ALKENES AND ALKYNES Alkenes contain one or more







- Slides: 19

ALKENES AND ALKYNES

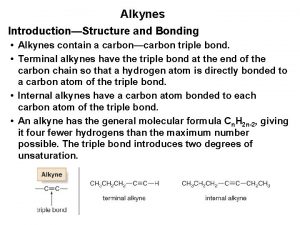
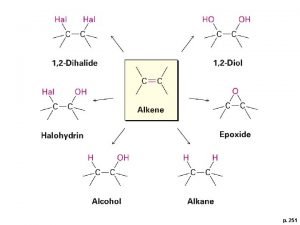
• Alkenes contain one or more double bonds • Alkynes contain one or more triple bonds • Since alkenes and alkynes are NOT bonded to the maximum possible number of atoms, these compounds are often referred to as unsaturated hydrocarbons Boiling points and melting points: alkynes < alkenes < alkanes Reactivity: alkynes > alkenes > alkanes

• alkenes are used to synthesize polymers because they are reactive • alkynes are very unstable. As a result, they rarely occur in nature • When naming these molecules, there a few extra rules to follow: your main chain must include double or triple bonds at lowest position number (indicate position #) when numbering the main chain, double and triple bonds have priority over alkyl groups

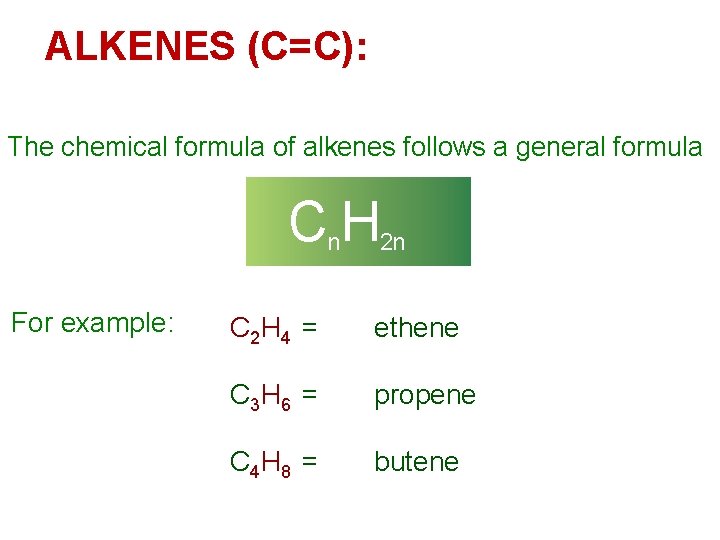
ALKENES (C=C): The chemical formula of alkenes follows a general formula Cn. H 2 n For example: C 2 H 4 = ethene C 3 H 6 = propene C 4 H 8 = butene

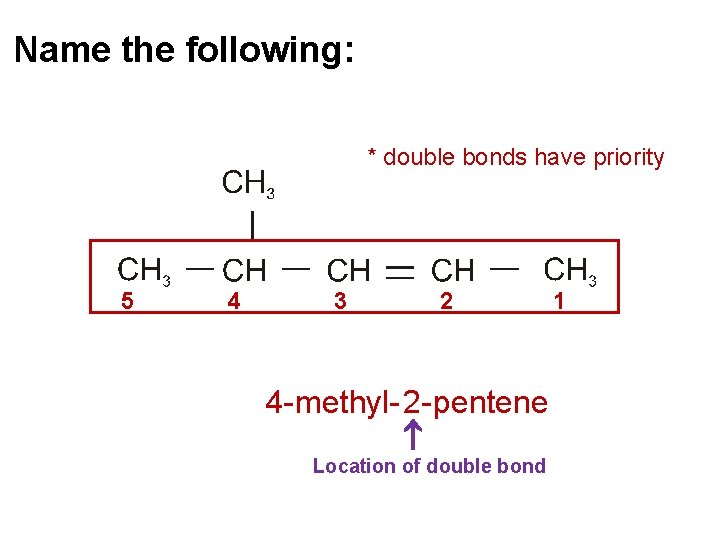
Name the following: * double bonds have priority 5 4 3 2 4 - methyl- 2 - pentene Location of double bond 1

