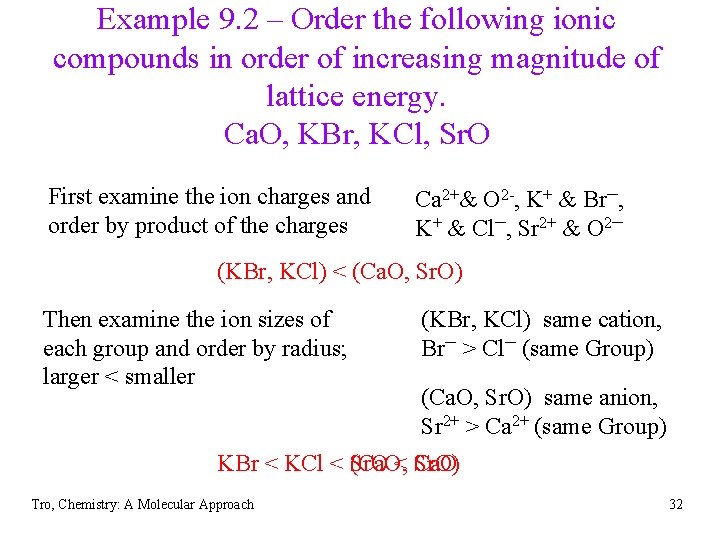
Example 9. 2 – Order the following ionic compounds in order of increasing magnitude of lattice energy. Ca. O, KBr, KCl, Sr. O First examine the ion charges and order by product of the charges Ca 2+& O 2 -, K+ & Br─, K+ & Cl─, Sr 2+ & O 2─ (KBr, KCl) < (Ca. O, Sr. O) Then examine the ion sizes of each group and order by radius; larger < smaller (KBr, KCl) same cation, Br─ > Cl─ (same Group) (Ca. O, Sr. O) same anion, Sr 2+ > Ca 2+ (same Group) KBr < KCl < (Ca. O, Sr. O < Sr. O) Ca. O Tro, Chemistry: A Molecular Approach 32

Ionic Bonding Model vs. Reality • ionic compounds have high melting points and boiling points ü MP generally > 300°C ü all ionic compounds are solids at room temperature • because the attractions between ions are strong, breaking down the crystal requires a lot of energy ü the stronger the attraction (larger the lattice energy), the higher the melting point Tro, Chemistry: A Molecular Approach 33

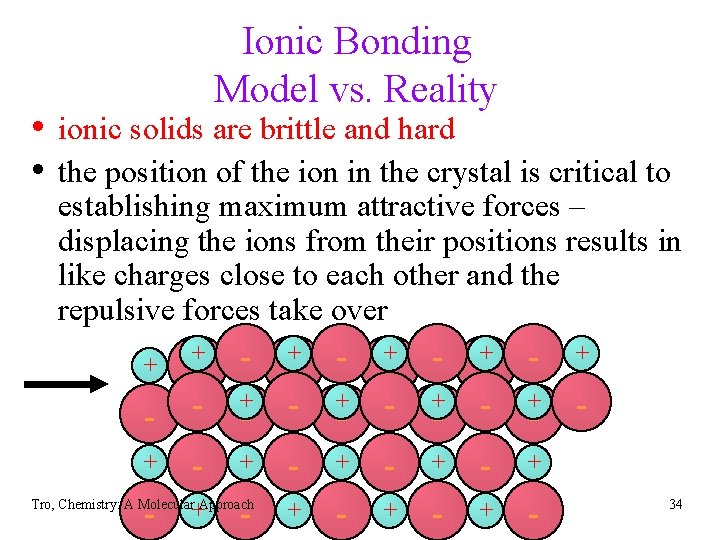
Ionic Bonding Model vs. Reality • ionic solids are brittle and hard • the position of the ion in the crystal is critical to establishing maximum attractive forces – displacing the ions from their positions results in like charges close to each other and the repulsive forces take over + + -+ - + + + Tro, Chemistry: A Molecular Approach -+ +- +- + + -+ + + - + - 34

Ionic Bonding Model vs. Reality • ionic compounds conduct electricity in the liquid state • • • or when dissolved in water, but not in the solid state to conduct electricity, a material must have charged particles that are able to flow through the material in the ionic solid, the charged particles are locked in position and cannot move around to conduct in the liquid state, or when dissolved in water, the ions have the ability to move through the structure and therefore conduct electricity Tro, Chemistry: A Molecular Approach 35

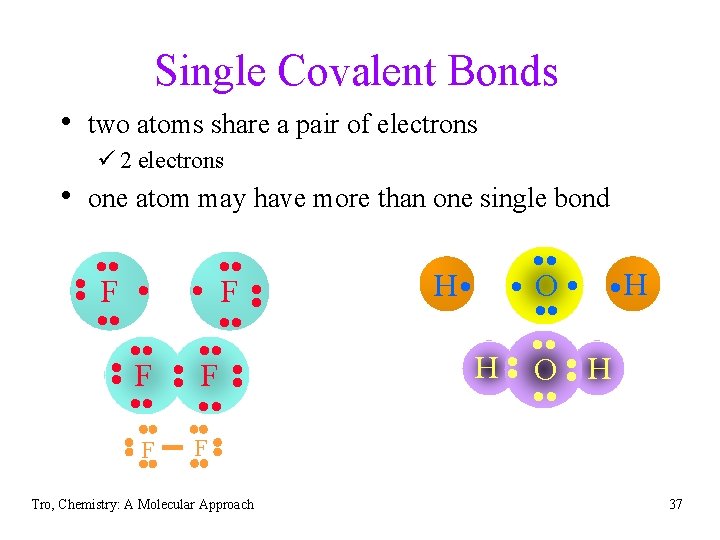
Single Covalent Bonds • two atoms share a pair of electrons ü 2 electrons • one atom may have more than one single bond • • F F • • F • • • • H • • O • H • • H O H • • • F • • • F Tro, Chemistry: A Molecular Approach 37

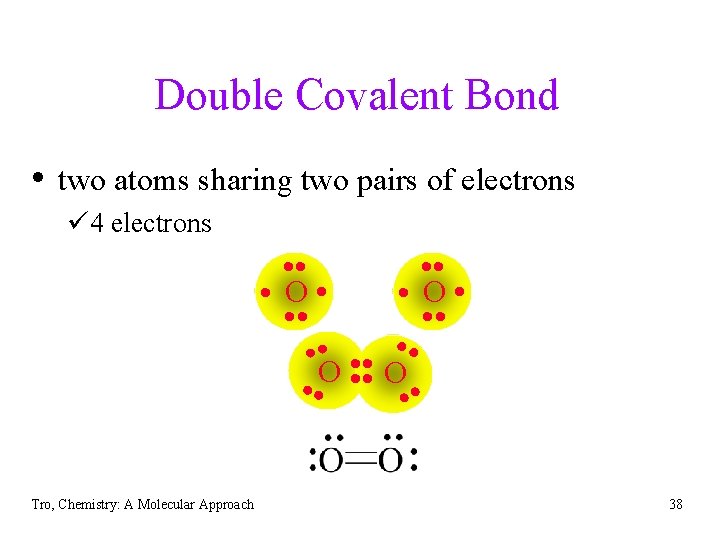
Double Covalent Bond • two atoms sharing two pairs of electrons ü 4 electrons Tro, Chemistry: A Molecular Approach • • • O • • • O O • • 38

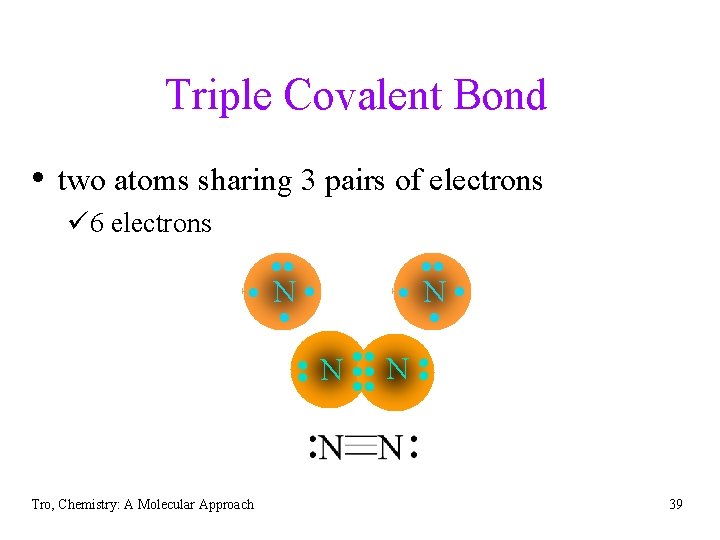
Triple Covalent Bond • two atoms sharing 3 pairs of electrons ü 6 electrons • • N • • Tro, Chemistry: A Molecular Approach • • • N • 39

Covalent Bonding Predictions from Lewis Theory • Lewis theory allows us to predict the formulas of • molecules Lewis theory predicts that some combinations should be stable, while others should not ü because the stable combinations result in “octets” • Lewis theory predicts in covalent bonding that the attractions between atoms are directional ü the shared electrons are most stable between the bonding atoms ü resulting in molecules rather than an array Tro, Chemistry: A Molecular Approach 40

Covalent Bonding Model vs. Reality • molecular compounds have low melting points and boiling points ü MP generally < 300°C ü molecular compounds are found in all 3 states at room temperature • melting and boiling involve breaking the attractions between the molecules, but not the bonds between the atoms ü the covalent bonds are strong ü the attractions between the molecules are generally weak ü the polarity of the covalent bonds influences the strength of the intermolecular attractions Tro, Chemistry: A Molecular Approach 41

