Chapter 9 Molecular Geometry and Bonding Theories Lewis
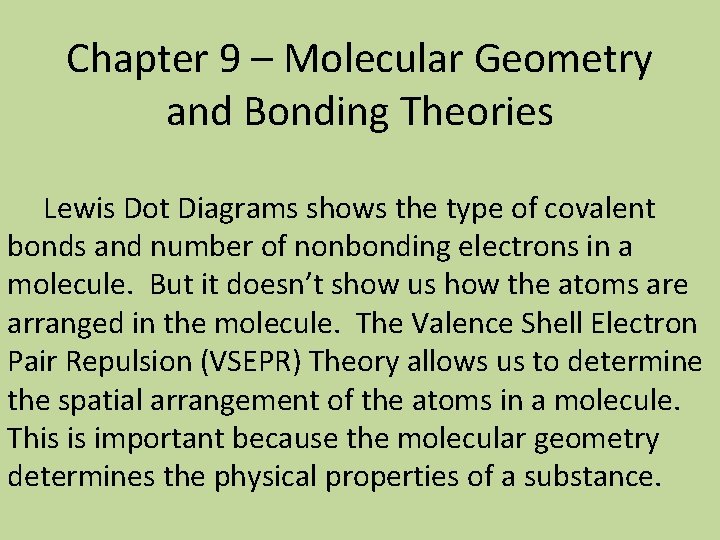
Chapter 9 – Molecular Geometry and Bonding Theories Lewis Dot Diagrams shows the type of covalent bonds and number of nonbonding electrons in a molecule. But it doesn’t show us how the atoms are arranged in the molecule. The Valence Shell Electron Pair Repulsion (VSEPR) Theory allows us to determine the spatial arrangement of the atoms in a molecule. This is important because the molecular geometry determines the physical properties of a substance.
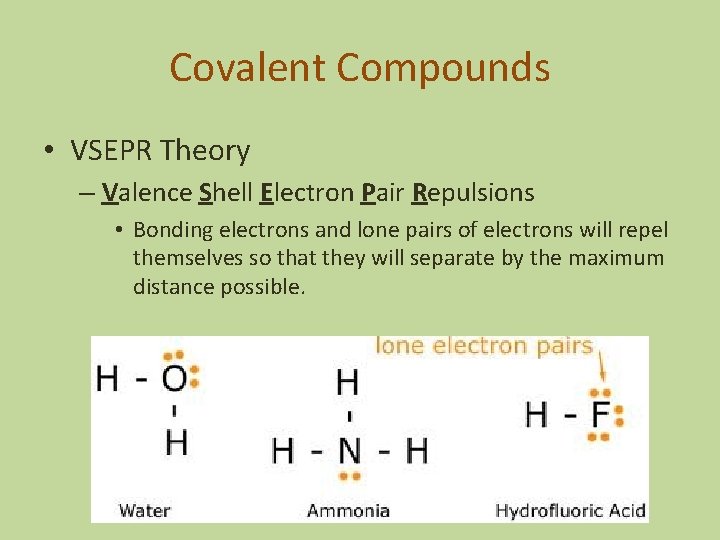
Covalent Compounds • VSEPR Theory – Valence Shell Electron Pair Repulsions • Bonding electrons and lone pairs of electrons will repel themselves so that they will separate by the maximum distance possible.
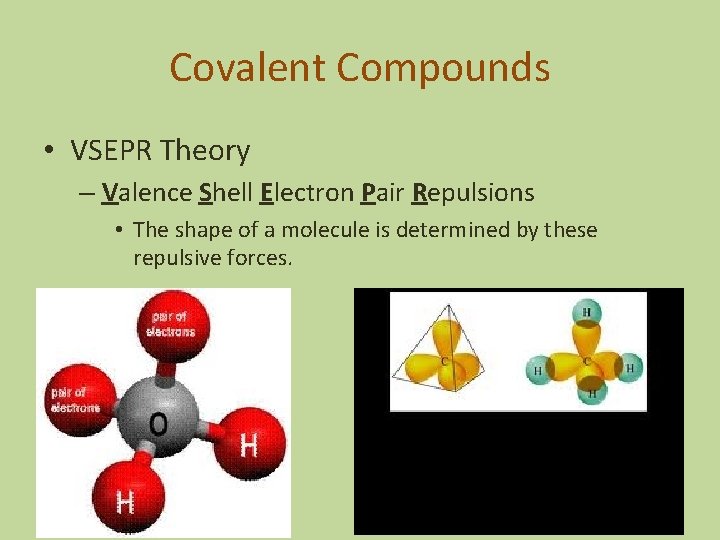
Covalent Compounds • VSEPR Theory – Valence Shell Electron Pair Repulsions • The shape of a molecule is determined by these repulsive forces.