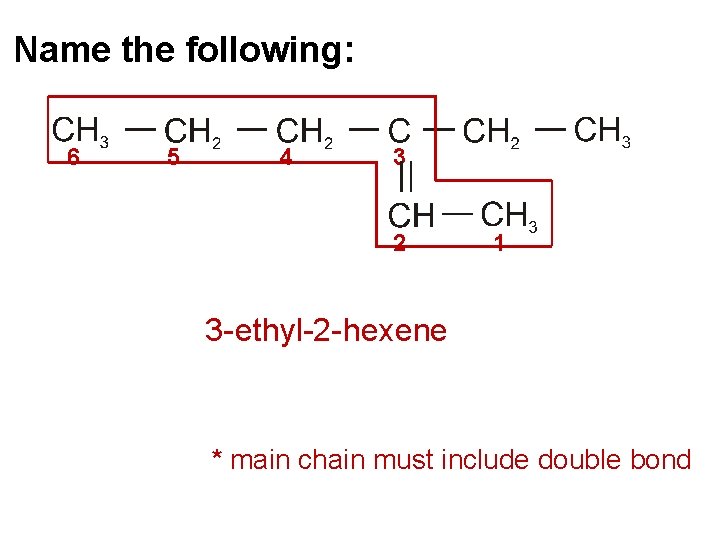
Name the following: 6 5 4 3 2 1 3 - ethyl-2 - hexene * main chain must include double bond

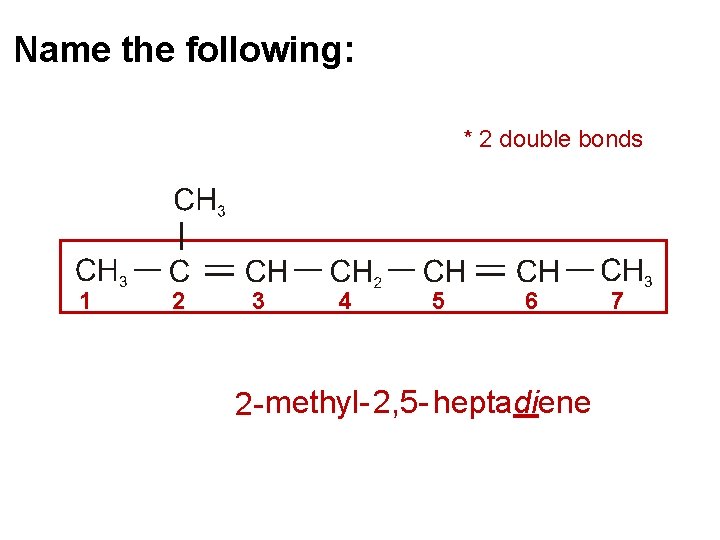
Name the following: * 2 double bonds 1 2 3 4 5 6 2 - methyl- 2, 5 - heptadiene 7

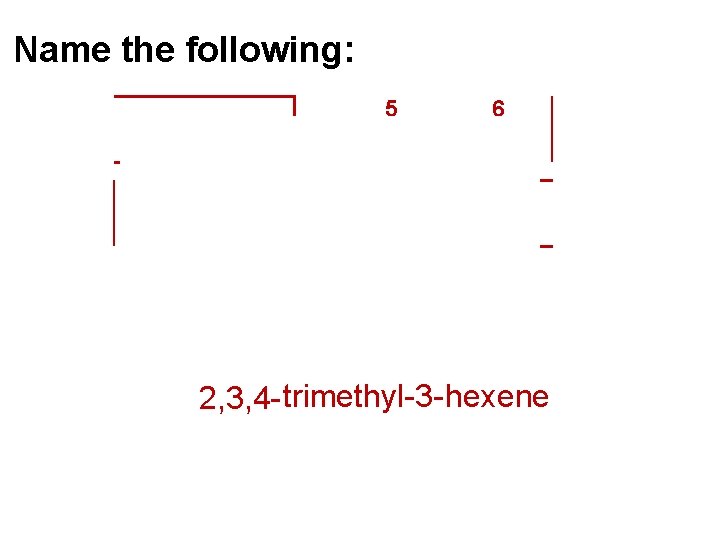
Name the following: 5 1 2 3 6 4 2, 3, 4 - trimethyl-3 - hexene