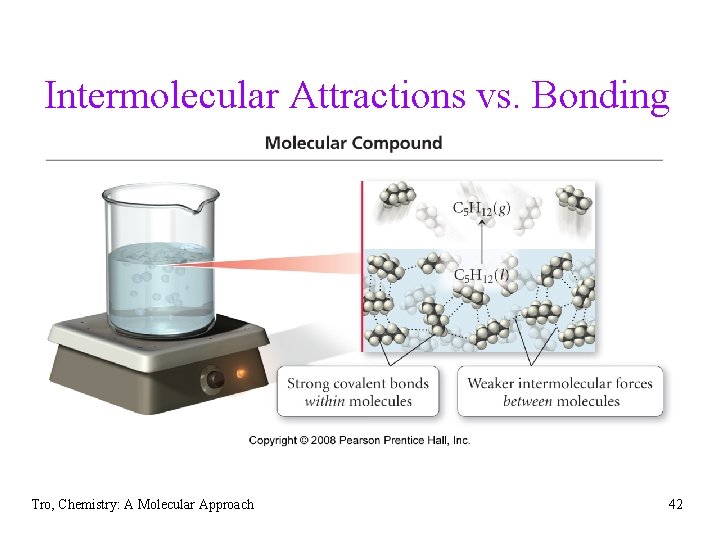
Intermolecular Attractions vs. Bonding Tro, Chemistry: A Molecular Approach 42

Ionic Bonding Model vs. Reality • some molecular solids are brittle and hard, but • • many are soft and waxy the kind and strength of the intermolecular attractions varies based on many factors the covalent bonds are not broken, however, the polarity of the bonds has influence on these attractive forces Tro, Chemistry: A Molecular Approach 43

Ionic Bonding Model vs. Reality • molecular compounds do not conduct electricity in the • • • liquid state molecular acids conduct electricity when dissolved in water, but not in the solid state in molecular solids, there are no charged particles around to allow the material to conduct when dissolved in water, molecular acids are ionized, and have the ability to move through the structure and therefore conduct electricity Tro, Chemistry: A Molecular Approach 44

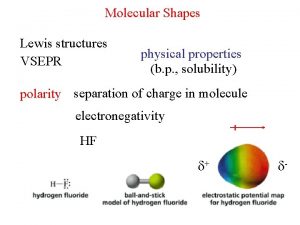
Bond Polarity • covalent bonding between unlike atoms results in unequal sharing of the electrons üone atom pulls the electrons in the bond closer to its side üone end of the bond has larger electron density than the other • the result is a polar covalent bond übond polarity üthe end with the larger electron density gets a partial negative charge üthe end that is electron deficient gets a partial positive charge Tro, Chemistry: A Molecular Approach 45

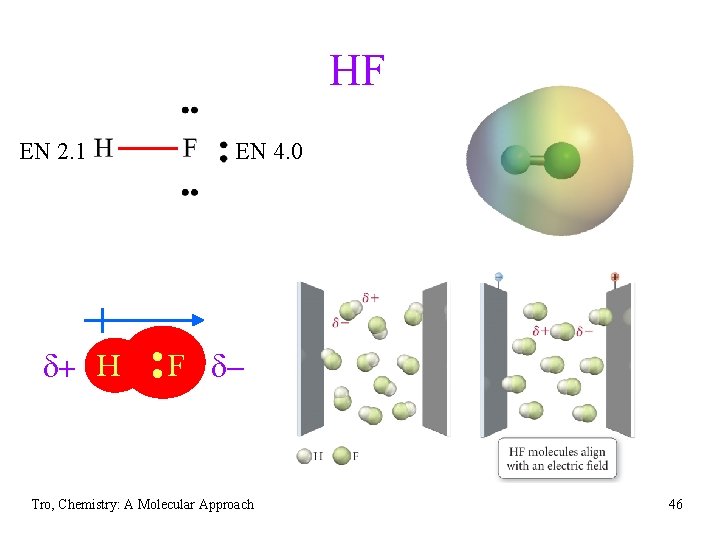
HF EN 2. 1 EN 4. 0 d+ H • • F d Tro, Chemistry: A Molecular Approach 46

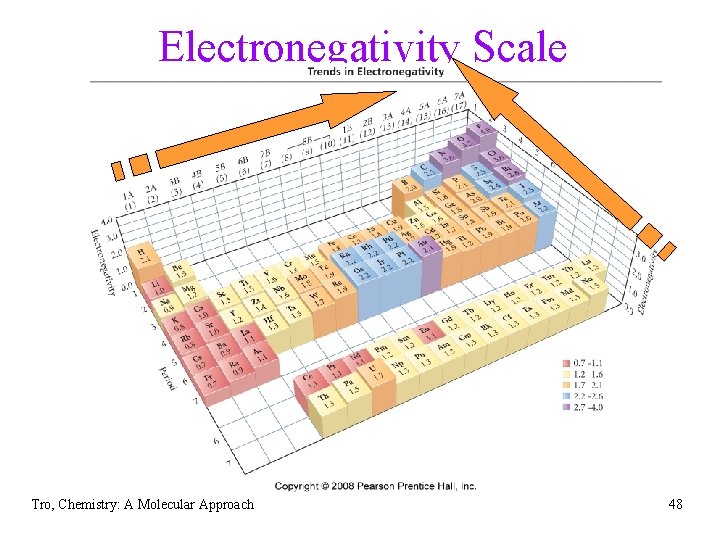
Electronegativity • measure of the pull an atom has on bonding • • electrons increases across period (left to right) and decreases down group (top to bottom) üfluorine is the most electronegative element üfrancium is the least electronegative element • the larger the difference in electronegativity, the more polar the bond ünegative end toward more electronegative atom Tro, Chemistry: A Molecular Approach 47

Electronegativity Scale Tro, Chemistry: A Molecular Approach 48

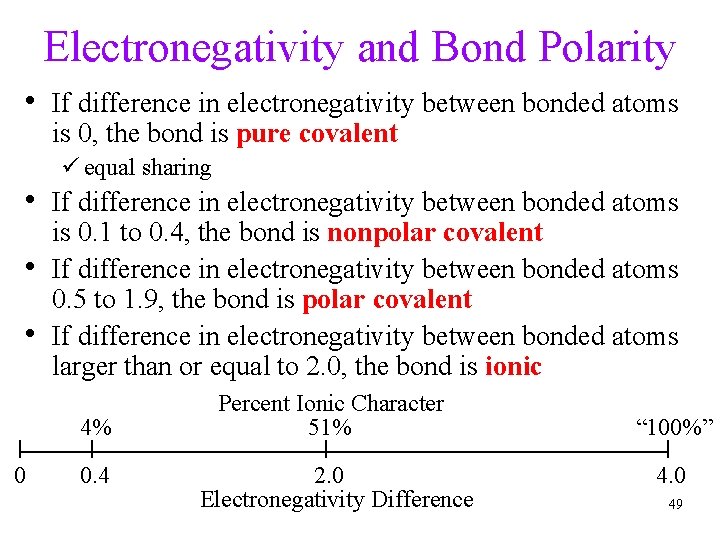
Electronegativity and Bond Polarity • If difference in electronegativity between bonded atoms is 0, the bond is pure covalent ü equal sharing • If difference in electronegativity between bonded atoms • • is 0. 1 to 0. 4, the bond is nonpolar covalent If difference in electronegativity between bonded atoms 0. 5 to 1. 9, the bond is polar covalent If difference in electronegativity between bonded atoms larger than or equal to 2. 0, the bond is ionic 4% 0 0. 4 Percent Ionic Character 51% 2. 0 Electronegativity Difference “ 100%” 4. 0 49

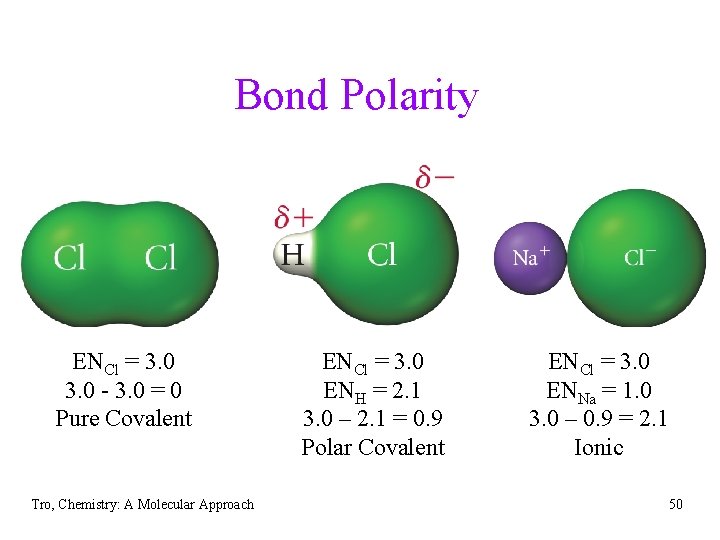
Bond Polarity ENCl = 3. 0 - 3. 0 = 0 Pure Covalent Tro, Chemistry: A Molecular Approach ENCl = 3. 0 ENH = 2. 1 3. 0 – 2. 1 = 0. 9 Polar Covalent ENCl = 3. 0 ENNa = 1. 0 3. 0 – 0. 9 = 2. 1 Ionic 50