Covalent Compounds • Molecular Shapes – The geometry of most molecules can be explained using 9 geometric shapes • • • Linear Trigonal Planar Tetrahedral Trigonal Bipyrimidal See-Saw T-Shaped Square Planar Square Pyramidal Octahedral
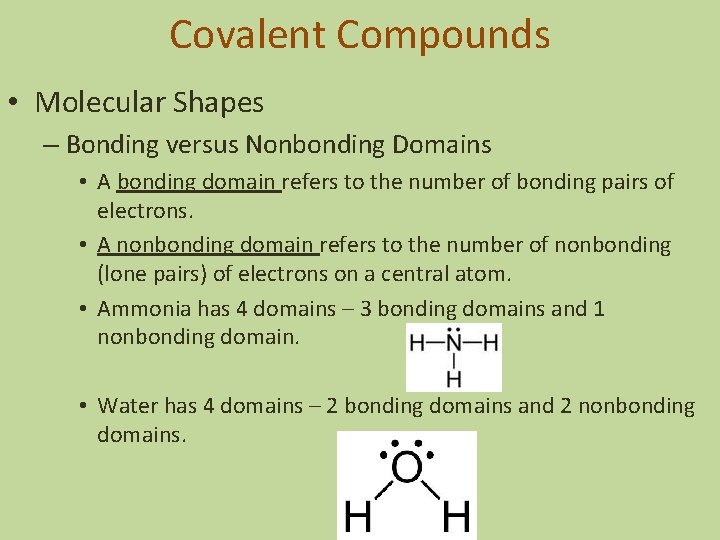
Covalent Compounds • Molecular Shapes – Bonding versus Nonbonding Domains • A bonding domain refers to the number of bonding pairs of electrons. • A nonbonding domain refers to the number of nonbonding (lone pairs) of electrons on a central atom. • Ammonia has 4 domains – 3 bonding domains and 1 nonbonding domain. • Water has 4 domains – 2 bonding domains and 2 nonbonding domains.
Covalent Compounds • Molecular Shapes – Bonding Domains How many domains does CO 2 have? How many domains does SO 3 have?
Covalent Compounds • Molecular Shapes – Electron Domain Geometry • Predicts the shapes of molecules based on the geometric arrangement of electrons. • This method allows us to predict the shapes of many molecules.
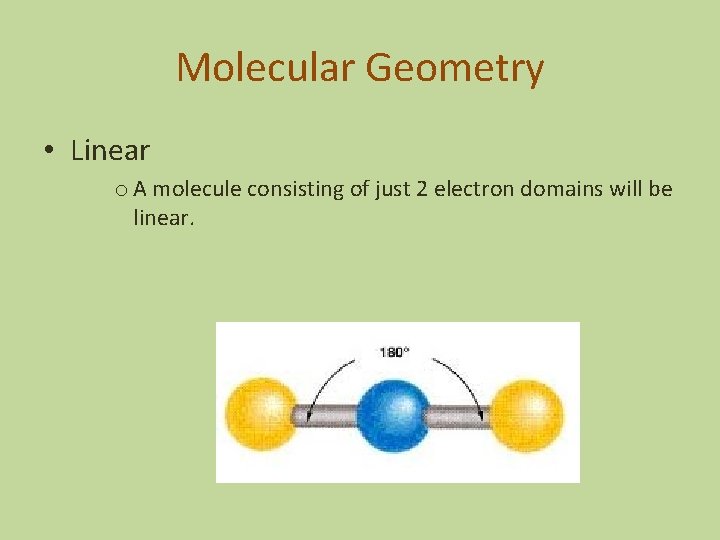
Molecular Geometry • Linear o A molecule consisting of just 2 electron domains will be linear.
