Chapter 14 The Chemistry of Alkynes Alkynes Also



- Slides: 32

Chapter 14 The Chemistry of Alkynes

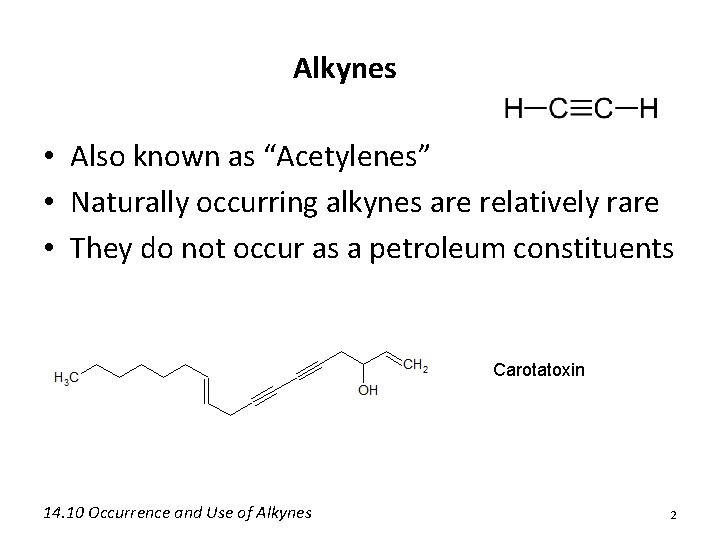
Alkynes • Also known as “Acetylenes” • Naturally occurring alkynes are relatively rare • They do not occur as a petroleum constituents Carotatoxin 14. 10 Occurrence and Use of Alkynes 2

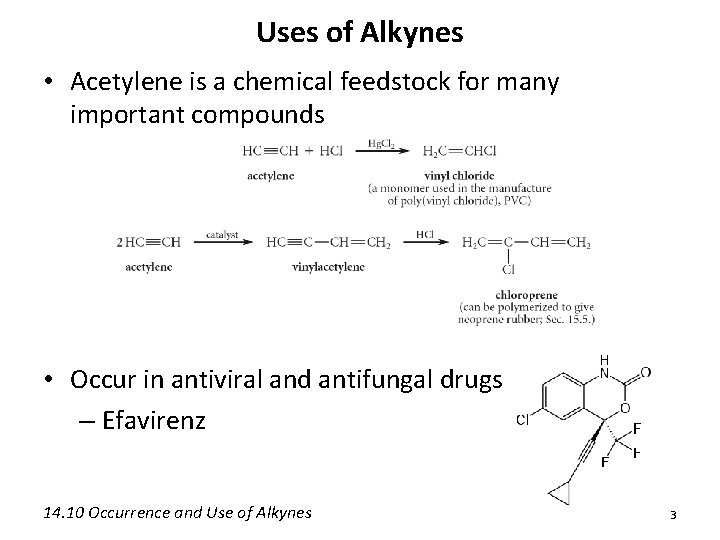
Uses of Alkynes • Acetylene is a chemical feedstock for many important compounds • Occur in antiviral and antifungal drugs – Efavirenz 14. 10 Occurrence and Use of Alkynes 3

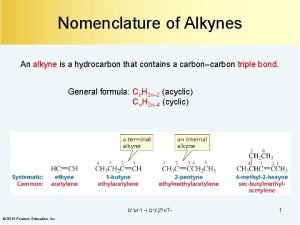
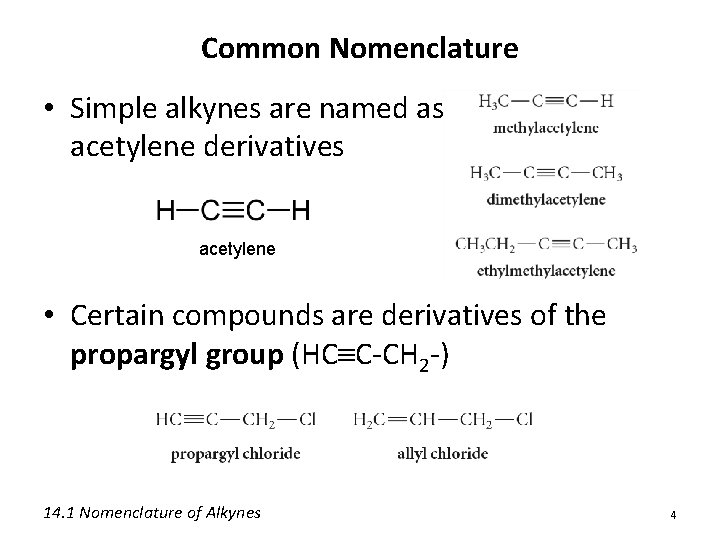
Common Nomenclature • Simple alkynes are named as acetylene derivatives acetylene • Certain compounds are derivatives of the propargyl group (HC C-CH 2 -) 14. 1 Nomenclature of Alkynes 4

