Empirical and Molecular Formulas Chemical Compounds Unit Notes









- Slides: 14

Empirical and Molecular Formulas Chemical Compounds Unit Notes #5

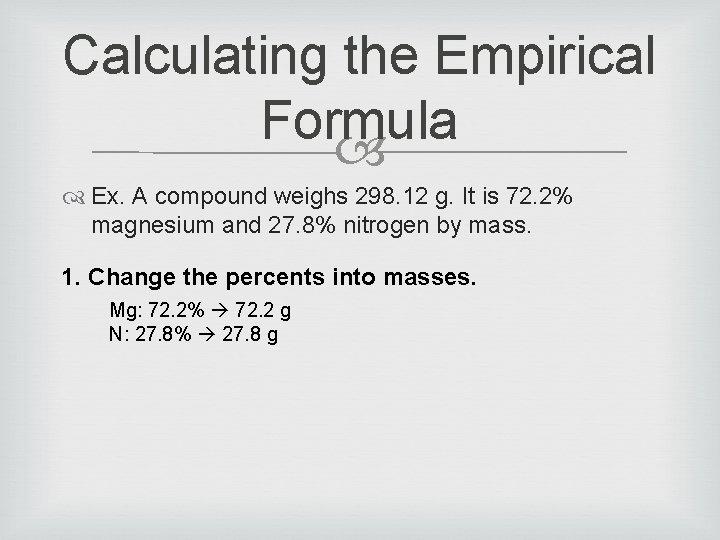
Writing a Formula What if we wanted to be able to give a name/formula to an unknown compound, based on data? Ex. A compound weighs 298. 12 g. It is 72. 2% magnesium and 27. 8% nitrogen by mass. How could we determine its formula?

Empirical Formula A compound’s empirical formula is the smallest whole number ratio of atoms in the compound. The ratio is shown in the subscripts. Ex) NO 2 vs. N 2 O 2 It does not necessarily tell us how many atoms are in the compound.

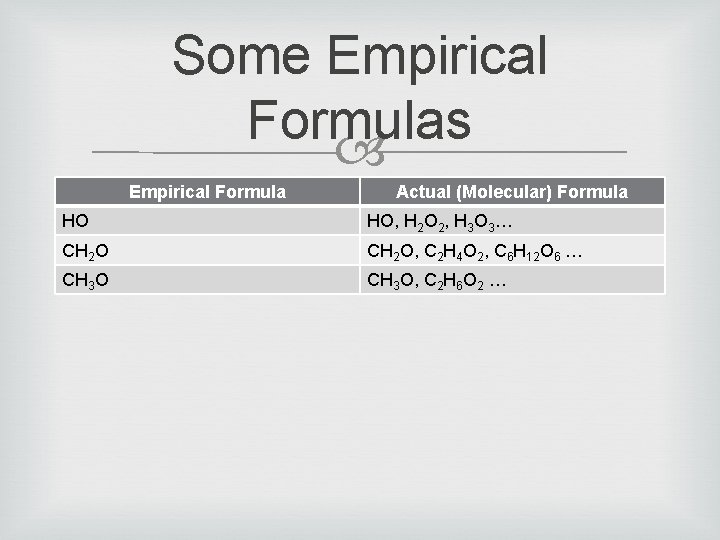
Some Empirical Formulas Empirical Formula Actual (Molecular) Formula HO HO, H 2 O 2, H 3 O 3… CH 2 O, C 2 H 4 O 2, C 6 H 12 O 6 … CH 3 O, C 2 H 6 O 2 …

Calculating the Empirical Formula Ex. A compound weighs 298. 12 g. It is 72. 2% magnesium and 27. 8% nitrogen by mass. 1. Change the percents into masses. Mg: 72. 2% 72. 2 g N: 27. 8% 27. 8 g

Calculating the Empirical Formula 2. Find the moles of each element. Mg: 72. 2 g x 1 mole = 2. 97 moles 24. 3 g N: 27. 8 g x 1 mole = 1. 97 moles 14. 01 g

Calculating the Empirical Formula 3. Divide both numbers of moles by the smaller number. We have 2. 97 moles Mg and 1. 97 moles N Mg : 2. 97 moles / 1. 97 moles = 1. 50 N: 1. 97 moles / 1. 97 moles = 1. 00

Calculating the Empirical Formula 4. Multiply both quotients by the same number until they are both whole numbers. Mg : 1. 50 x 2 = 3. 00 N: 1. 00 x 2 = 2. 00 5. The final answers become the subscripts in the empirical formula. Mg 3 N 2