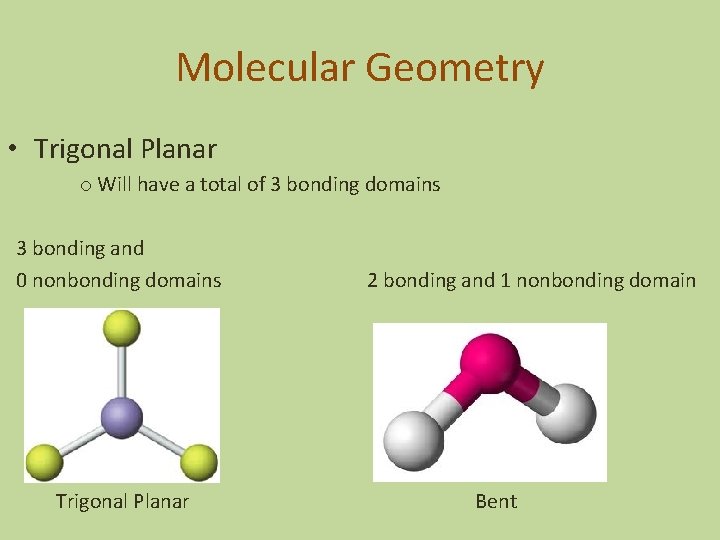
Molecular Geometry • Trigonal Planar o Will have a total of 3 bonding domains 3 bonding and 0 nonbonding domains Trigonal Planar 2 bonding and 1 nonbonding domain Bent
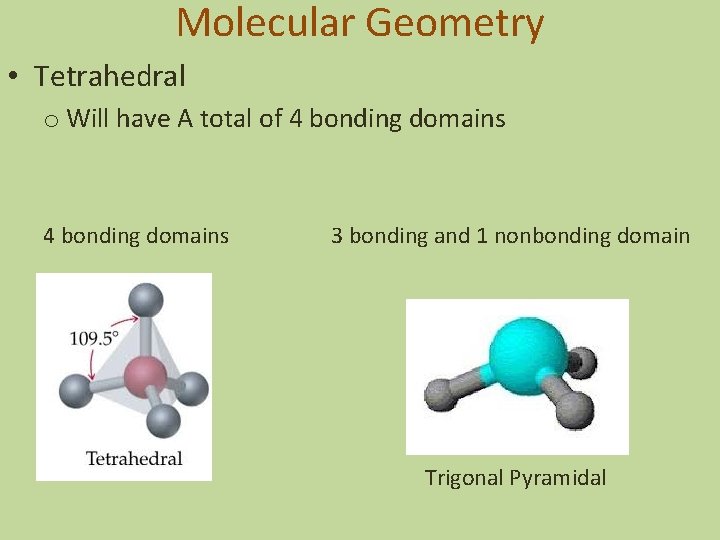
Molecular Geometry • Tetrahedral o Will have A total of 4 bonding domains 3 bonding and 1 nonbonding domain Trigonal Pyramidal
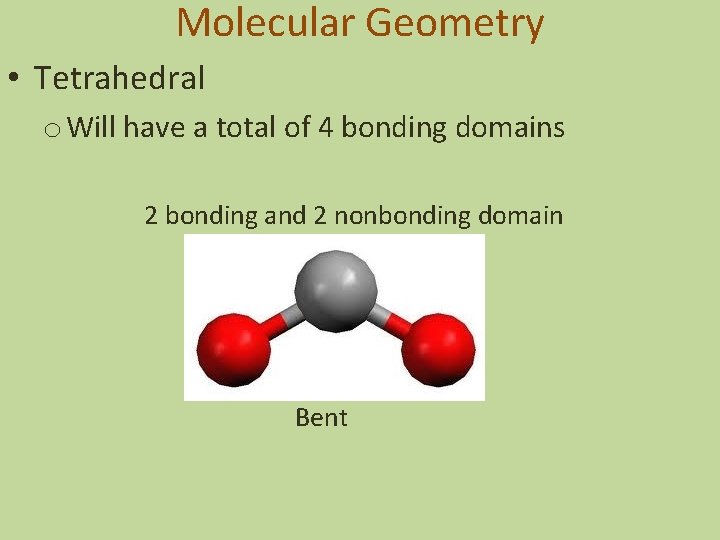
Molecular Geometry • Tetrahedral o Will have a total of 4 bonding domains 2 bonding and 2 nonbonding domain Bent
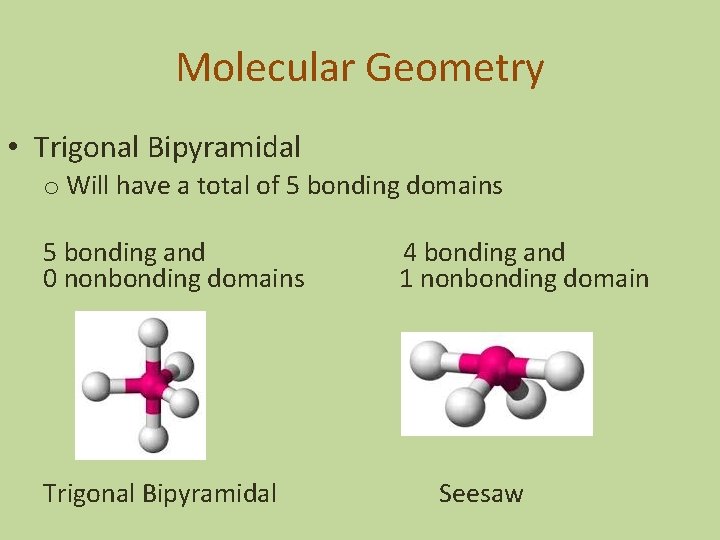
Molecular Geometry • Trigonal Bipyramidal o Will have a total of 5 bonding domains 5 bonding and 0 nonbonding domains Trigonal Bipyramidal 4 bonding and 1 nonbonding domain Seesaw