Tro, Chemistry: A Molecular Approach 51

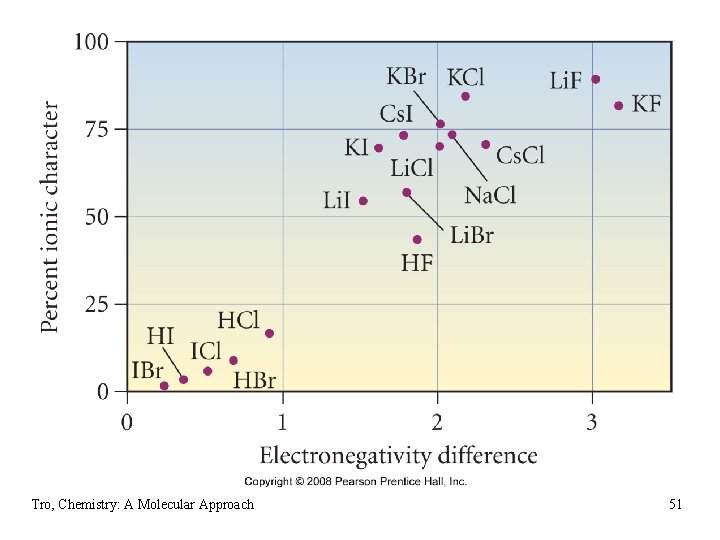
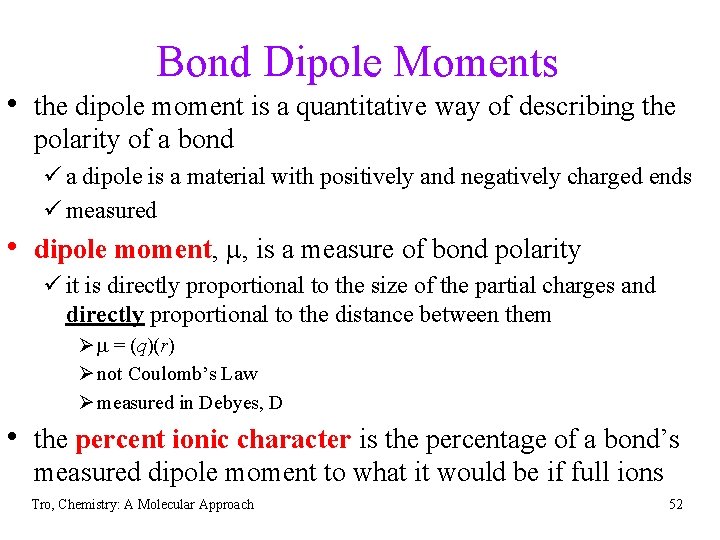
Bond Dipole Moments • the dipole moment is a quantitative way of describing the polarity of a bond ü a dipole is a material with positively and negatively charged ends ü measured • dipole moment, m, is a measure of bond polarity ü it is directly proportional to the size of the partial charges and directly proportional to the distance between them Ø m = (q)(r) Ø not Coulomb’s Law Ø measured in Debyes, D • the percent ionic character is the percentage of a bond’s measured dipole moment to what it would be if full ions Tro, Chemistry: A Molecular Approach 52

Dipole Moments Tro, Chemistry: A Molecular Approach 53

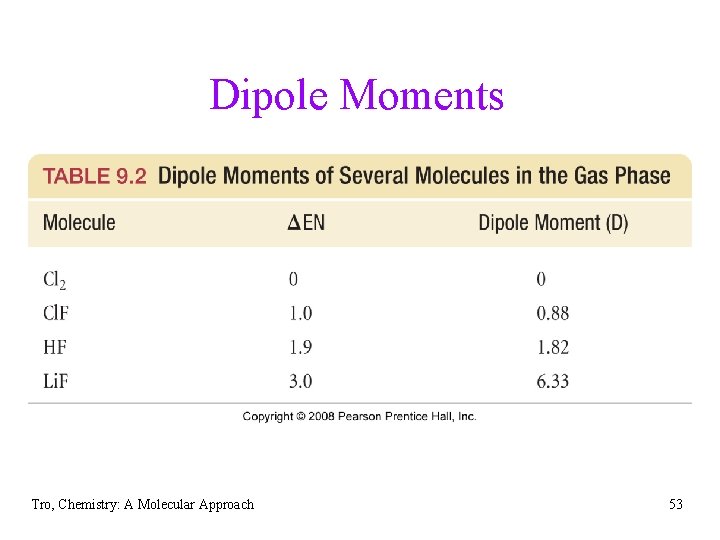
Water – a Polar Molecule stream of water attracted to a charged glass rod Tro, Chemistry: A Molecular Approach stream of hexane not attracted to a charged glass rod 54

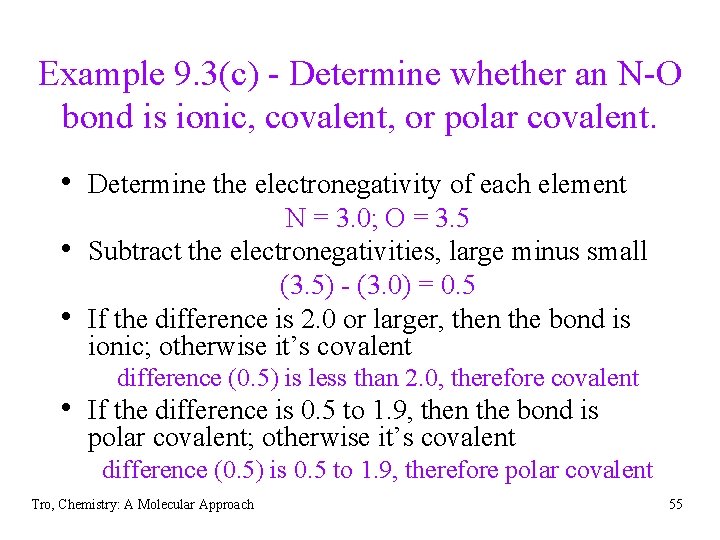
Example 9. 3(c) - Determine whether an N-O bond is ionic, covalent, or polar covalent. • Determine the electronegativity of each element • • N = 3. 0; O = 3. 5 Subtract the electronegativities, large minus small (3. 5) - (3. 0) = 0. 5 If the difference is 2. 0 or larger, then the bond is ionic; otherwise it’s covalent difference (0. 5) is less than 2. 0, therefore covalent • If the difference is 0. 5 to 1. 9, then the bond is polar covalent; otherwise it’s covalent difference (0. 5) is 0. 5 to 1. 9, therefore polar covalent Tro, Chemistry: A Molecular Approach 55

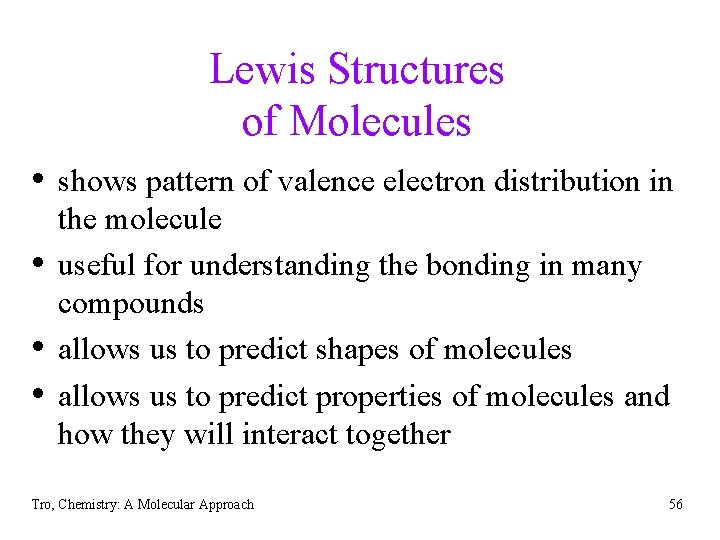
Lewis Structures of Molecules • shows pattern of valence electron distribution in • • • the molecule useful for understanding the bonding in many compounds allows us to predict shapes of molecules allows us to predict properties of molecules and how they will interact together Tro, Chemistry: A Molecular Approach 56

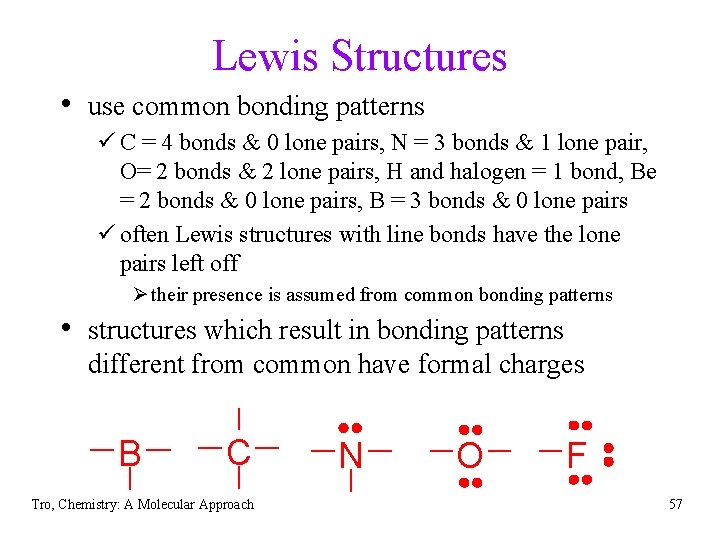
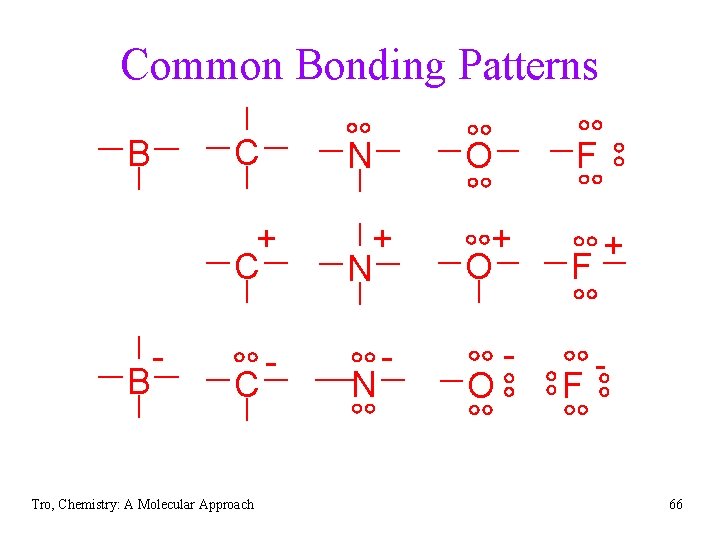
Lewis Structures • use common bonding patterns ü C = 4 bonds & 0 lone pairs, N = 3 bonds & 1 lone pair, O= 2 bonds & 2 lone pairs, H and halogen = 1 bond, Be = 2 bonds & 0 lone pairs, B = 3 bonds & 0 lone pairs ü often Lewis structures with line bonds have the lone pairs left off Ø their presence is assumed from common bonding patterns • structures which result in bonding patterns different from common have formal charges B C Tro, Chemistry: A Molecular Approach N O F 57

