Chapter 3 Alkenes and Alkynes Alkenes and Alkynes








- Slides: 27

Chapter 3 Alkenes and Alkynes

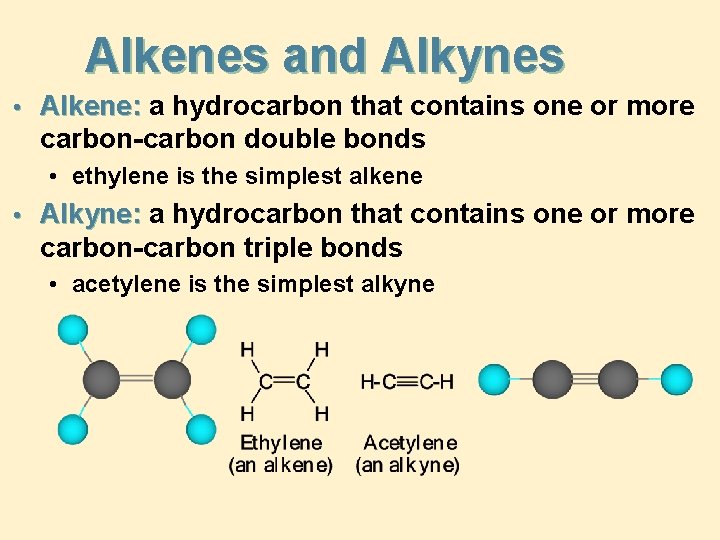
Alkenes and Alkynes • Alkene: a hydrocarbon that contains one or more carbon-carbon double bonds • ethylene is the simplest alkene • Alkyne: a hydrocarbon that contains one or more carbon-carbon triple bonds • acetylene is the simplest alkyne



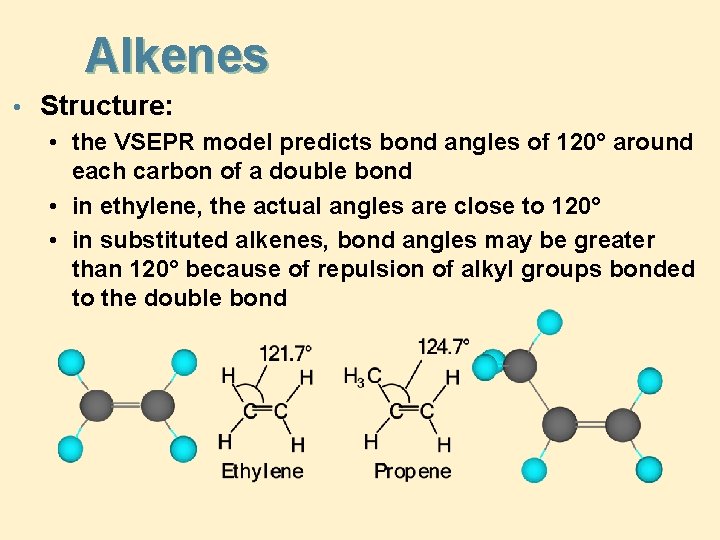
Alkenes • Structure: • the VSEPR model predicts bond angles of 120° around each carbon of a double bond • in ethylene, the actual angles are close to 120° • in substituted alkenes, bond angles may be greater than 120° because of repulsion of alkyl groups bonded to the double bond

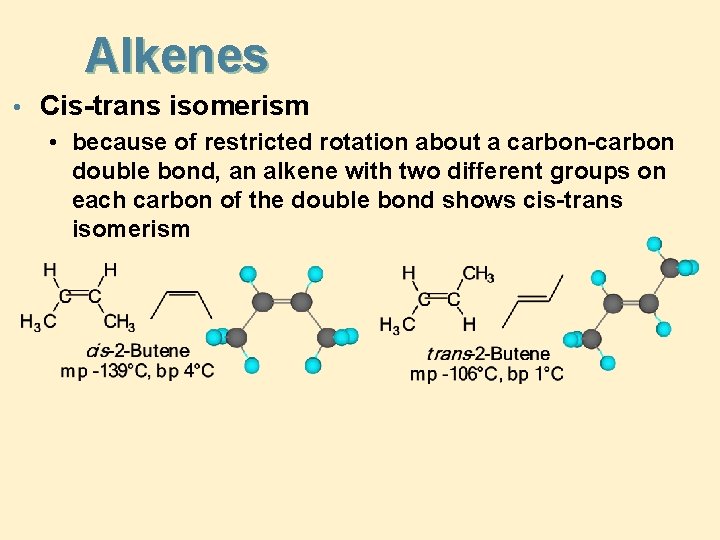
Alkenes • Cis-trans isomerism • because of restricted rotation about a carbon-carbon double bond, an alkene with two different groups on each carbon of the double bond shows cis-trans isomerism