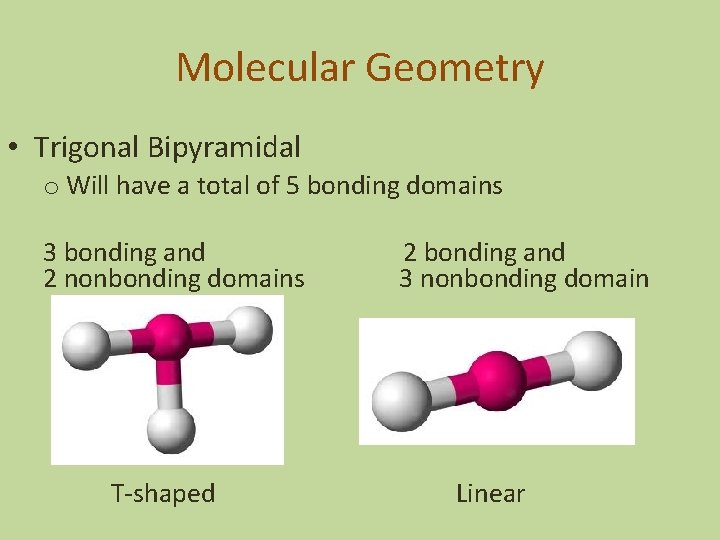
Molecular Geometry • Trigonal Bipyramidal o Will have a total of 5 bonding domains 3 bonding and 2 nonbonding domains T-shaped 2 bonding and 3 nonbonding domain Linear
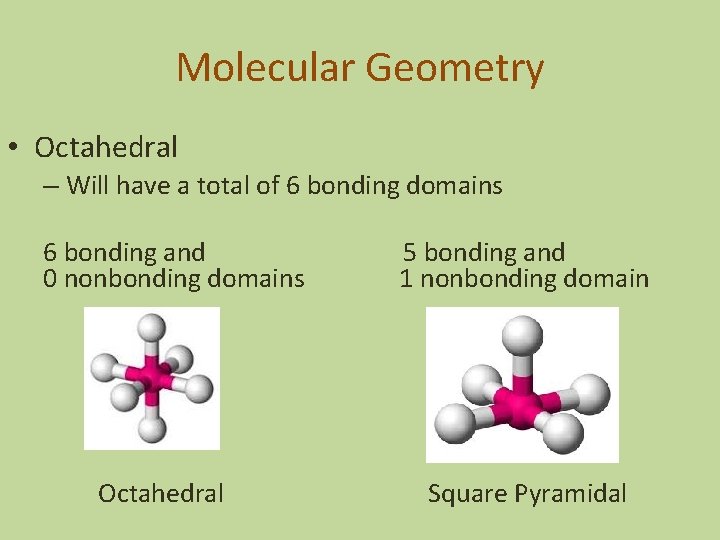
Molecular Geometry • Octahedral – Will have a total of 6 bonding domains 6 bonding and 0 nonbonding domains Octahedral 5 bonding and 1 nonbonding domain Square Pyramidal
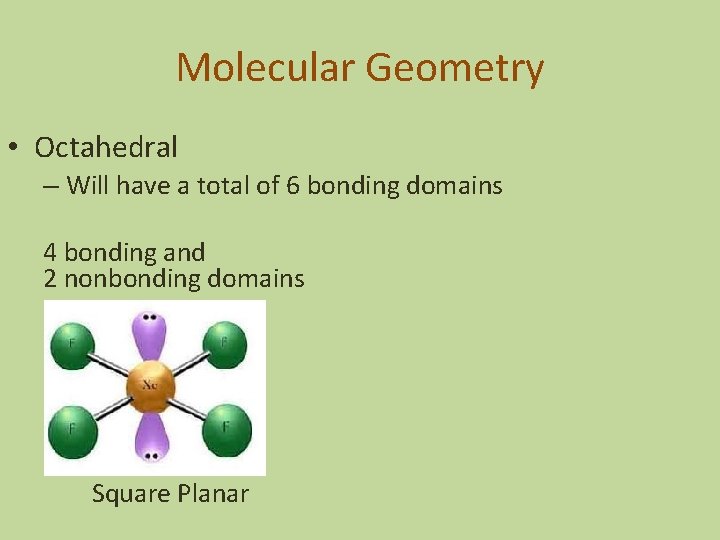
Molecular Geometry • Octahedral – Will have a total of 6 bonding domains 4 bonding and 2 nonbonding domains Square Planar
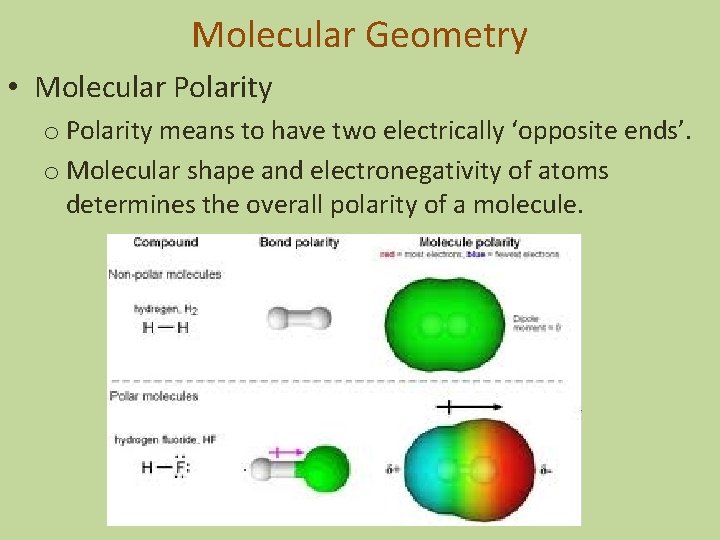