Try it! A sample is 25. 9% nitrogen and 74. 1% oxygen. 1. 2. 3. 4. 5. In grams: In moles: Divided: Multiplied: Final: 25. 9 g N; 74. 1 g O 1. 85 moles N; 4. 63 moles O N: 1. 00; O: 2. 50 N: 1. 00 x 2 = 2. 00; O: 2. 50 x 2 = 5. 00 N 2 O 5

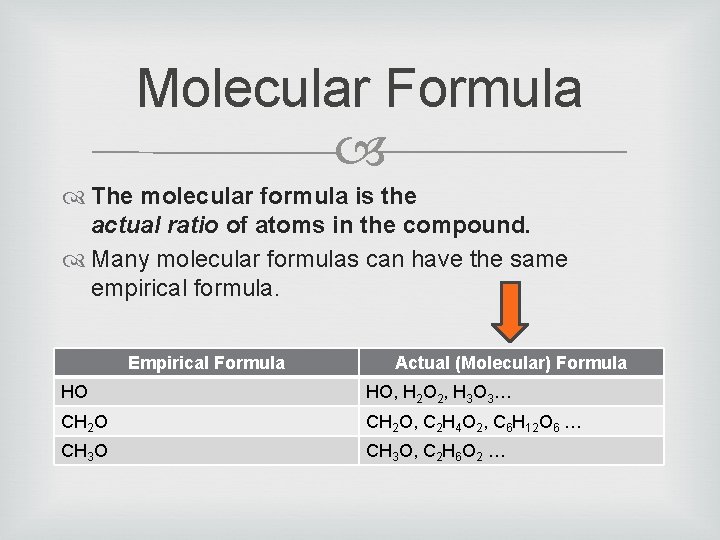
Molecular Formula The molecular formula is the actual ratio of atoms in the compound. Many molecular formulas can have the same empirical formula. Empirical Formula Actual (Molecular) Formula HO HO, H 2 O 2, H 3 O 3… CH 2 O, C 2 H 4 O 2, C 6 H 12 O 6 … CH 3 O, C 2 H 6 O 2 …

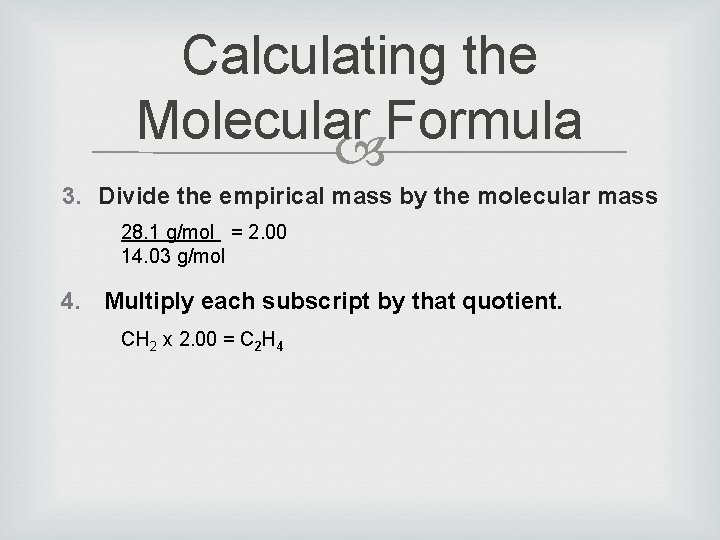
Calculating the Molecular Formula A compound has the empirical formula CH 2 and a molecular mass of 28. 1 g/mol.

Calculating the Molecular Formula 1. Find the empirical formula CH 2 2. Find the molar mass of the empirical formula 1(12. 01) + 2(1. 01) = 14. 03 g/mol

Calculating the Molecular Formula 3. Divide the empirical mass by the molecular mass 28. 1 g/mol = 2. 00 14. 03 g/mol 4. Multiply each subscript by that quotient. CH 2 x 2. 00 = C 2 H 4

Try it! A sample is 25. 9% nitrogen and 74. 1% oxygen, and has a molecular mass of 216 g/mol. 1. Empirical formula: 2. Empirical mass: 3. Molecular mass divided by empirical mass: 4. Subscripts multiplied: N 2 O 5 108 g/mol 216 / 108 = 2 (N 2 O 5) * 2 = N 2 O 5