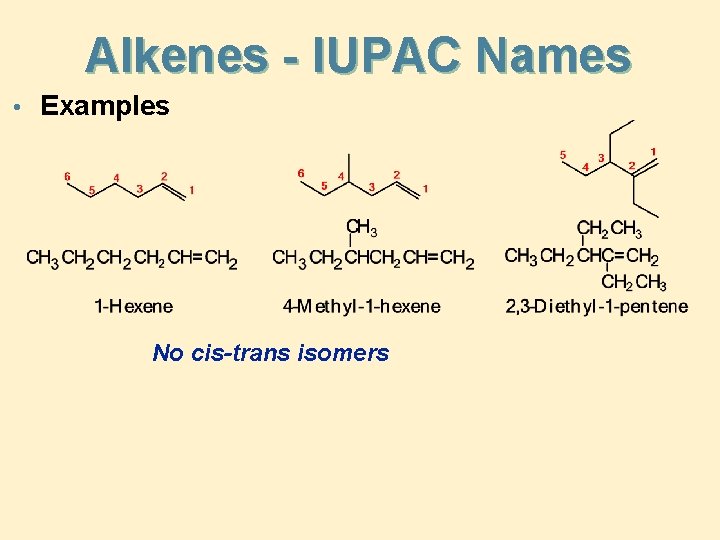
Alkenes - IUPAC Names • To name an alkene • the parent name is that of the longest chain that contains the C=C • number the chain from the end that gives the lower numbers to the carbons of the C=C • locate the C=C by the number of its first carbon • use the ending -ene to show the presence of the C=C • branched-chain alkenes are named in a manner similar to alkanes; substituted groups are located and named

Alkenes - IUPAC Names • Examples No cis-trans isomers

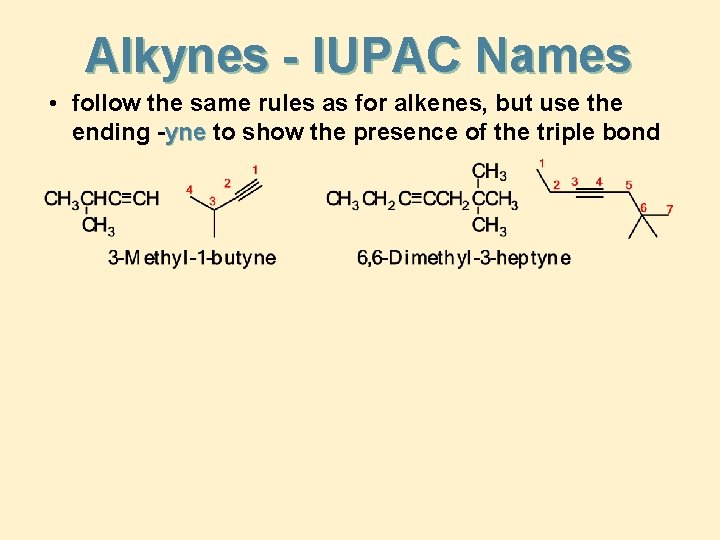
Alkynes - IUPAC Names • follow the same rules as for alkenes, but use the ending -yne to show the presence of the triple bond

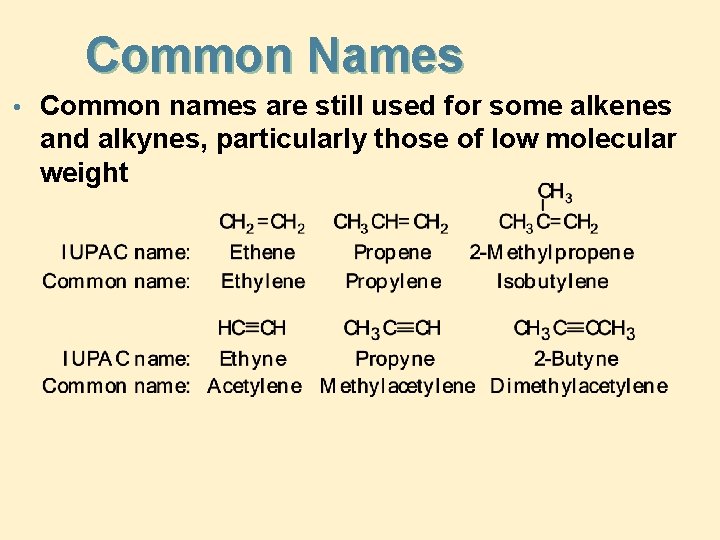
Common Names • Common names are still used for some alkenes and alkynes, particularly those of low molecular weight