Molecular Geometry • Molecular Polarity o Polarity means to have two electrically ‘opposite ends’. o Molecular shape and electronegativity of atoms determines the overall polarity of a molecule.
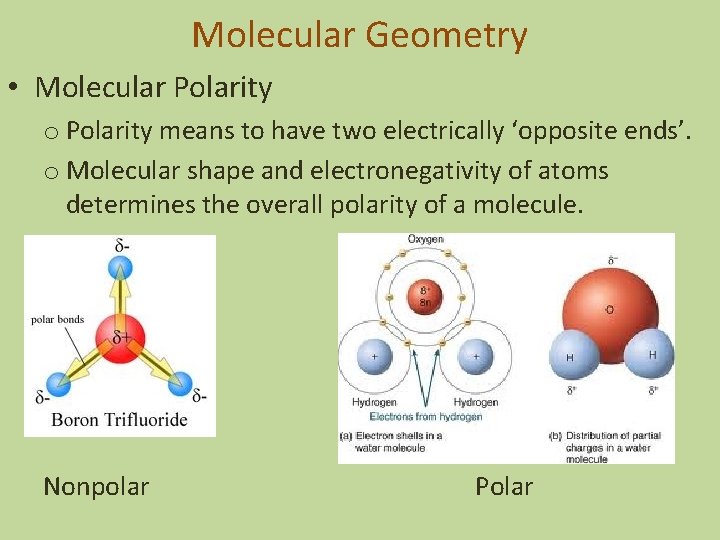
Molecular Geometry • Molecular Polarity o Polarity means to have two electrically ‘opposite ends’. o Molecular shape and electronegativity of atoms determines the overall polarity of a molecule. Nonpolar Polar
Molecular Geometry • Molecular Polarity o Identify the following substances as either polar or nonpolar; o SO 2 o SF 6 o NF 3 o BCl 3
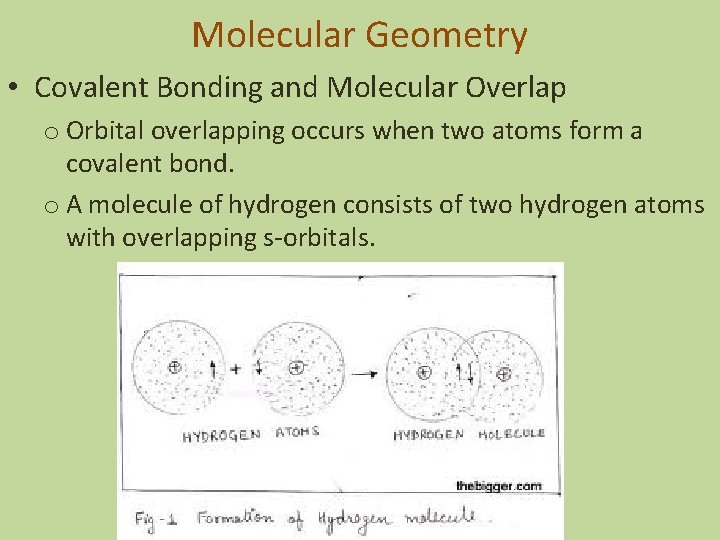
Molecular Geometry • Covalent Bonding and Molecular Overlap o Orbital overlapping occurs when two atoms form a covalent bond. o A molecule of hydrogen consists of two hydrogen atoms with overlapping s-orbitals.