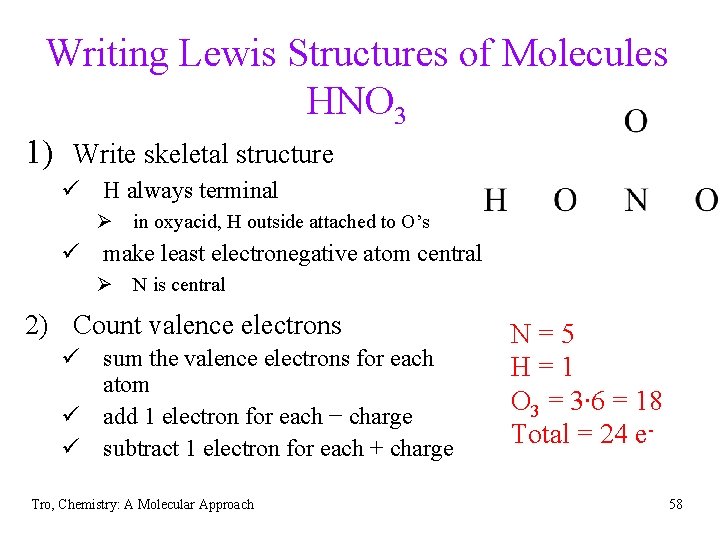
Writing Lewis Structures of Molecules HNO 3 1) Write skeletal structure ü H always terminal Ø in oxyacid, H outside attached to O’s ü make least electronegative atom central Ø N is central 2) Count valence electrons ü sum the valence electrons for each atom ü add 1 electron for each − charge ü subtract 1 electron for each + charge Tro, Chemistry: A Molecular Approach N=5 H=1 O 3 = 3∙ 6 = 18 Total = 24 e 58

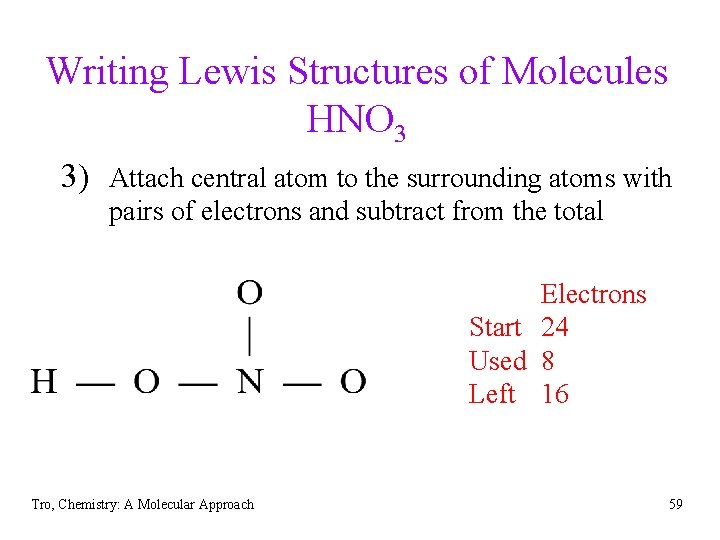
Writing Lewis Structures of Molecules HNO 3 3) Attach central atom to the surrounding atoms with pairs of electrons and subtract from the total Electrons Start 24 Used 8 Left 16 Tro, Chemistry: A Molecular Approach 59

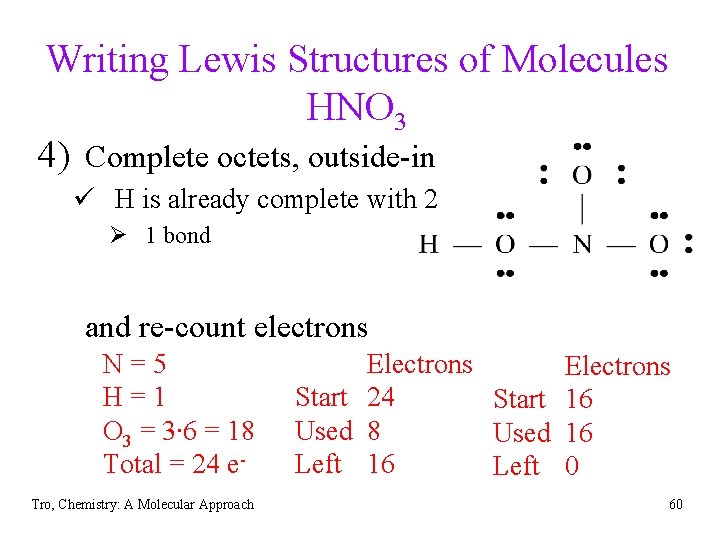
Writing Lewis Structures of Molecules HNO 3 4) Complete octets, outside-in ü H is already complete with 2 Ø 1 bond and re-count electrons N=5 H=1 O 3 = 3∙ 6 = 18 Total = 24 e. Tro, Chemistry: A Molecular Approach Electrons Start 24 Start 16 Used 8 Used 16 Left 0 60

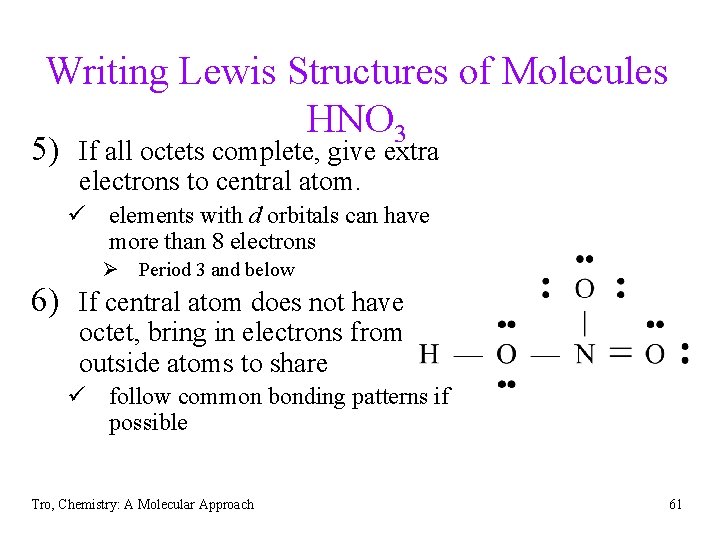
Writing Lewis Structures of Molecules HNO 3 5) If all octets complete, give extra electrons to central atom. ü elements with d orbitals can have more than 8 electrons Ø Period 3 and below 6) If central atom does not have octet, bring in electrons from outside atoms to share ü follow common bonding patterns if possible Tro, Chemistry: A Molecular Approach 61

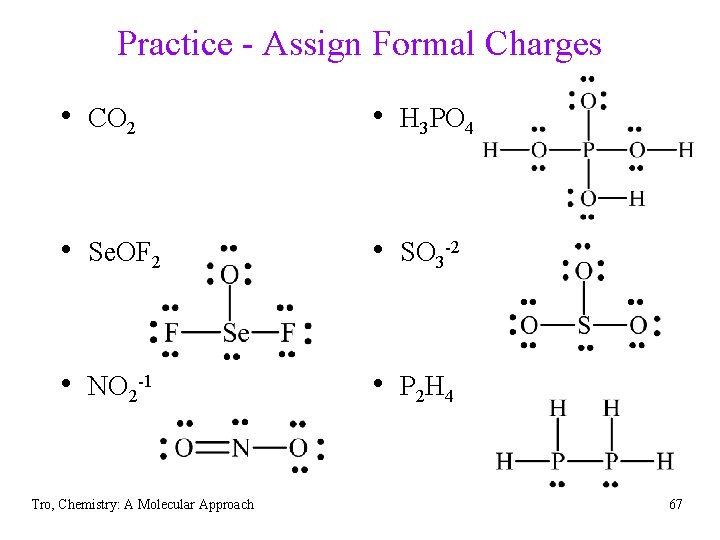
Practice - Lewis Structures • CO 2 • H 3 PO 4 • Se. OF 2 • SO 3 -2 • NO 2 -1 • P 2 H 4 Tro, Chemistry: A Molecular Approach 62