Cycloalkenes • To name a cycloalkene • number the carbon atoms of the ring double bond 1 and 2 in the direction that gives the lower number to the substituent encountered first • number and list substituents in alphabetical order

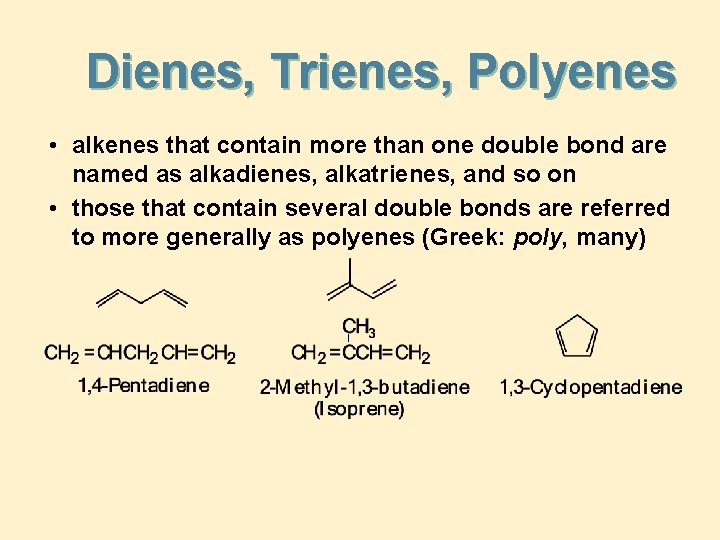
Dienes, Trienes, Polyenes • alkenes that contain more than one double bond are named as alkadienes, alkatrienes, and so on • those that contain several double bonds are referred to more generally as polyenes (Greek: poly, many)

Physical Properties • alkenes and alkynes are nonpolar compounds • the only attractive forces between their molecules are London dispersion forces • their physical properties are similar to those of alkanes with the same carbon skeletons • alkenes and alkynes are insoluble in water but soluble in one another and in nonpolar organic liquids • alkenes and alkynes that are liquid or solid at room temperature have densities less than 1 g/m. L; they float on water

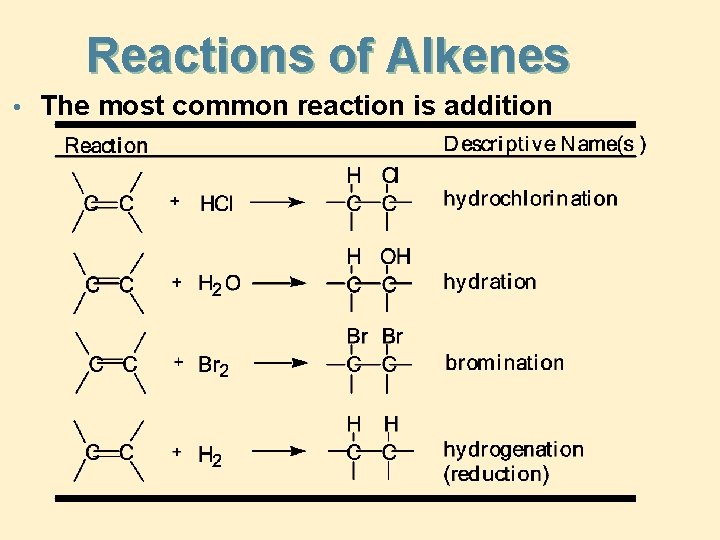
Reactions of Alkenes • The most common reaction is addition