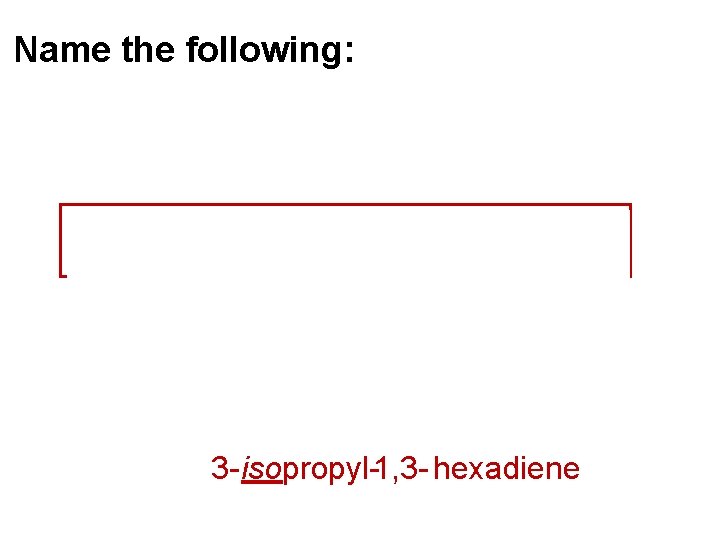
Name the following: 1 2 3 4 5 6 3 - isopropyl-1, 3 - hexadiene

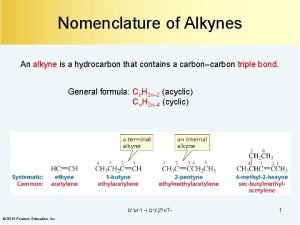
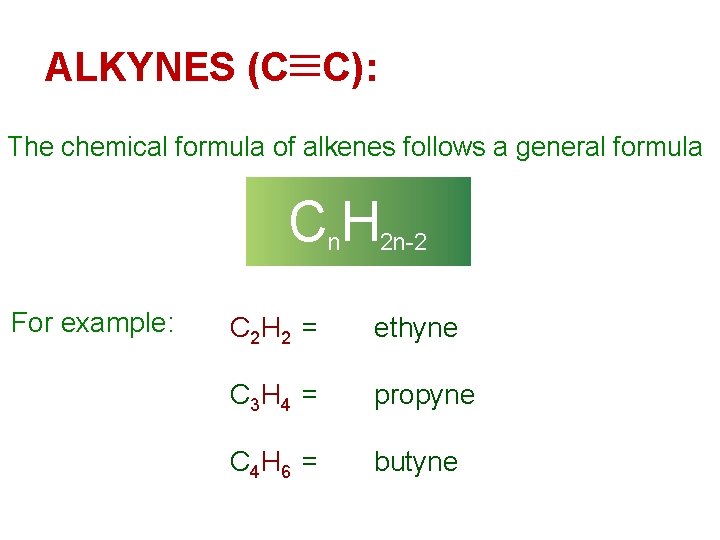
ALKYNES (C C): The chemical formula of alkenes follows a general formula Cn. H 2 n-2 For example: C 2 H 2 = ethyne C 3 H 4 = propyne C 4 H 6 = butyne

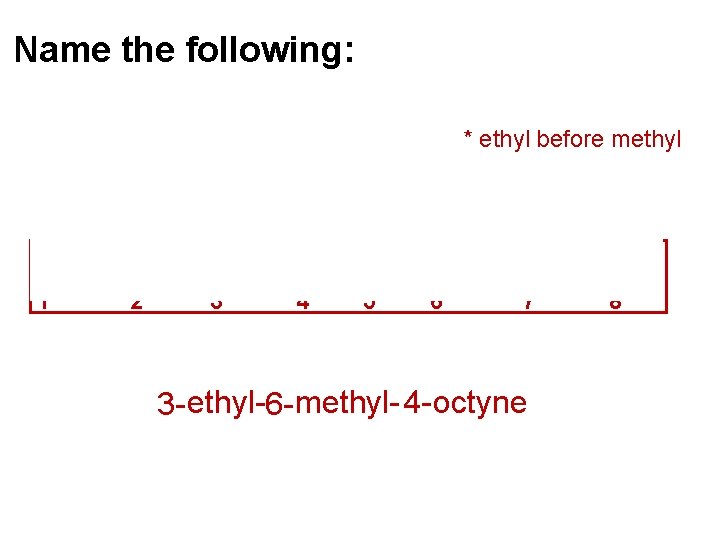
Name the following: * ethyl before methyl 1 2 3 4 5 6 7 3 - ethyl-6 - methyl- 4 - octyne 8