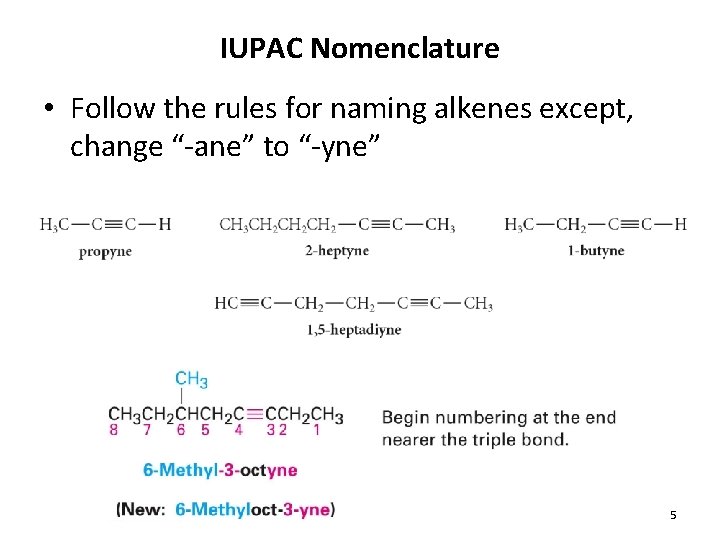
IUPAC Nomenclature • Follow the rules for naming alkenes except, change “-ane” to “-yne” 5

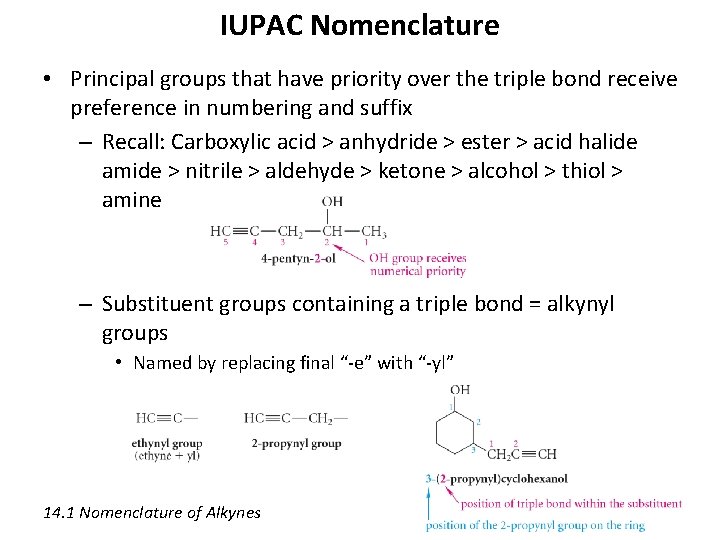
IUPAC Nomenclature • Principal groups that have priority over the triple bond receive preference in numbering and suffix – Recall: Carboxylic acid > anhydride > ester > acid halide amide > nitrile > aldehyde > ketone > alcohol > thiol > amine – Substituent groups containing a triple bond = alkynyl groups • Named by replacing final “-e” with “-yl” 14. 1 Nomenclature of Alkynes 6

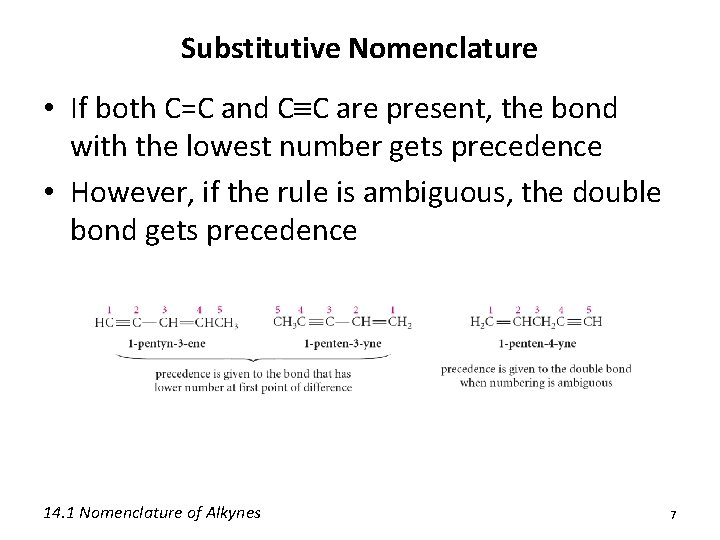
Substitutive Nomenclature • If both C=C and C C are present, the bond with the lowest number gets precedence • However, if the rule is ambiguous, the double bond gets precedence 14. 1 Nomenclature of Alkynes 7

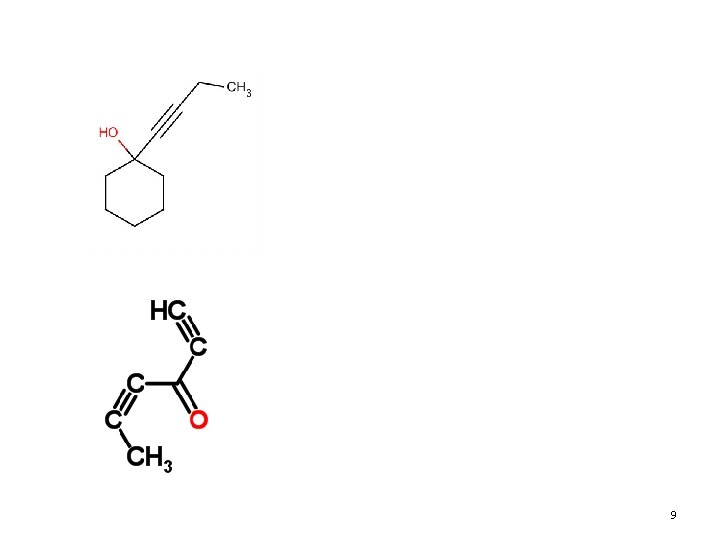
Problems • Name the following compounds: 8