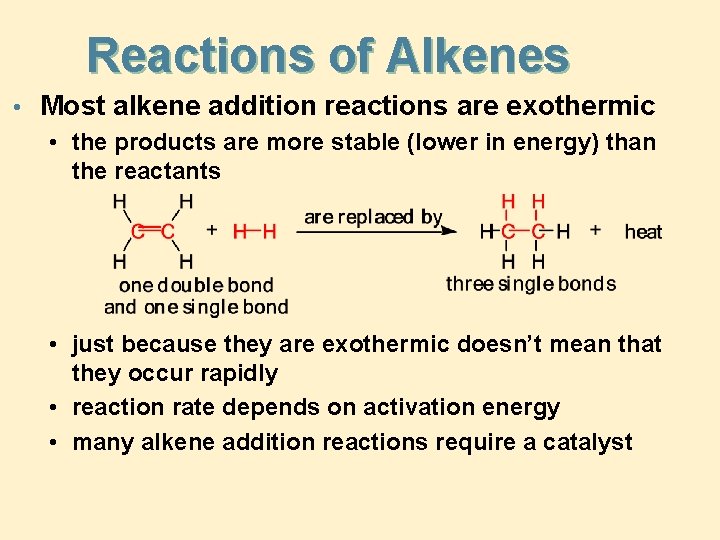
Reactions of Alkenes • Most alkene addition reactions are exothermic • the products are more stable (lower in energy) than the reactants • just because they are exothermic doesn’t mean that they occur rapidly • reaction rate depends on activation energy • many alkene addition reactions require a catalyst

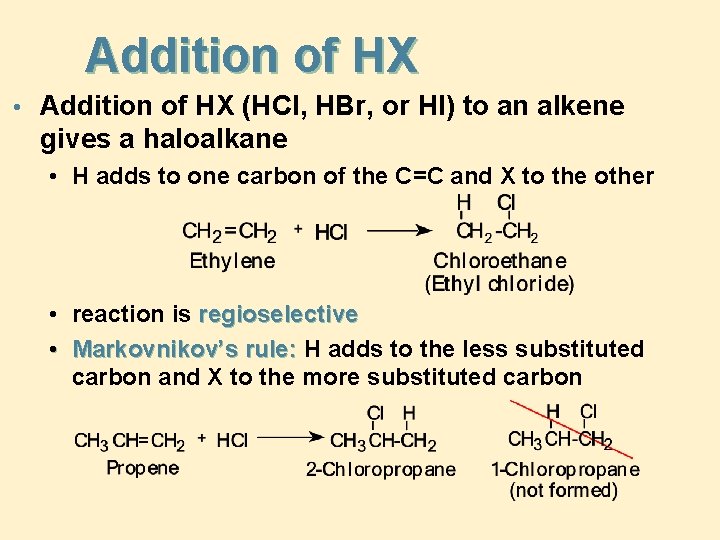
Addition of HX • Addition of HX (HCl, HBr, or HI) to an alkene gives a haloalkane • H adds to one carbon of the C=C and X to the other • reaction is regioselective • Markovnikov’s rule: H adds to the less substituted carbon and X to the more substituted carbon


Addition of HX • Chemists account for the addition of HX to an alkene by a two-step reaction mechanism • we use curved arrows to show the movement of electron pairs during a chemical reaction • the tail of an arrow shows the origin of the electron pair (either on an atom of in a bond) • the head of the arrow shows its new position • curved arrows show us which bonds break and which new ones form

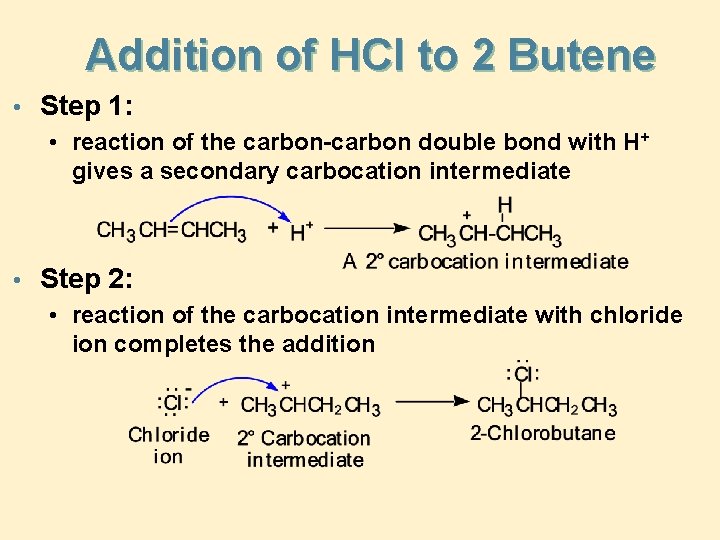
Addition of HCl to 2 Butene • Step 1: • reaction of the carbon-carbon double bond with H+ gives a secondary carbocation intermediate • Step 2: • reaction of the carbocation intermediate with chloride ion completes the addition