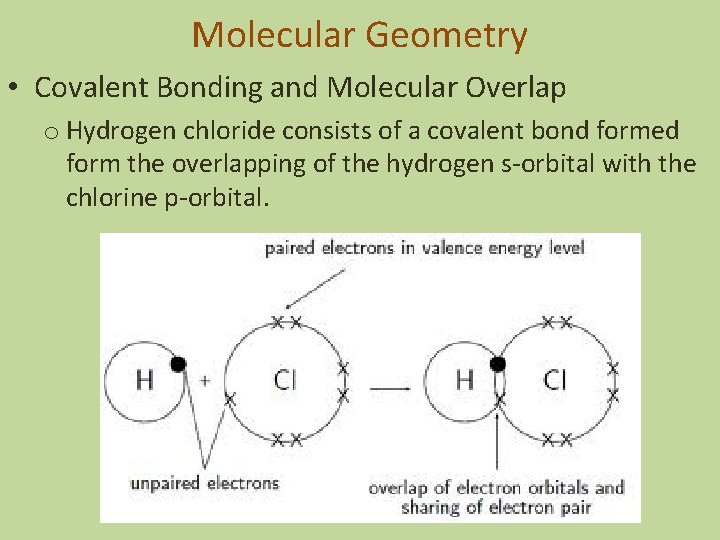
Molecular Geometry • Covalent Bonding and Molecular Overlap o Hydrogen chloride consists of a covalent bond formed form the overlapping of the hydrogen s-orbital with the chlorine p-orbital.
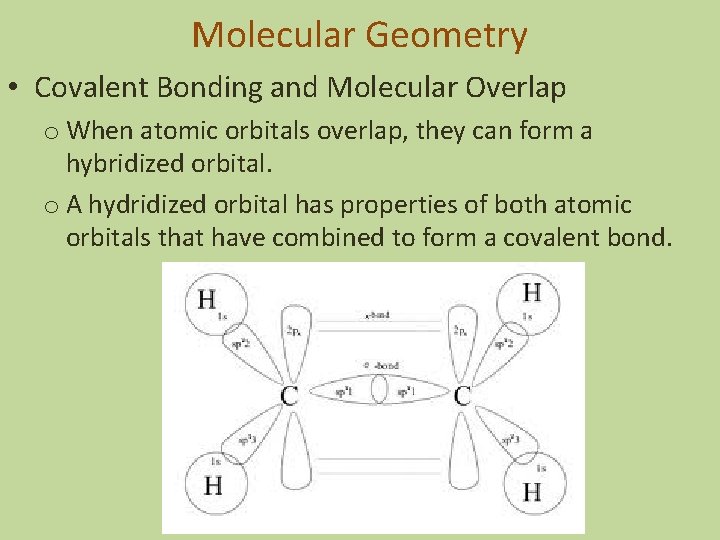
Molecular Geometry • Covalent Bonding and Molecular Overlap o When atomic orbitals overlap, they can form a hybridized orbital. o A hydridized orbital has properties of both atomic orbitals that have combined to form a covalent bond.
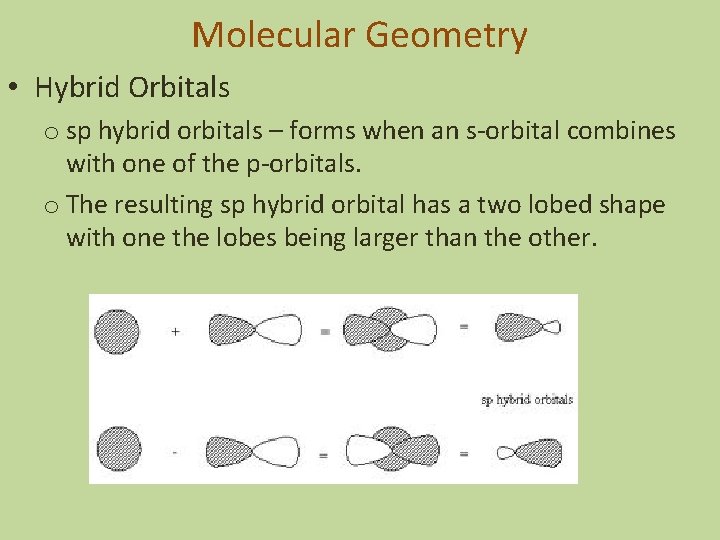
Molecular Geometry • Hybrid Orbitals o sp hybrid orbitals – forms when an s-orbital combines with one of the p-orbitals. o The resulting sp hybrid orbital has a two lobed shape with one the lobes being larger than the other.