9

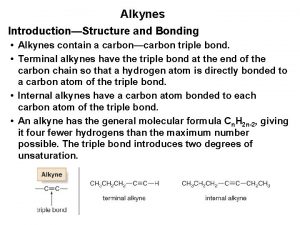
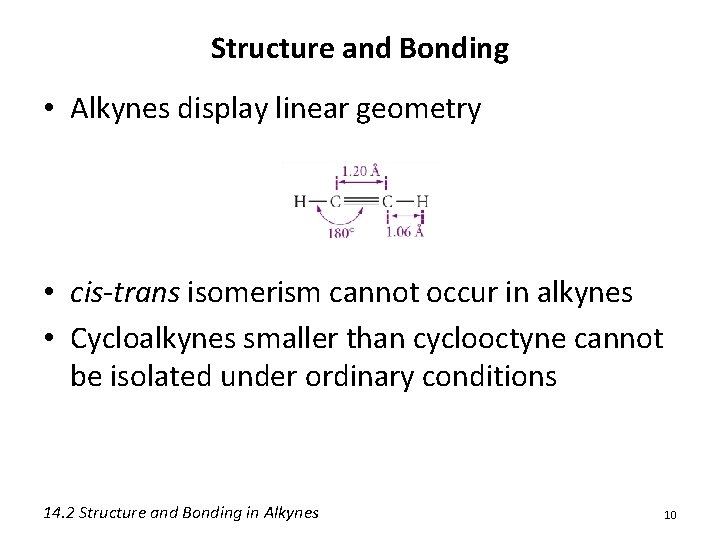
Structure and Bonding • Alkynes display linear geometry • cis-trans isomerism cannot occur in alkynes • Cycloalkynes smaller than cyclooctyne cannot be isolated under ordinary conditions 14. 2 Structure and Bonding in Alkynes 10

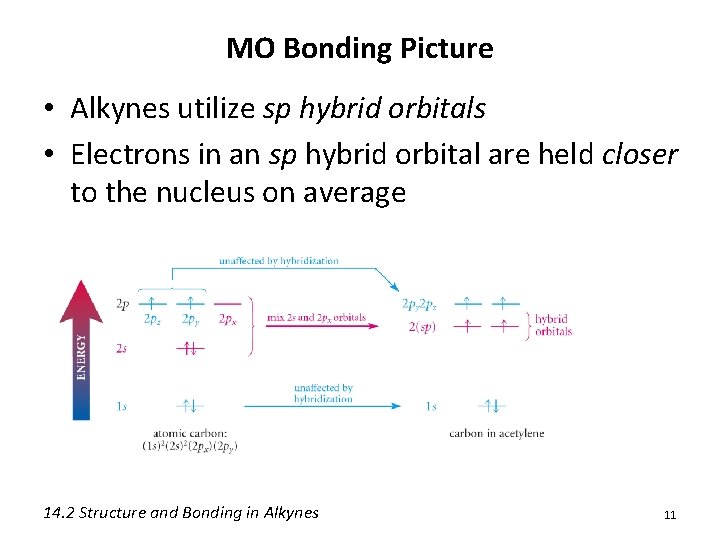
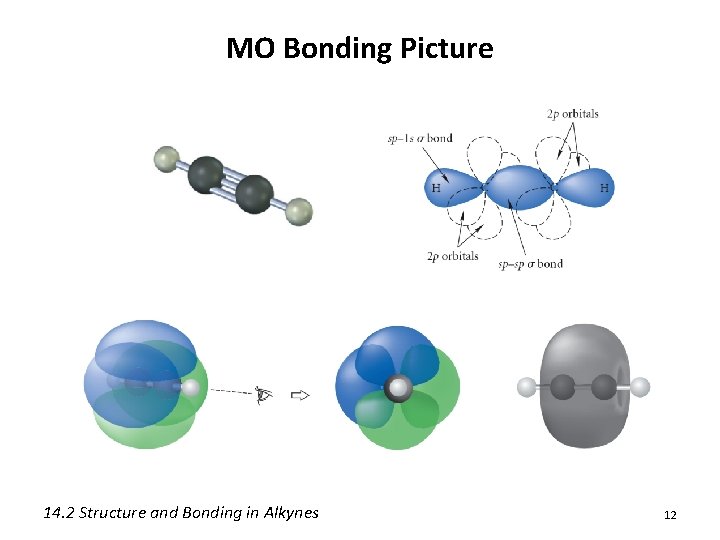
MO Bonding Picture • Alkynes utilize sp hybrid orbitals • Electrons in an sp hybrid orbital are held closer to the nucleus on average 14. 2 Structure and Bonding in Alkynes 11