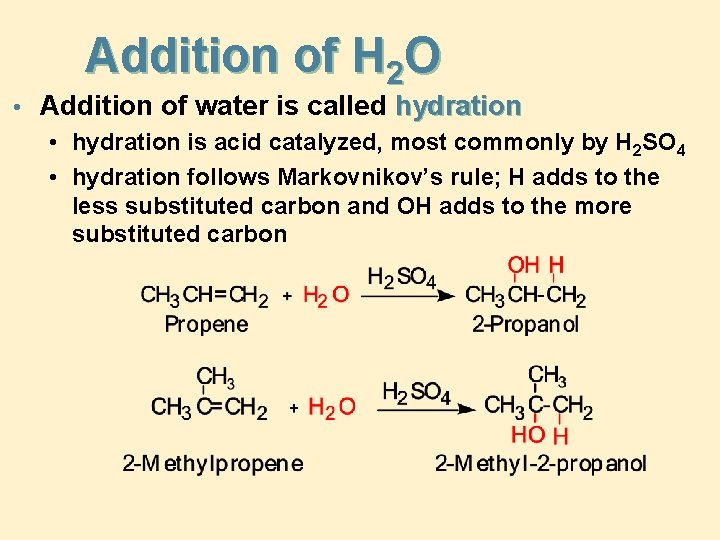
Addition of H 2 O • Addition of water is called hydration • hydration is acid catalyzed, most commonly by H 2 SO 4 • hydration follows Markovnikov’s rule; H adds to the less substituted carbon and OH adds to the more substituted carbon

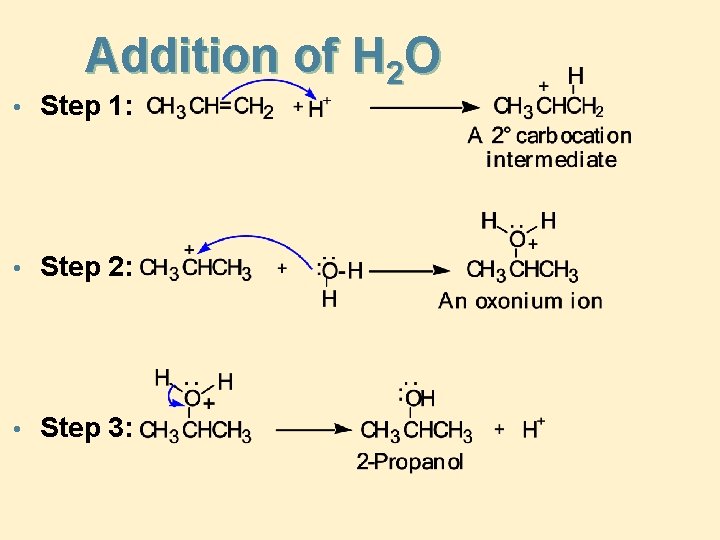
Addition of H 2 O • Step 1: • Step 2: • Step 3:

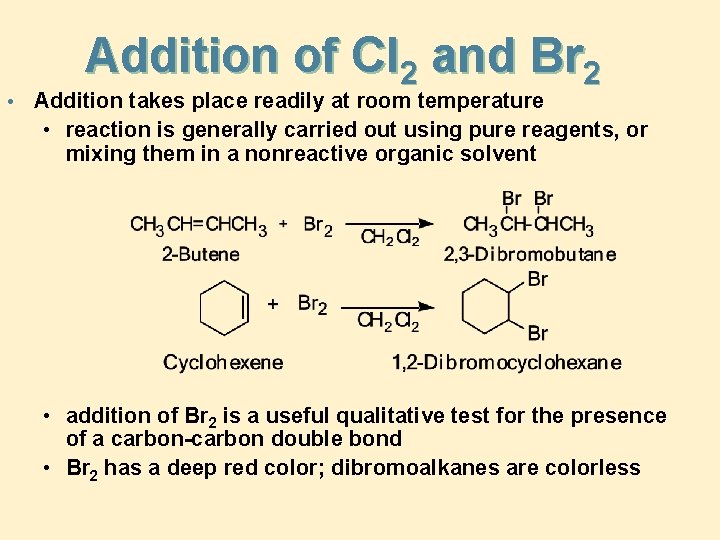
• Addition of Cl 2 and Br 2 Addition takes place readily at room temperature • reaction is generally carried out using pure reagents, or mixing them in a nonreactive organic solvent • addition of Br 2 is a useful qualitative test for the presence of a carbon-carbon double bond • Br 2 has a deep red color; dibromoalkanes are colorless