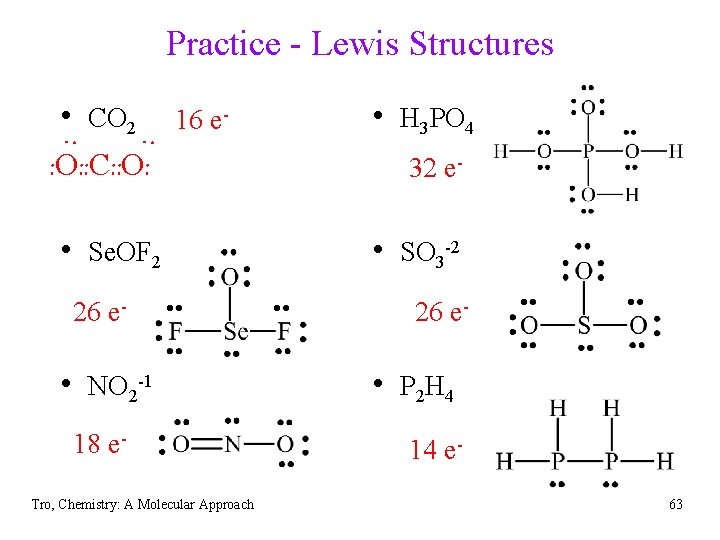
Practice - Lewis Structures • CO 2 16 e- • H 3 PO 4 : : : O: : C: : O: • Se. OF 2 26 e- • NO 2 -1 18 e. Tro, Chemistry: A Molecular Approach 32 e- • SO 3 -2 26 e- • P 2 H 4 14 e 63

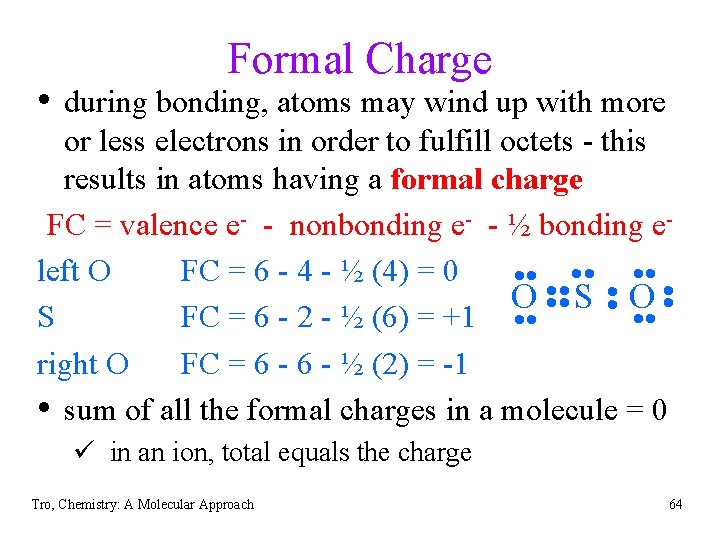
Formal Charge • during bonding, atoms may wind up with more or less electrons in order to fulfill octets - this results in atoms having a formal charge FC = valence e- - nonbonding e- - ½ bonding eleft O FC = 6 - 4 - ½ (4) = 0 • • • • • • O • • S • O • S FC = 6 - 2 - ½ (6) = +1 • • right O FC = 6 - ½ (2) = -1 • sum of all the formal charges in a molecule = 0 ü in an ion, total equals the charge Tro, Chemistry: A Molecular Approach 64

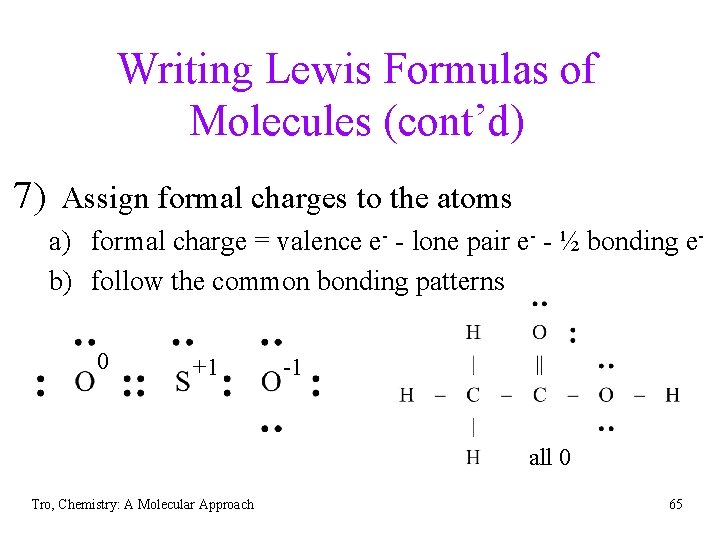
Writing Lewis Formulas of Molecules (cont’d) 7) Assign formal charges to the atoms a) formal charge = valence e- - lone pair e- - ½ bonding eb) follow the common bonding patterns 0 +1 -1 all 0 Tro, Chemistry: A Molecular Approach 65

Common Bonding Patterns B B - C N O + C + N + O - - - C Tro, Chemistry: A Molecular Approach N O F F F + - 66

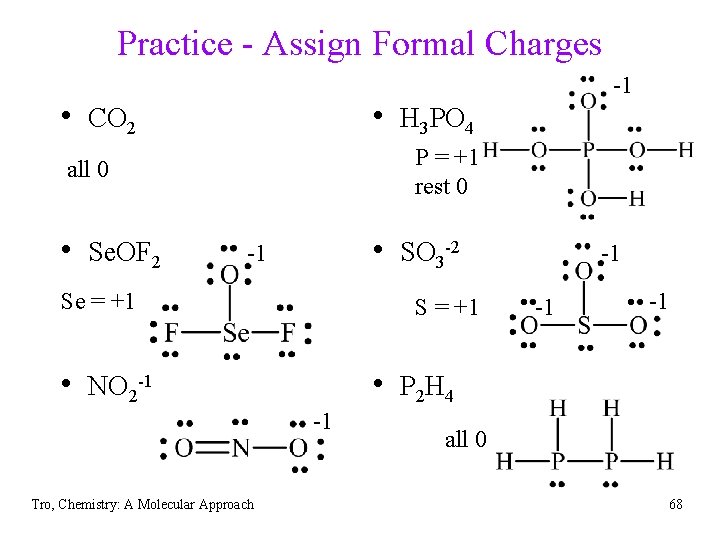
Practice - Assign Formal Charges • CO 2 • H 3 PO 4 • Se. OF 2 • SO 3 -2 • NO 2 -1 • P 2 H 4 Tro, Chemistry: A Molecular Approach 67

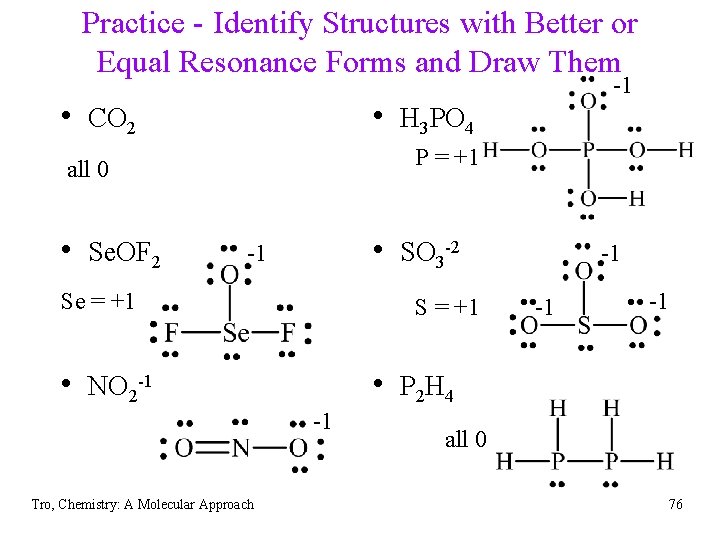
Practice - Assign Formal Charges • CO 2 • H 3 PO 4 P = +1 rest 0 all 0 • Se. OF 2 -1 • SO 3 -2 -1 Se = +1 S = +1 • NO 2 -1 -1 -1 • P 2 H 4 -1 Tro, Chemistry: A Molecular Approach -1 all 0 68

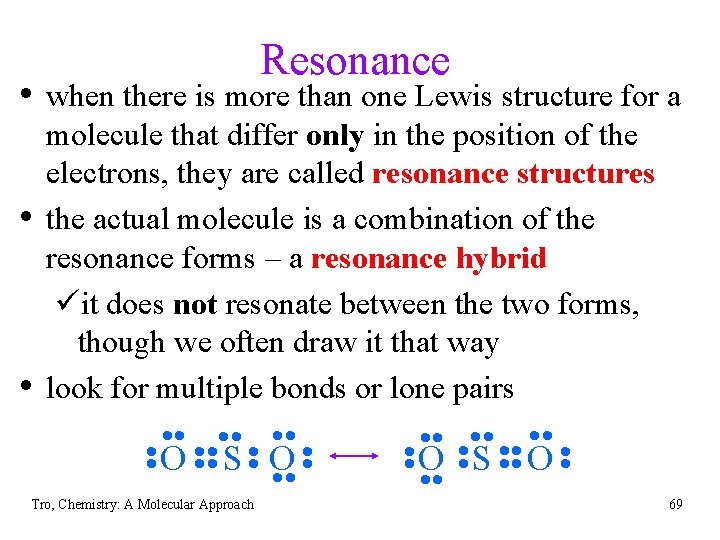
Resonance • when there is more than one Lewis structure for a • • molecule that differ only in the position of the electrons, they are called resonance structures the actual molecule is a combination of the resonance forms – a resonance hybrid üit does not resonate between the two forms, though we often draw it that way look for multiple bonds or lone pairs • • O • • S • • O • • Tro, Chemistry: A Molecular Approach • • O • • S • • O • • 69