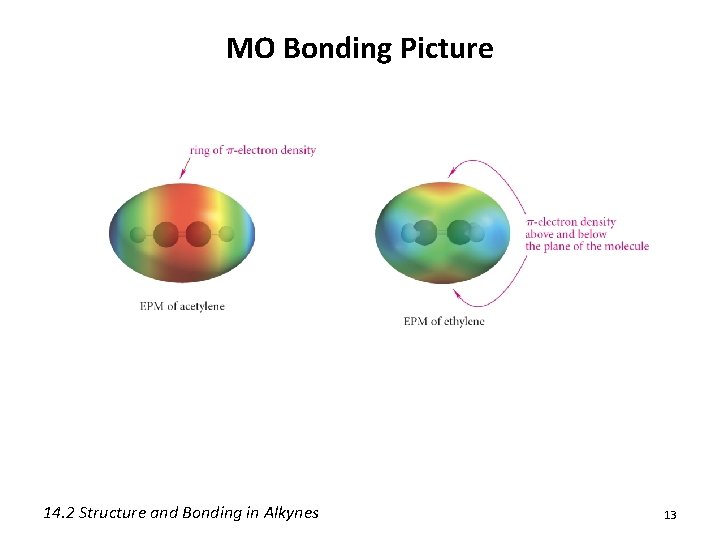
MO Bonding Picture 14. 2 Structure and Bonding in Alkynes 12

MO Bonding Picture 14. 2 Structure and Bonding in Alkynes 13

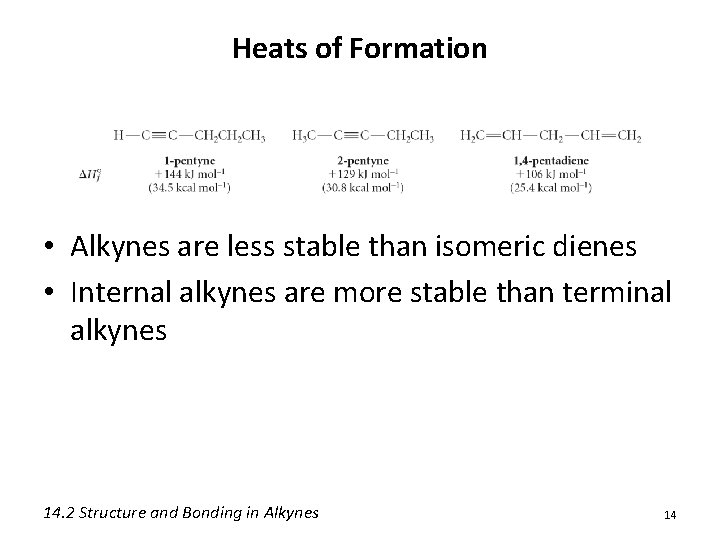
Heats of Formation • Alkynes are less stable than isomeric dienes • Internal alkynes are more stable than terminal alkynes 14. 2 Structure and Bonding in Alkynes 14

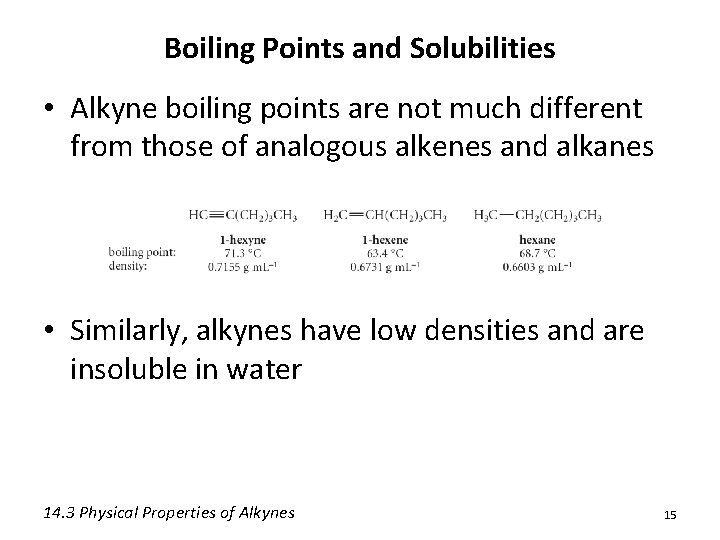
Boiling Points and Solubilities • Alkyne boiling points are not much different from those of analogous alkenes and alkanes • Similarly, alkynes have low densities and are insoluble in water 14. 3 Physical Properties of Alkynes 15

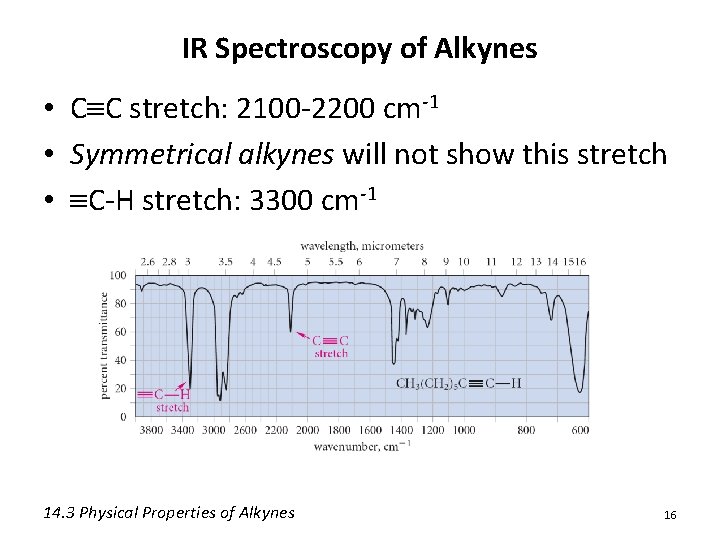
IR Spectroscopy of Alkynes • C C stretch: 2100 -2200 cm-1 • Symmetrical alkynes will not show this stretch • C-H stretch: 3300 cm-1 14. 3 Physical Properties of Alkynes 16