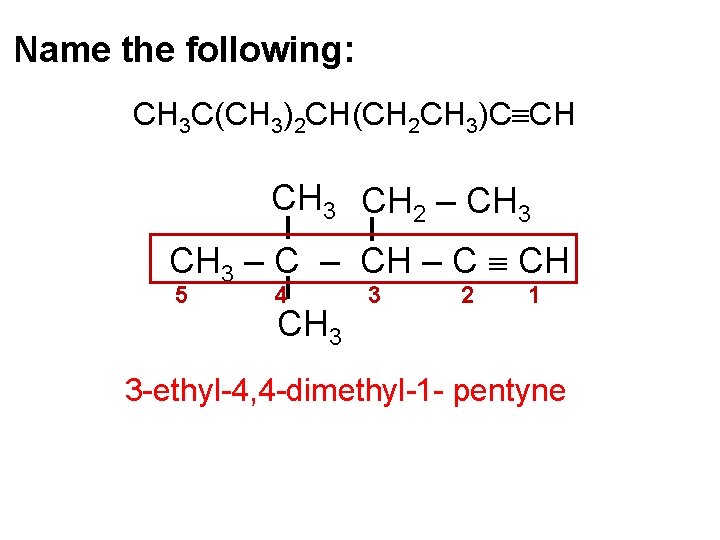
Name the following: CH 3 C(CH 3)2 CH(CH 2 CH 3)C CH CH 3 CH 2 – CH 3 – CH – C CH 5 4 CH 3 3 2 1 3 -ethyl-4, 4 -dimethyl-1 - pentyne

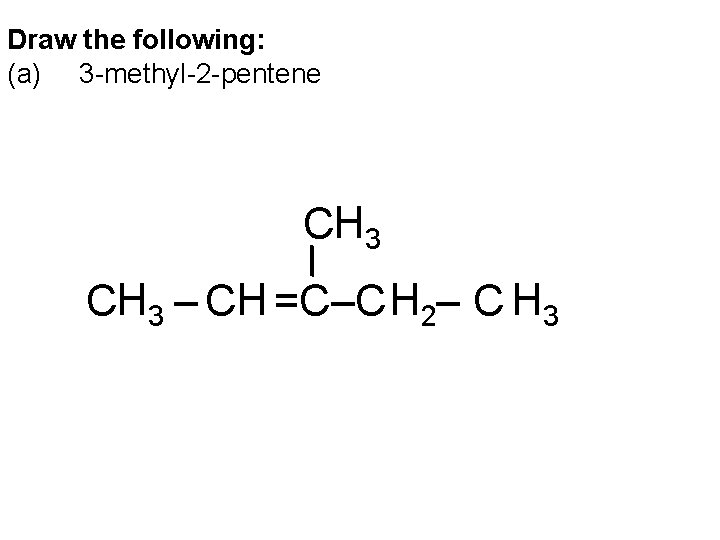
Draw the following: (a) 3 -methyl-2 -pentene CH 3 – CH =C–C H 2– C H 3