Resonance Tro, Chemistry: A Molecular Approach 70

Ozone Layer Tro, Chemistry: A Molecular Approach 71

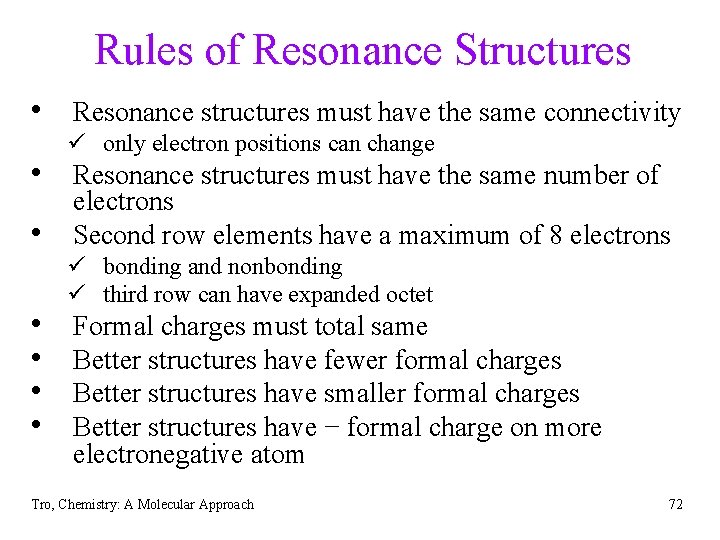
Rules of Resonance Structures • Resonance structures must have the same connectivity ü only electron positions can change • Resonance structures must have the same number of • • • electrons Second row elements have a maximum of 8 electrons ü bonding and nonbonding ü third row can have expanded octet Formal charges must total same Better structures have fewer formal charges Better structures have smaller formal charges Better structures have − formal charge on more electronegative atom Tro, Chemistry: A Molecular Approach 72

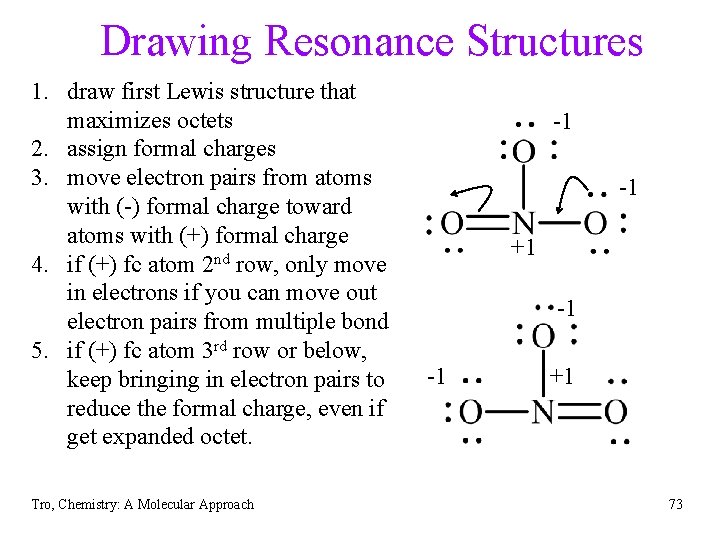
Drawing Resonance Structures 1. draw first Lewis structure that maximizes octets 2. assign formal charges 3. move electron pairs from atoms with (-) formal charge toward atoms with (+) formal charge 4. if (+) fc atom 2 nd row, only move in electrons if you can move out electron pairs from multiple bond 5. if (+) fc atom 3 rd row or below, keep bringing in electron pairs to reduce the formal charge, even if get expanded octet. Tro, Chemistry: A Molecular Approach -1 -1 +1 73

Exceptions to the Octet Rule • expanded octets üelements with empty d orbitals can have more than 8 electrons • odd number electron species e. g. , NO üwill have 1 unpaired electron üfree-radical üvery reactive • incomplete octets üB, Al Tro, Chemistry: A Molecular Approach 74

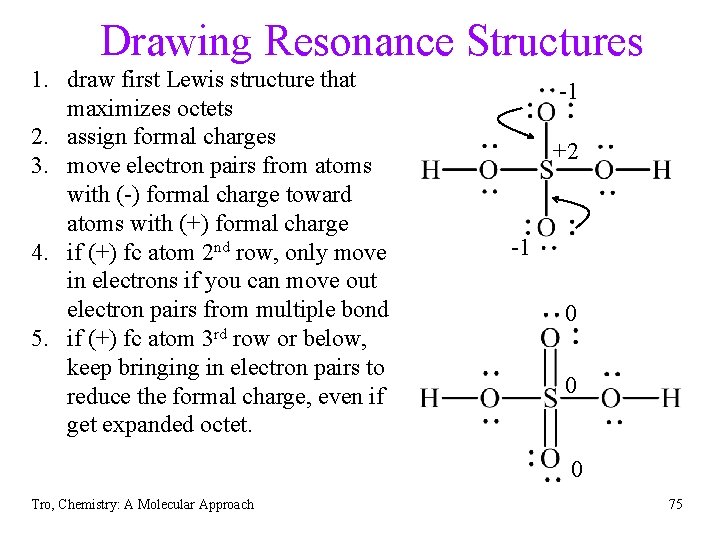
Drawing Resonance Structures 1. draw first Lewis structure that maximizes octets 2. assign formal charges 3. move electron pairs from atoms with (-) formal charge toward atoms with (+) formal charge 4. if (+) fc atom 2 nd row, only move in electrons if you can move out electron pairs from multiple bond 5. if (+) fc atom 3 rd row or below, keep bringing in electron pairs to reduce the formal charge, even if get expanded octet. -1 +2 -1 0 0 0 Tro, Chemistry: A Molecular Approach 75

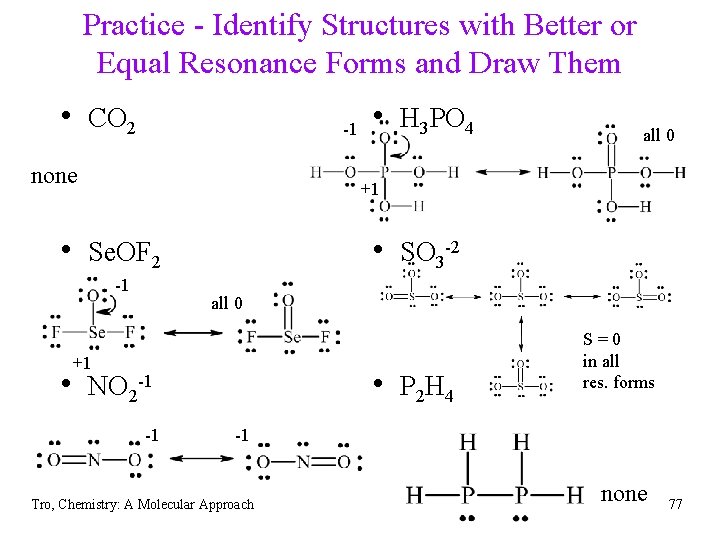
Practice - Identify Structures with Better or Equal Resonance Forms and Draw Them • CO 2 • H 3 PO 4 P = +1 all 0 • Se. OF 2 -1 • SO 3 -2 -1 Se = +1 S = +1 • NO 2 -1 -1 -1 • P 2 H 4 -1 Tro, Chemistry: A Molecular Approach -1 all 0 76

Practice - Identify Structures with Better or Equal Resonance Forms and Draw Them • CO 2 -1 none • H 3 PO 4 all 0 +1 • Se. OF 2 -1 • SO 3 -2 all 0 +1 • NO 2 -1 -1 • P 2 H 4 S=0 in all res. forms -1 Tro, Chemistry: A Molecular Approach none 77

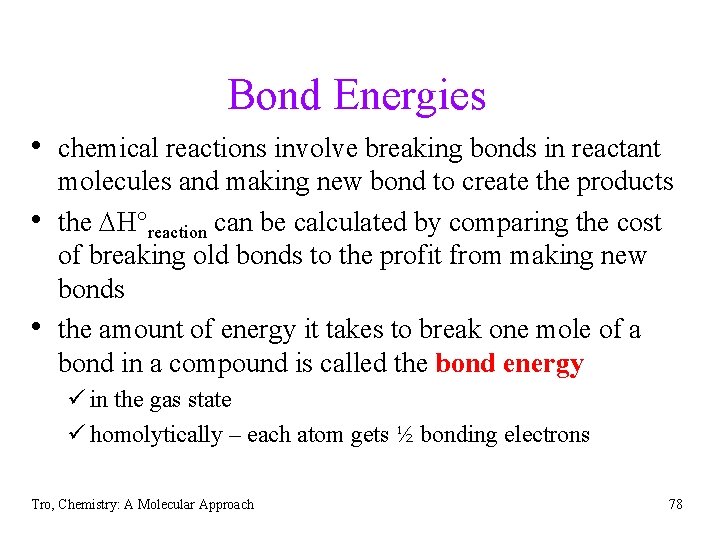
Bond Energies • chemical reactions involve breaking bonds in reactant • • molecules and making new bond to create the products the DH°reaction can be calculated by comparing the cost of breaking old bonds to the profit from making new bonds the amount of energy it takes to break one mole of a bond in a compound is called the bond energy ü in the gas state ü homolytically – each atom gets ½ bonding electrons Tro, Chemistry: A Molecular Approach 78