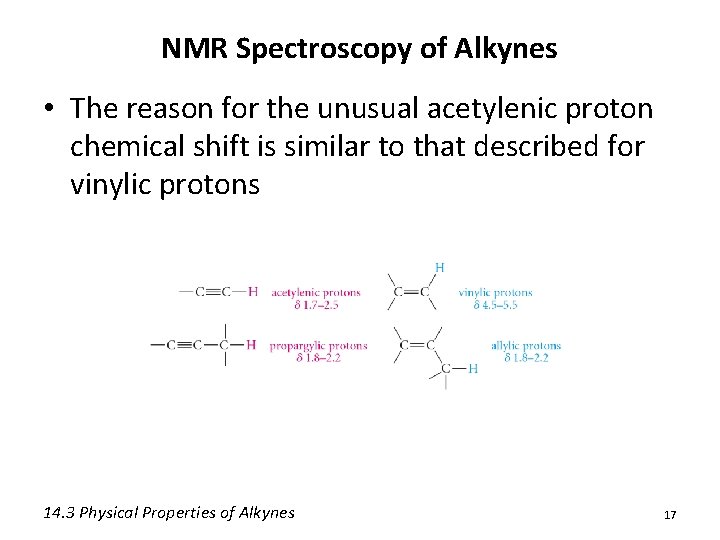
NMR Spectroscopy of Alkynes • The reason for the unusual acetylenic proton chemical shift is similar to that described for vinylic protons 14. 3 Physical Properties of Alkynes 17

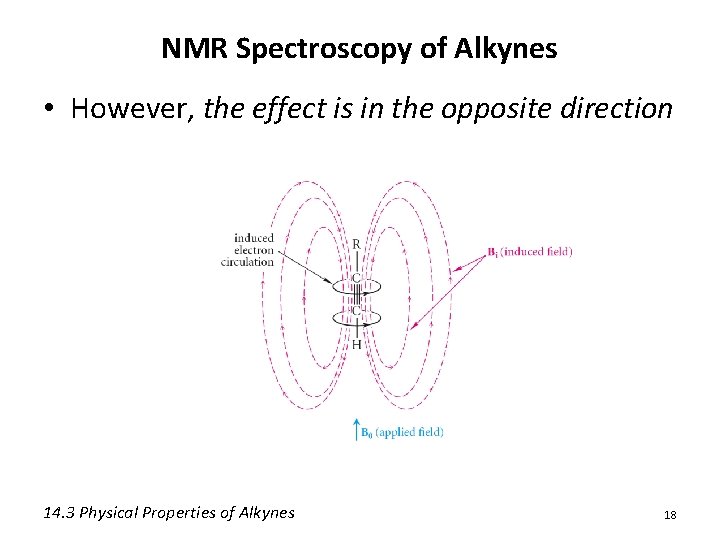
NMR Spectroscopy of Alkynes • However, the effect is in the opposite direction 14. 3 Physical Properties of Alkynes 18

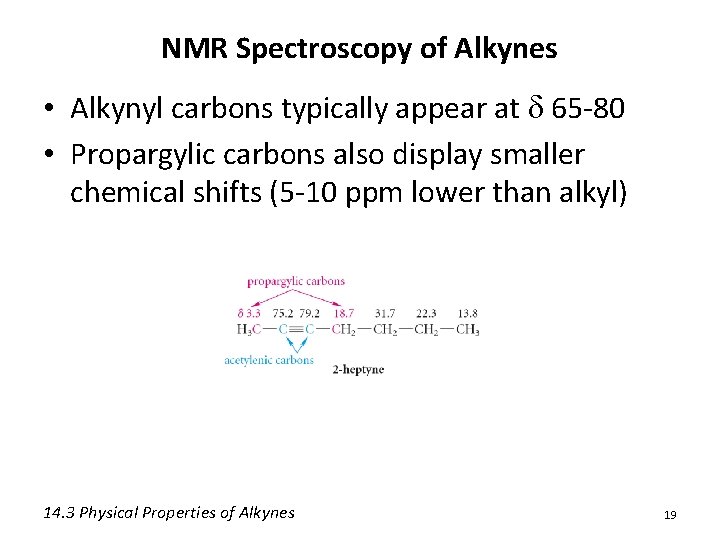
NMR Spectroscopy of Alkynes • Alkynyl carbons typically appear at d 65 -80 • Propargylic carbons also display smaller chemical shifts (5 -10 ppm lower than alkyl) 14. 3 Physical Properties of Alkynes 19