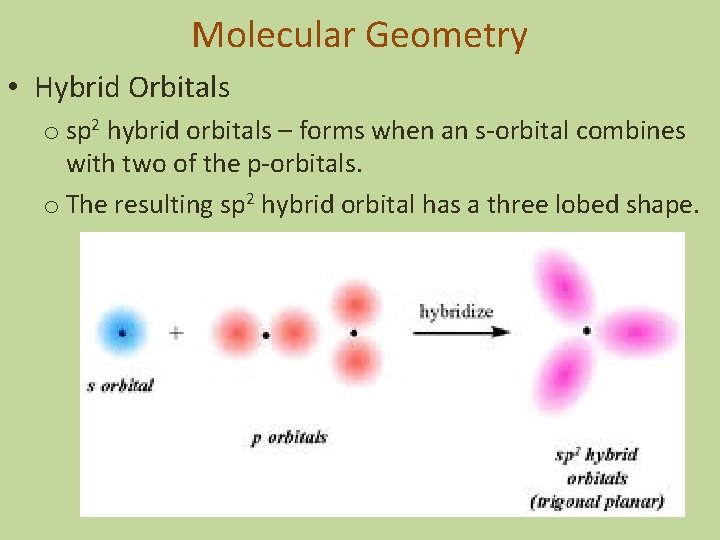
Molecular Geometry • Hybrid Orbitals o sp 2 hybrid orbitals – forms when an s-orbital combines with two of the p-orbitals. o The resulting sp 2 hybrid orbital has a three lobed shape.
Molecular Geometry • Hybrid Orbitals o sp 3 d hybrid orbitals – forms when an s-orbital combines with three of the p-orbitals and one d-orbital. o sp 3 d 2 hybrid orbitals – forms when an s-orbital combines with three p-orbitals, and two d-orbitals. What does this all mean?
Molecular Geometry • Hybrid Orbitals o The hybridization of orbitals explains how an atom can form a covalent bond with other atoms and have a specific geometric shape. o If a central atom forms covalent bonds with 4 other atoms, then it must have hybridized its orbitals to accommodate 4 bonding spaces. Therefore one s and three p-orbitals must have hybridized to form an sp 3.
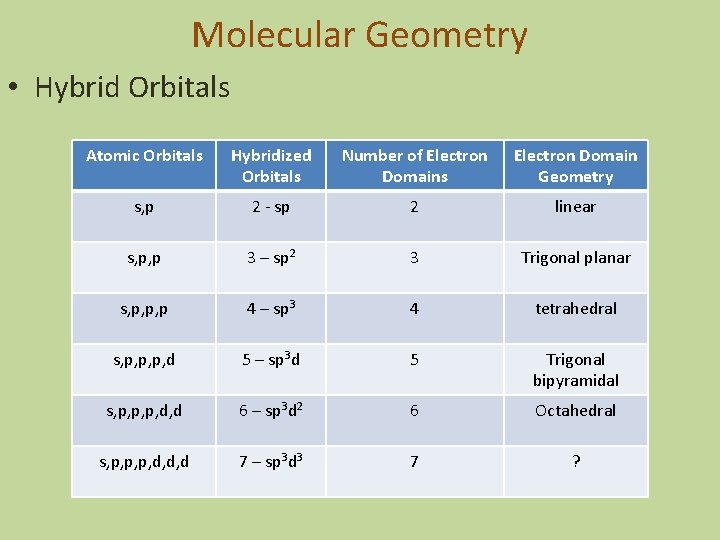
Molecular Geometry • Hybrid Orbitals Atomic Orbitals Hybridized Orbitals Number of Electron Domains Electron Domain Geometry s, p 2 - sp 2 linear s, p, p 3 – sp 2 3 Trigonal planar s, p, p, p 4 – sp 3 4 tetrahedral s, p, p, p, d 5 – sp 3 d 5 Trigonal bipyramidal s, p, p, p, d, d 6 – sp 3 d 2 6 Octahedral s, p, p, p, d, d, d 7 – sp 3 d 3 7 ?