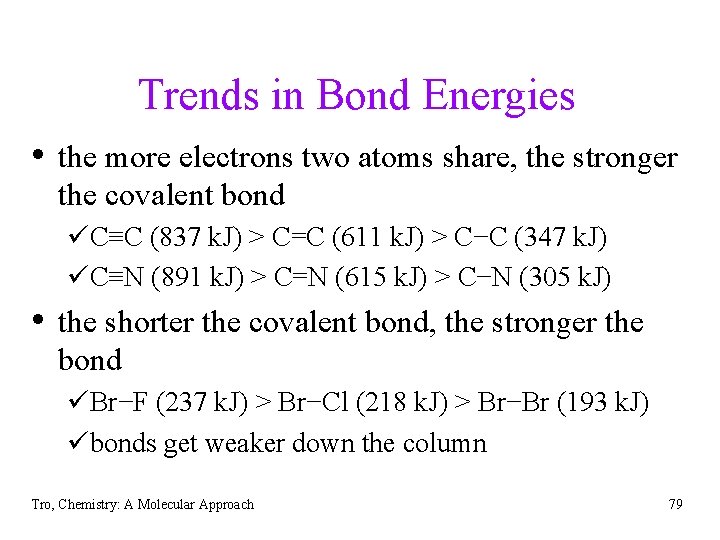
Trends in Bond Energies • the more electrons two atoms share, the stronger the covalent bond üC≡C (837 k. J) > C=C (611 k. J) > C−C (347 k. J) üC≡N (891 k. J) > C=N (615 k. J) > C−N (305 k. J) • the shorter the covalent bond, the stronger the bond üBr−F (237 k. J) > Br−Cl (218 k. J) > Br−Br (193 k. J) übonds get weaker down the column Tro, Chemistry: A Molecular Approach 79

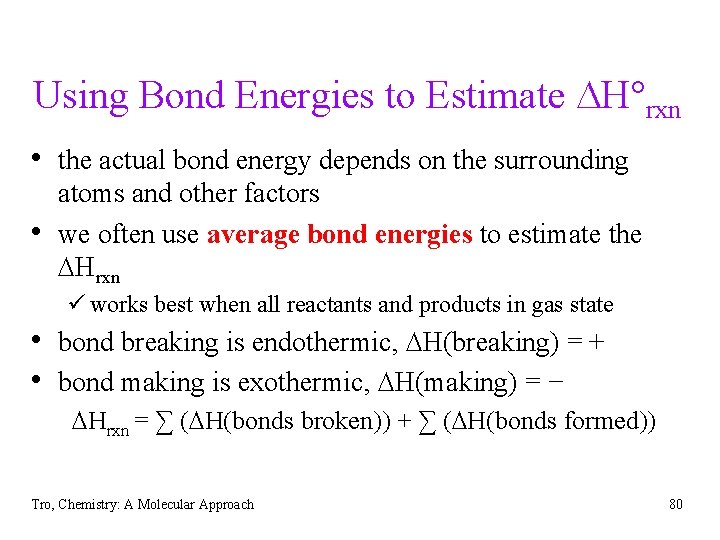
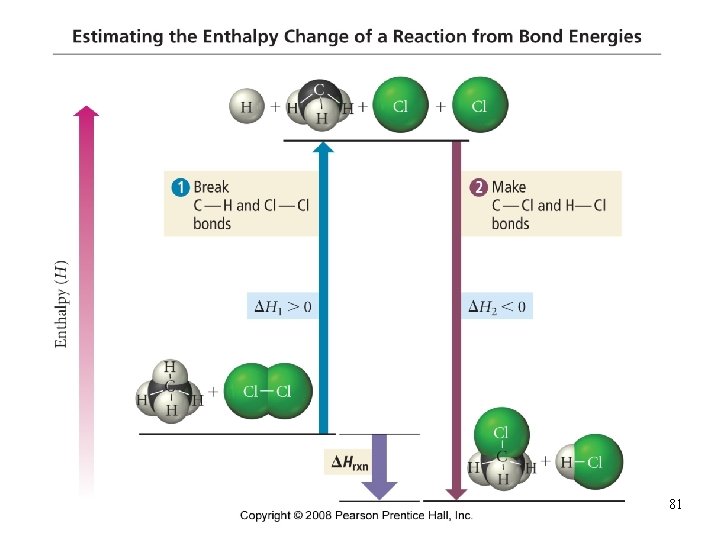
Using Bond Energies to Estimate DH°rxn • the actual bond energy depends on the surrounding • atoms and other factors we often use average bond energies to estimate the DHrxn ü works best when all reactants and products in gas state • bond breaking is endothermic, DH(breaking) = + • bond making is exothermic, DH(making) = − DHrxn = ∑ (DH(bonds broken)) + ∑ (DH(bonds formed)) Tro, Chemistry: A Molecular Approach 80

81

Estimate the Enthalpy of the Following Reaction 82

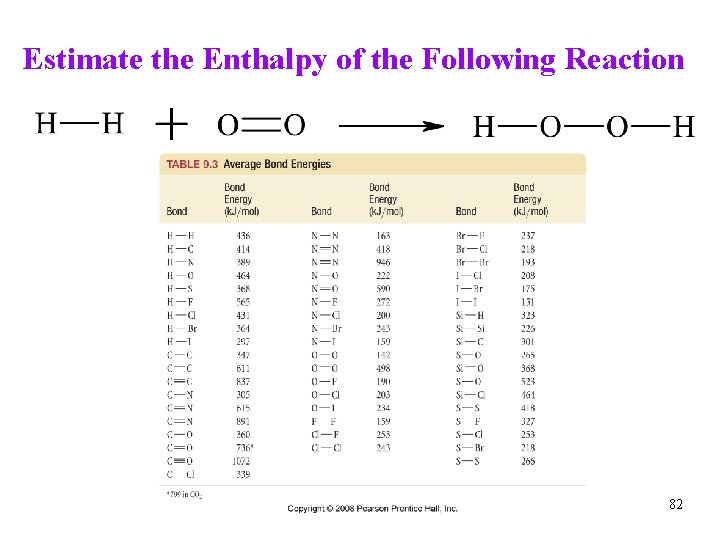
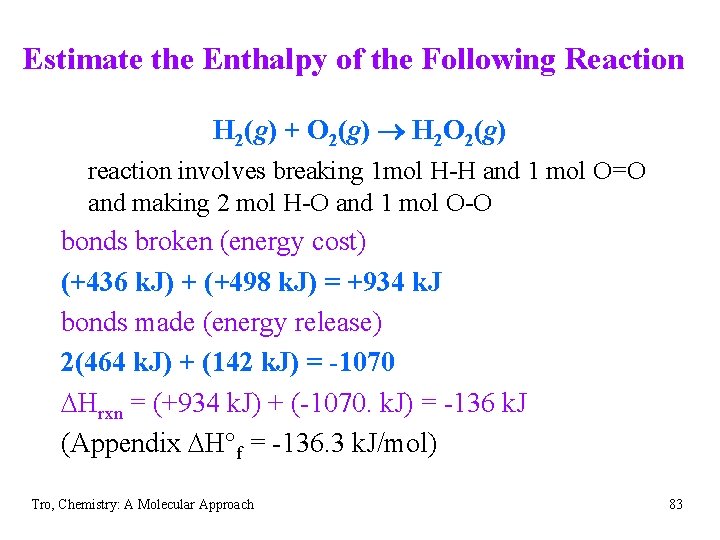
Estimate the Enthalpy of the Following Reaction H 2(g) + O 2(g) ® H 2 O 2(g) reaction involves breaking 1 mol H-H and 1 mol O=O and making 2 mol H-O and 1 mol O-O bonds broken (energy cost) (+436 k. J) + (+498 k. J) = +934 k. J bonds made (energy release) 2(464 k. J) + (142 k. J) = -1070 DHrxn = (+934 k. J) + (-1070. k. J) = -136 k. J (Appendix DH°f = -136. 3 k. J/mol) Tro, Chemistry: A Molecular Approach 83

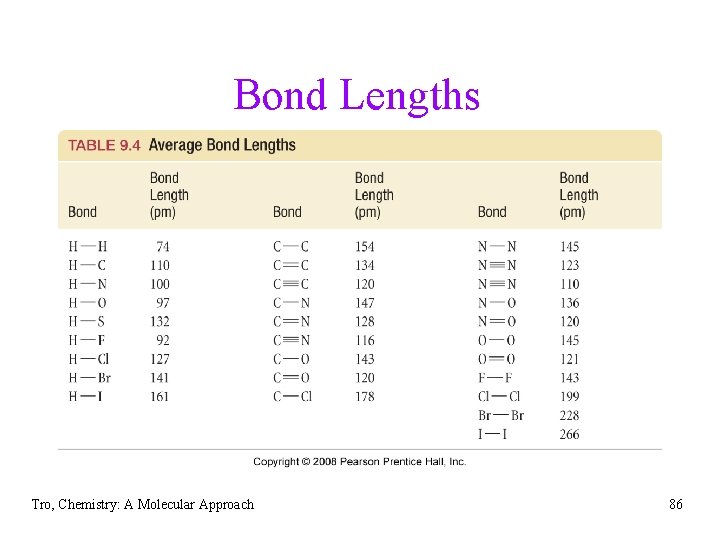
Bond Lengths • the distance between the nuclei of • bonded atoms is called the bond length because the actual bond length depends on the other atoms around the bond we often use the average bond length üaveraged for similar bonds from many compounds Tro, Chemistry: A Molecular Approach 84

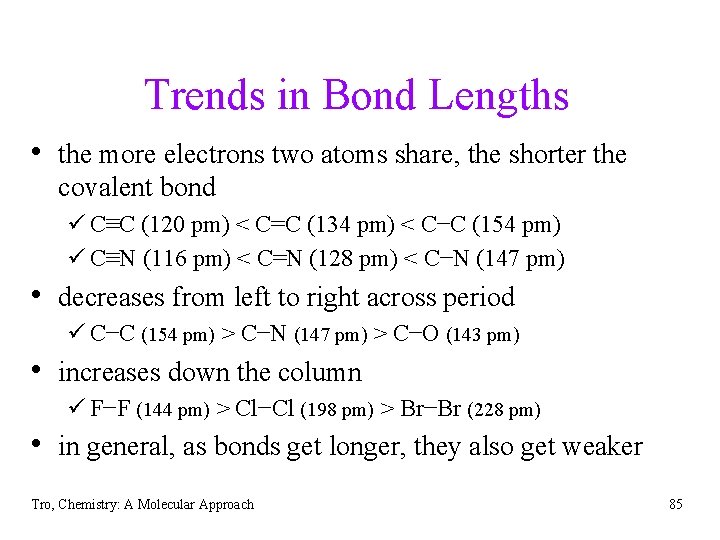
Trends in Bond Lengths • the more electrons two atoms share, the shorter the covalent bond ü C≡C (120 pm) < C=C (134 pm) < C−C (154 pm) ü C≡N (116 pm) < C=N (128 pm) < C−N (147 pm) • decreases from left to right across period ü C−C (154 pm) > C−N (147 pm) > C−O (143 pm) • increases down the column ü F−F (144 pm) > Cl−Cl (198 pm) > Br−Br (228 pm) • in general, as bonds get longer, they also get weaker Tro, Chemistry: A Molecular Approach 85