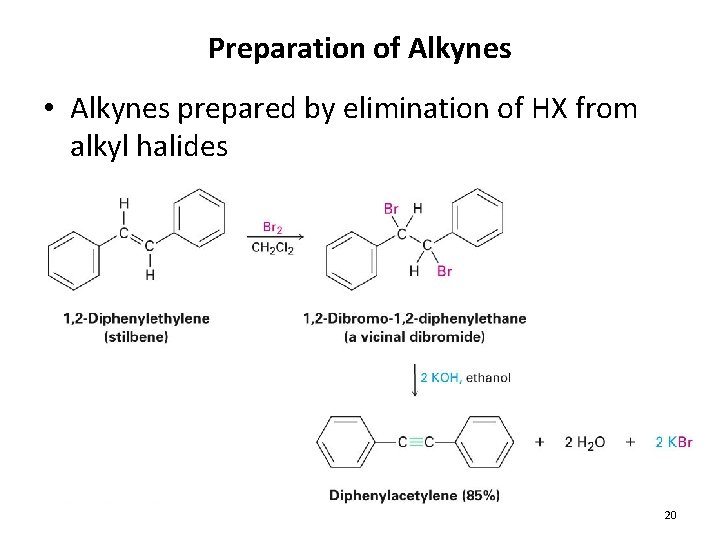
Preparation of Alkynes • Alkynes prepared by elimination of HX from alkyl halides 20

21

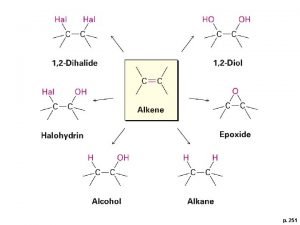
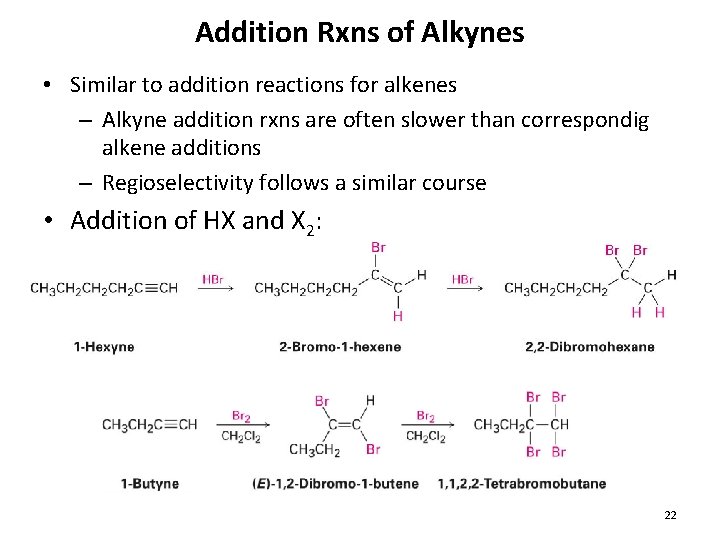
Addition Rxns of Alkynes • Similar to addition reactions for alkenes – Alkyne addition rxns are often slower than correspondig alkene additions – Regioselectivity follows a similar course • Addition of HX and X 2: 22

23

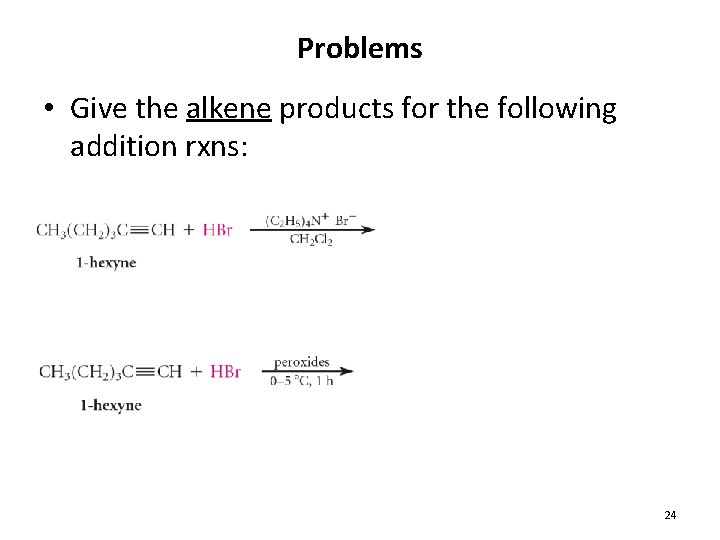
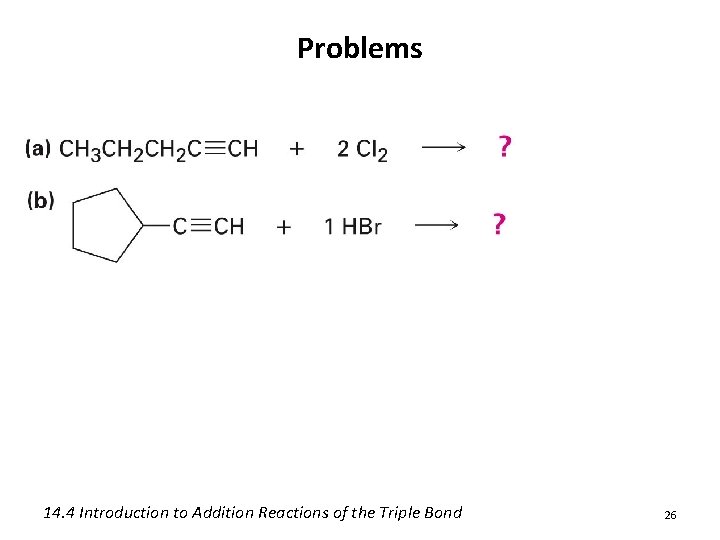
Problems • Give the alkene products for the following addition rxns: 24