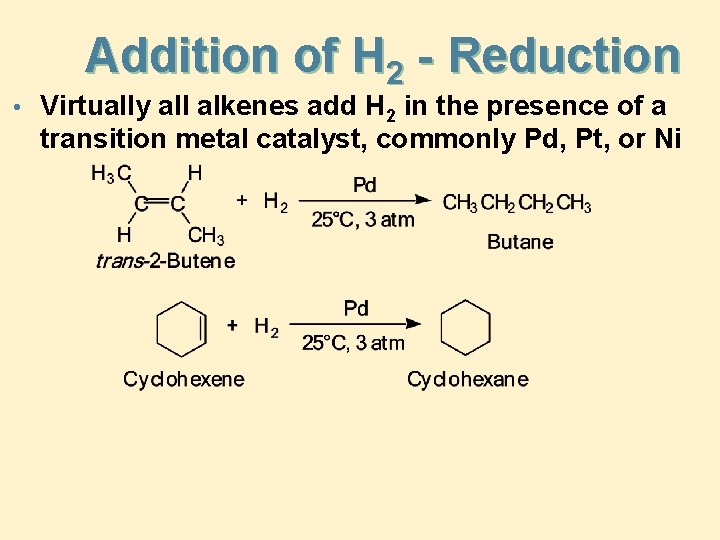
Addition of H 2 - Reduction • Virtually all alkenes add H 2 in the presence of a transition metal catalyst, commonly Pd, Pt, or Ni

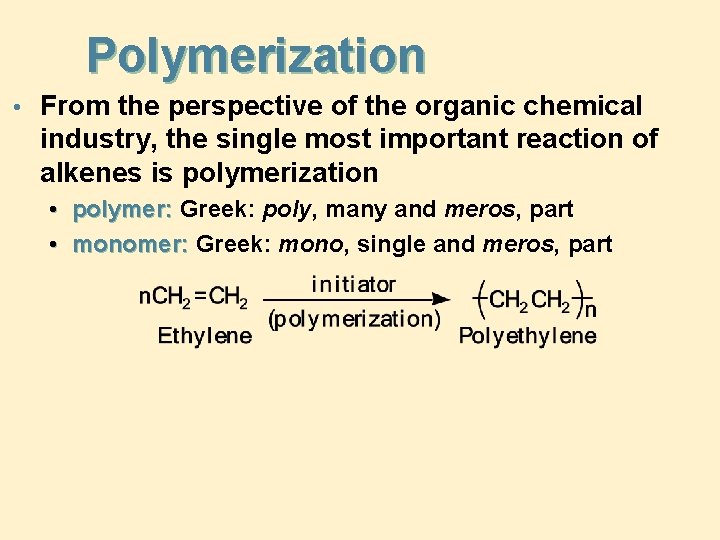
Polymerization • From the perspective of the organic chemical industry, the single most important reaction of alkenes is polymerization • polymer: Greek: poly, many and meros, part • monomer: Greek: mono, single and meros, part

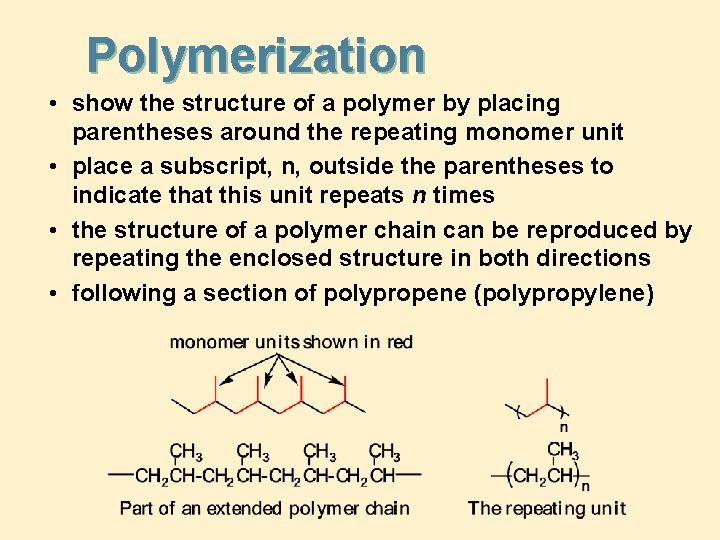
Polymerization • show the structure of a polymer by placing parentheses around the repeating monomer unit • place a subscript, n, outside the parentheses to indicate that this unit repeats n times • the structure of a polymer chain can be reproduced by repeating the enclosed structure in both directions • following a section of polypropene (polypropylene)


Chapter 3 End Chapter 3