Bond Lengths Tro, Chemistry: A Molecular Approach 86

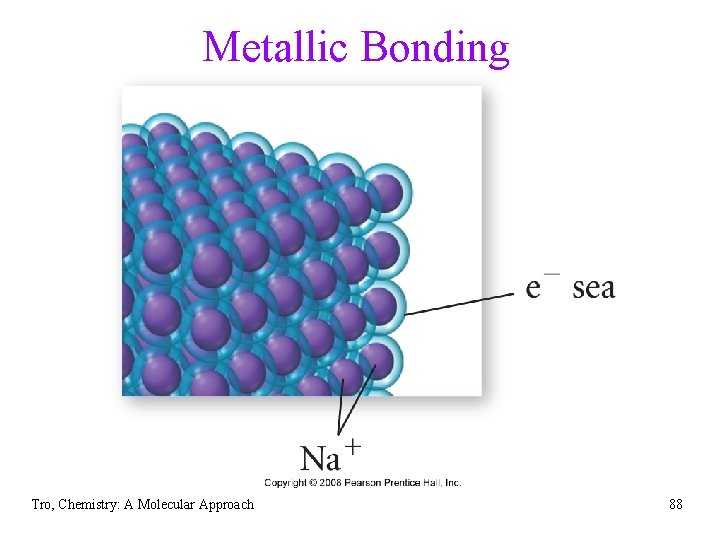
Metallic Bonds • low ionization energy of metals allows them to lose electrons easily • the simplest theory of metallic bonding involves the metals atoms releasing their valence electrons to be shared by all to atoms/ions in the metal üan organization of metal cation islands in a sea of electrons üelectrons delocalized throughout the metal structure • bonding results from attraction of cation for the delocalized electrons Tro, Chemistry: A Molecular Approach 87

Metallic Bonding Tro, Chemistry: A Molecular Approach 88

Metallic Bonding Model vs. Reality • metallic solids conduct electricity • because the free electrons are mobile, it • • allows the electrons to move through the metallic crystal and conduct electricity as temperature increases, electrical conductivity decreases heating causes the metal ions to vibrate faster, making it harder for electrons to make their way through the crystal Tro, Chemistry: A Molecular Approach 89

Metallic Bonding Model vs. Reality • metallic solids conduct heat • the movement of the small, light electrons • • through the solid can transfer kinetic energy quicker than larger particles metallic solids reflect light the mobile electrons on the surface absorb the outside light and then emit it at the same frequency Tro, Chemistry: A Molecular Approach 90

Metallic Bonding Model vs. Reality • metallic solids are malleable and ductile • because the free electrons are mobile, the • direction of the attractive force between the metal cation and free electrons is adjustable this allows the position of the metal cation islands to move around in the sea of electrons without breaking the attractions and the crystal structure Tro, Chemistry: A Molecular Approach 91

Metallic Bonding Model vs. Reality • metals generally have high melting points and boiling points ü all but Hg are solids at room temperature • the attractions of the metal cations for the free electrons • • is strong and hard to overcome melting points generally increase to right across period the charge on the metal cation increases across the period, causing stronger attractions melting points generally decrease down column the cations get larger down the column, resulting in a larger distance from the nucleus to the free electrons Tro, Chemistry: A Molecular Approach 92
 Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro Nivaldo j. tro introductory chemistry
Nivaldo j. tro introductory chemistry Democritus atomic model diagram
Democritus atomic model diagram Covalent bond melting point
Covalent bond melting point Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Ap chemistry molecular geometry
Ap chemistry molecular geometry Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Approach chemistry chalk chapter
Approach chemistry chalk chapter Datagram switching and virtual circuit switching
Datagram switching and virtual circuit switching Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach Waterfall market entry strategy
Waterfall market entry strategy Approach approach conflict
Approach approach conflict Cognitive approach vs behavioral approach
Cognitive approach vs behavioral approach What is research
What is research Diagram for traditional approach
Diagram for traditional approach Tony wagner's seven survival skills
Tony wagner's seven survival skills Formula of vsepr theory
Formula of vsepr theory Chemistry geometry
Chemistry geometry Pf3 number of vsepr electron groups
Pf3 number of vsepr electron groups Crash course molecular biology
Crash course molecular biology Molecular level vs cellular level
Molecular level vs cellular level Molecular absorption
Molecular absorption Ab6 molecular geometry
Ab6 molecular geometry Kmt law
Kmt law Properties of network covalent solids
Properties of network covalent solids Kinetic molecular theory of solids
Kinetic molecular theory of solids Significance of chemical formula
Significance of chemical formula Clasificacion molecular cancer de endometrio
Clasificacion molecular cancer de endometrio Unit chemical bonding molecular geometry
Unit chemical bonding molecular geometry 5 examples of palindromic dna sequences
5 examples of palindromic dna sequences Molecular rebar design
Molecular rebar design Covalent molecular substances
Covalent molecular substances Empirical formula of haemoglobin
Empirical formula of haemoglobin Number molecular weight
Number molecular weight Dicots
Dicots Percent composition of magnesium nitrate
Percent composition of magnesium nitrate Molecular mass of potassium permanganate
Molecular mass of potassium permanganate Potassium permanganate percent composition
Potassium permanganate percent composition How to find empirical formula from percentages
How to find empirical formula from percentages Molecular modelling laboratory
Molecular modelling laboratory Molecular replacement method
Molecular replacement method Condensed structural formula of alkenes
Condensed structural formula of alkenes Naming compounds and writing formulas
Naming compounds and writing formulas Binary molecule
Binary molecule N srinivasan iisc passed away
N srinivasan iisc passed away Relationship between bond dipoles and molecular dipoles
Relationship between bond dipoles and molecular dipoles Number-average molecular weight
Number-average molecular weight Molecular shape for ch2o
Molecular shape for ch2o Kahoot shapes
Kahoot shapes Molecular shapes polar or nonpolar
Molecular shapes polar or nonpolar Molecular replacement method
Molecular replacement method Patterson
Patterson Nh3 mo diagram
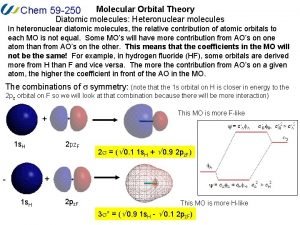
Nh3 mo diagram Heteronuclear mo diagram
Heteronuclear mo diagram B2 molecular orbital diagram
B2 molecular orbital diagram Molecular response
Molecular response Hnhhh
Hnhhh Molecular microbiology definition
Molecular microbiology definition 4 electron domains 2 lone pairs
4 electron domains 2 lone pairs Molecular geometry pogil
Molecular geometry pogil Lewis structure for pf3
Lewis structure for pf3 Scl molecular geometry
Scl molecular geometry Molecular geometry and bonding theories
Molecular geometry and bonding theories What is a bond order in chemistry
What is a bond order in chemistry Molecular gastronomy restaurants
Molecular gastronomy restaurants Define molecular formula
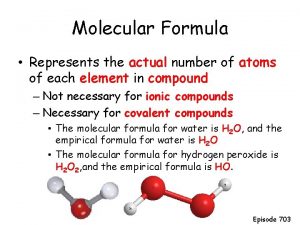
Define molecular formula Molecular farming definition
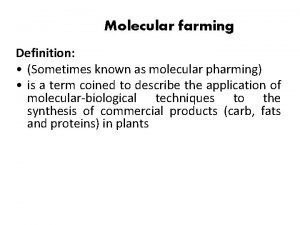
Molecular farming definition Molecular ecological network analyses
Molecular ecological network analyses Grams to molecules
Grams to molecules Concentration moles equation
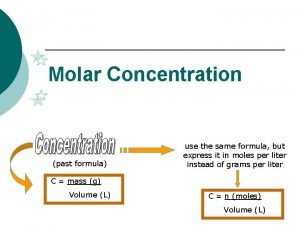
Concentration moles equation Cintico
Cintico Osometer
Osometer Molecula de sales minerales
Molecula de sales minerales Molecular dynamics limitations
Molecular dynamics limitations Cocl2 molecular geometry
Cocl2 molecular geometry Lewis dot structure trigonal pyramidal
Lewis dot structure trigonal pyramidal Lentils examples
Lentils examples Kinetic molecular theory of gases
Kinetic molecular theory of gases Molecular theory of gases and liquids
Molecular theory of gases and liquids The kinetic molecular theory
The kinetic molecular theory Nocl molecular shape
Nocl molecular shape Application of uv visible spectroscopy
Application of uv visible spectroscopy Molecular replacement method
Molecular replacement method Covalent network solid vs molecular solid
Covalent network solid vs molecular solid Bio molecular systems ltd
Bio molecular systems ltd Icl2 molecular geometry
Icl2 molecular geometry Section 14-3 human molecular genetics
Section 14-3 human molecular genetics History of molecular gastronomy
History of molecular gastronomy