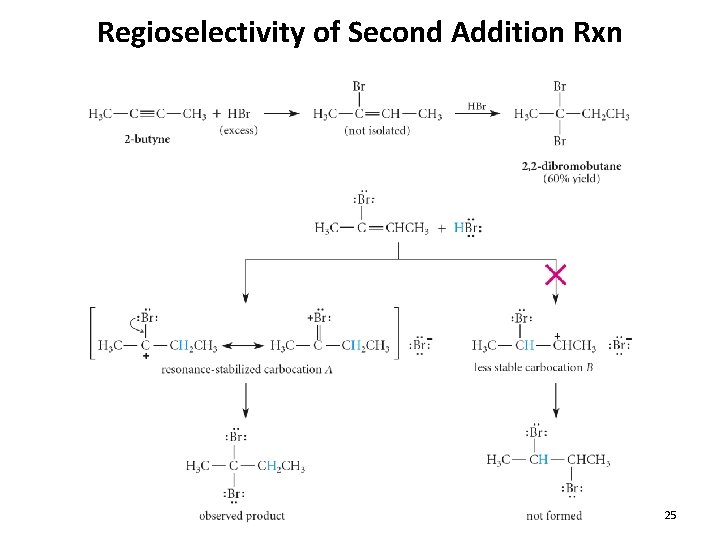
Regioselectivity of Second Addition Rxn 25

Problems 14. 4 Introduction to Addition Reactions of the Triple Bond 26

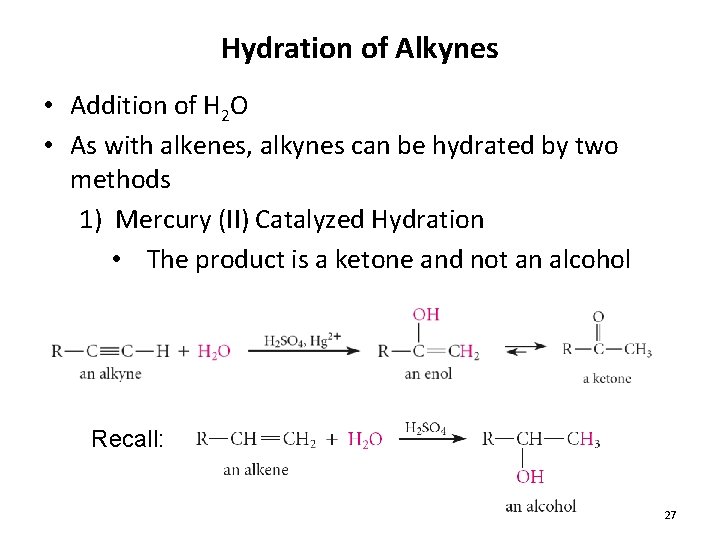
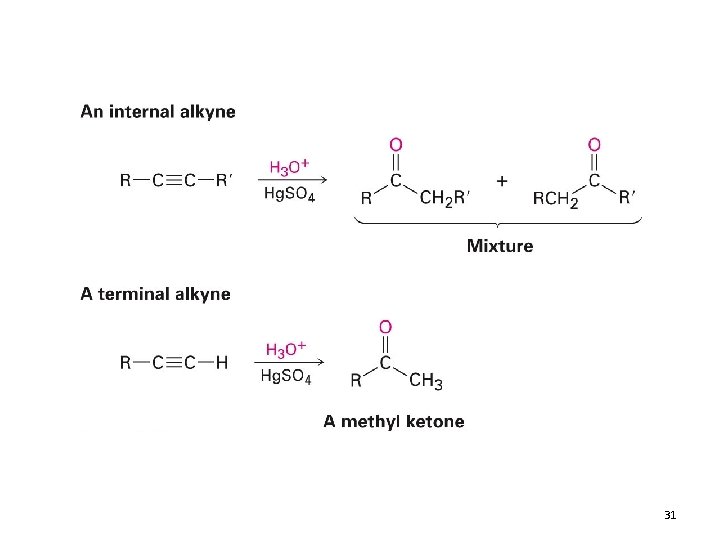
Hydration of Alkynes • Addition of H 2 O • As with alkenes, alkynes can be hydrated by two methods 1) Mercury (II) Catalyzed Hydration • The product is a ketone and not an alcohol Recall: 27

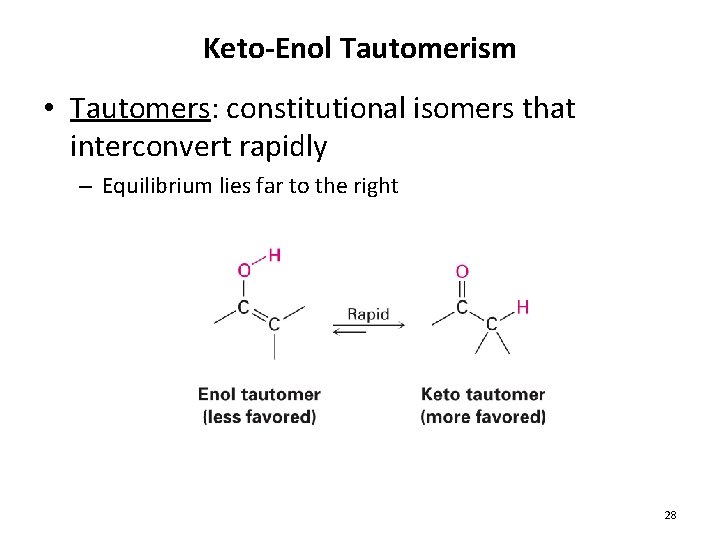
Keto-Enol Tautomerism • Tautomers: constitutional isomers that interconvert rapidly – Equilibrium lies far to the right 28