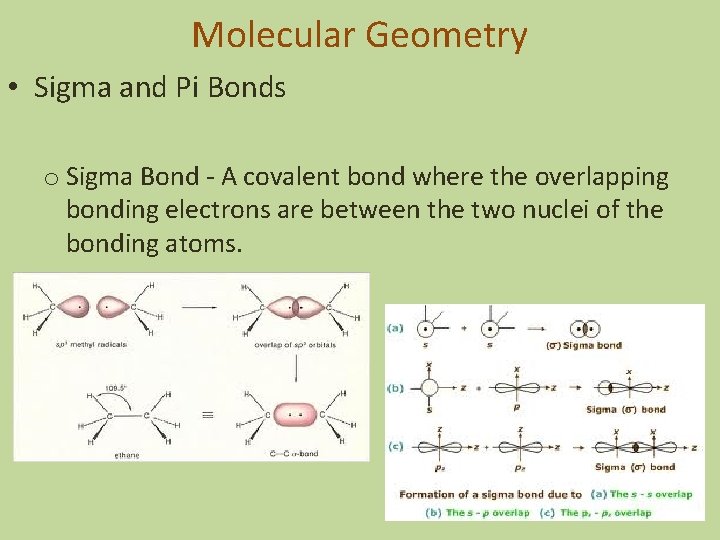
Molecular Geometry • Sigma and Pi Bonds o Sigma Bond - A covalent bond where the overlapping bonding electrons are between the two nuclei of the bonding atoms.
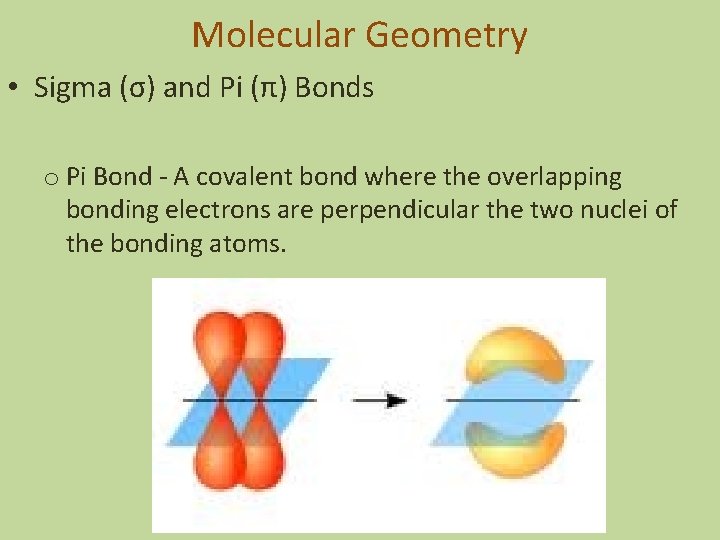
Molecular Geometry • Sigma (σ) and Pi (π) Bonds o Pi Bond - A covalent bond where the overlapping bonding electrons are perpendicular the two nuclei of the bonding atoms.
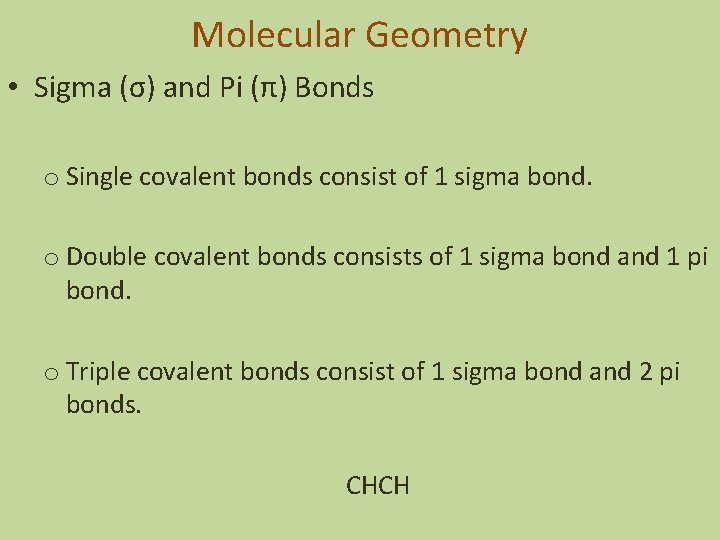
Molecular Geometry • Sigma (σ) and Pi (π) Bonds o Single covalent bonds consist of 1 sigma bond. o Double covalent bonds consists of 1 sigma bond and 1 pi bond. o Triple covalent bonds consist of 1 sigma bond and 2 pi bonds. CHCH
- Slides: 29