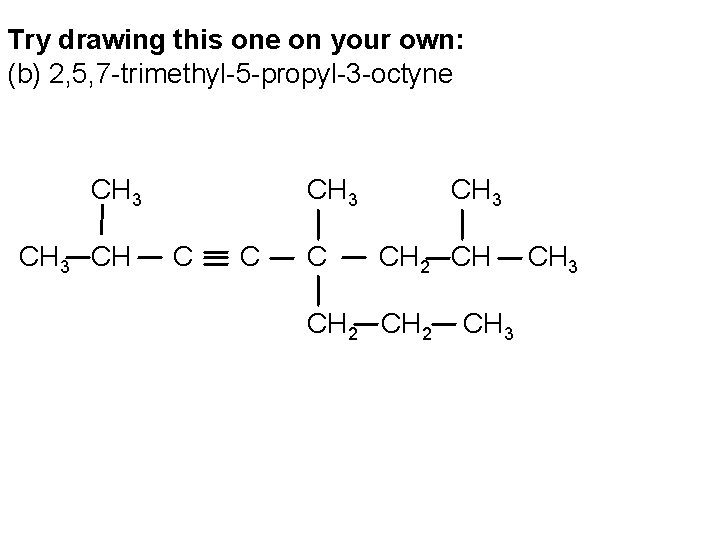
Try drawing this one on your own: (b) 2, 5, 7 -trimethyl-5 -propyl-3 -octyne CH 3 CH CH 3 CH 2 CH 3

Cyclic Hydrocarbons

• Cyclic hydrocarbons are hydrocarbon ring structures • When naming these molecules, the cyclic structure is always the main chain • Number the carbons, in either direction, so that the branches have the lowest possible position number • Double bonds have priority over alkyl groups • have the prefix “cyclo”

Name the following: (a) CH 3 2 3 4 5 CH 3 (b) 2 3 1 5 CH 2 CH 3 1 6 1 -ethyl- 2, 4 -dimethylcyclohexane CH 3 3, 4 -dimethyl -1 -cyclopentene 3, 4 -dimethylcyclopentene 4 CH 3 • Do not need to # double bond (it has to be between 1 & 2)

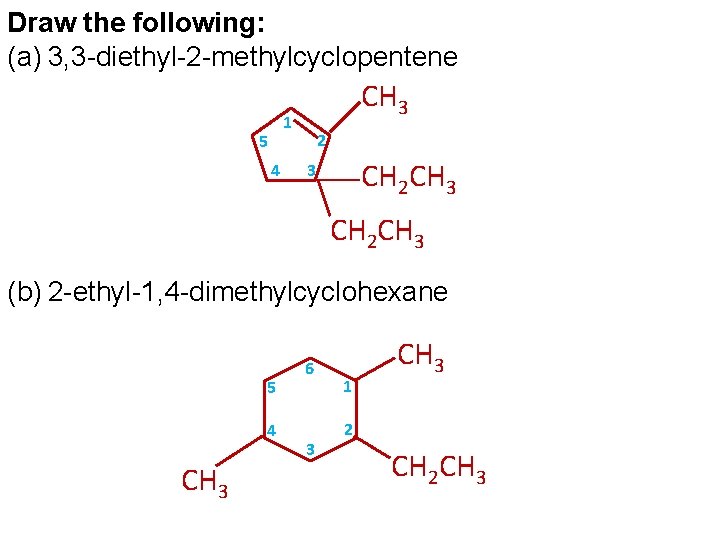
Draw the following: (a) 3, 3 -diethyl-2 -methylcyclopentene CH 3 1 5 4 2 CH 2 CH 3 3 CH 2 CH 3 (b) 2 -ethyl-1, 4 -dimethylcyclohexane 5 4 CH 3 6 3 1 CH 3 2 CH 2 CH 3

HOMEWORK WS “Naming and Drawing Alkenes” WS “Naming and Drawing Alkynes WS “Naming and Drawing Cyclic molecules”
 More more more i want more more more more we praise you
More more more i want more more more more we praise you More more more i want more more more more we praise you
More more more i want more more more more we praise you Alkanes alkenes alkynes
Alkanes alkenes alkynes Halogenation of alkynes
Halogenation of alkynes Triple bond nomenclature
Triple bond nomenclature 2 methylpropene + hbr
2 methylpropene + hbr Addition of halogens to alkenes
Addition of halogens to alkenes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes What is the general formula for alkenes?
What is the general formula for alkenes? Ozonolysis of alkynes
Ozonolysis of alkynes Alkynes structural formula
Alkynes structural formula Alkynes
Alkynes Alkynes
Alkynes Alkynes
Alkynes Wiley
Wiley First 10 members of alkynes
First 10 members of alkynes Benzalacetone
Benzalacetone Combustion of alkynes
Combustion of alkynes When was the poem she walks in beauty written
When was the poem she walks in beauty written