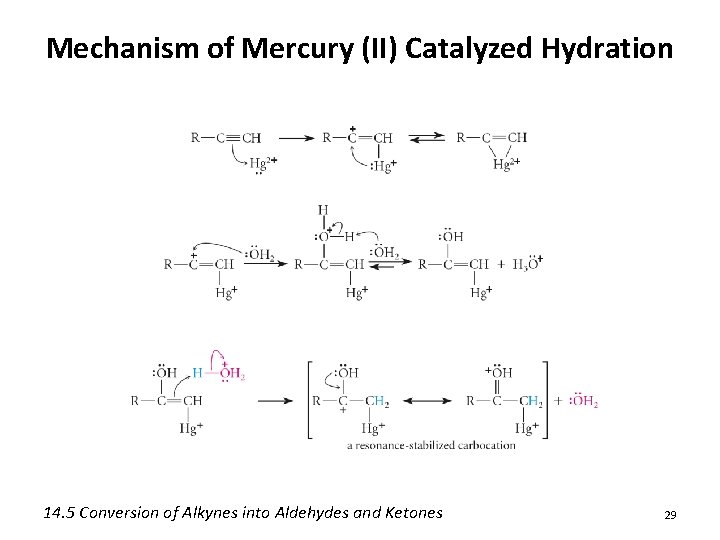
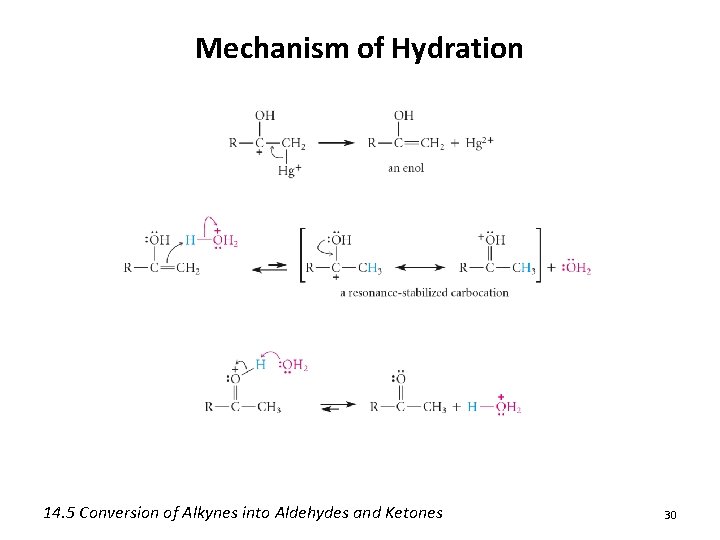
Mechanism of Mercury (II) Catalyzed Hydration 14. 5 Conversion of Alkynes into Aldehydes and Ketones 29

Mechanism of Hydration 14. 5 Conversion of Alkynes into Aldehydes and Ketones 30

31

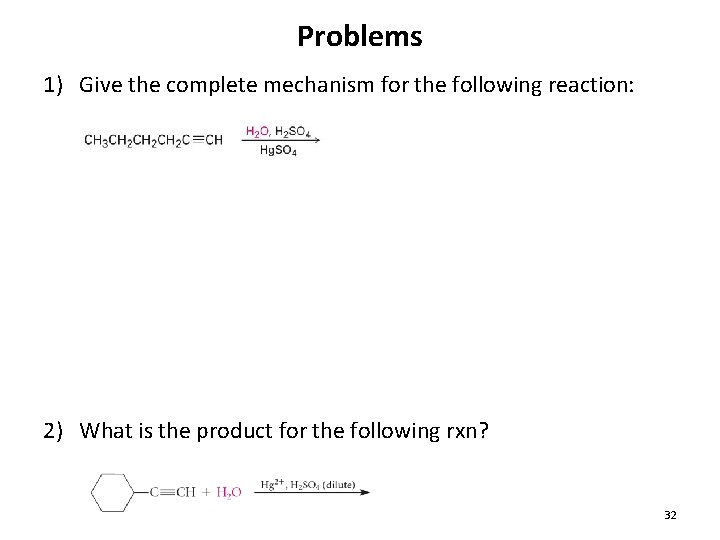
Problems 1) Give the complete mechanism for the following reaction: 2) What is the product for the following rxn? 32
 Halogenation of alkynes
Halogenation of alkynes Naming alkynes
Naming alkynes Anti markovnikov rule
Anti markovnikov rule Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes What is the general formula for alkenes?
What is the general formula for alkenes? Alkanes alkenes alkynes
Alkanes alkenes alkynes Hybridization of alkynes
Hybridization of alkynes Condensed structural formula of propyne
Condensed structural formula of propyne Preparation of alkynes
Preparation of alkynes Alkynes
Alkynes Alkynes
Alkynes Alkane to alkyne
Alkane to alkyne First 10 members of alkynes
First 10 members of alkynes Aldol condensation aldehyde
Aldol condensation aldehyde Combustion reaction of alkanes
Combustion reaction of alkanes Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng