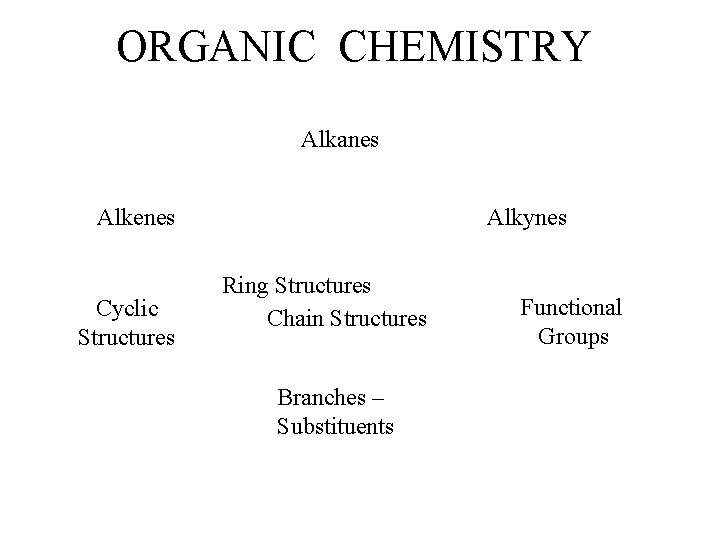
ORGANIC CHEMISTRY Alkanes Alkenes Cyclic Structures Alkynes Ring




- Slides: 37

ORGANIC CHEMISTRY Alkanes Alkenes Cyclic Structures Alkynes Ring Structures Chain Structures Branches – Substituents Functional Groups

TERMS ORGANIC CHEMISTRY carbon containing molecules HYDROCARBONS carbon & hydrogen molecules

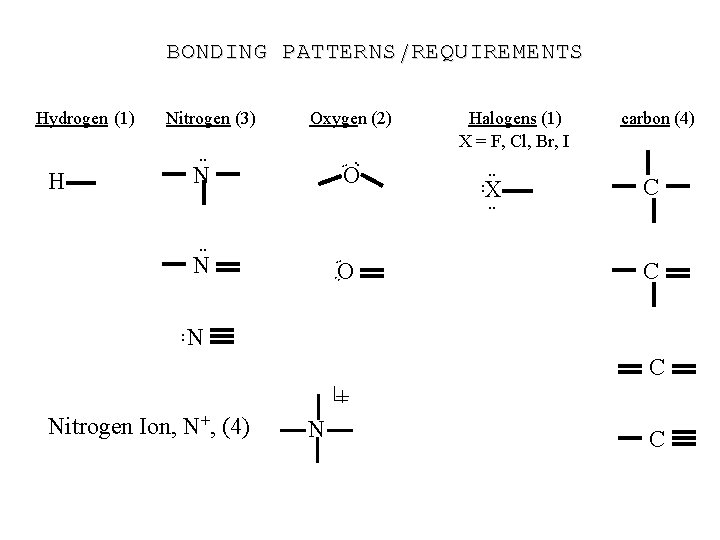
BONDING PATTERNS/REQUIREMENTS Hydrogen (1) Nitrogen (3) Oxygen (2) . . H O . . N O . . : . . N Halogens (1) X = F, Cl, Br, I. . X. . : carbon (4) C C N C + Nitrogen Ion, N+, (4) N C

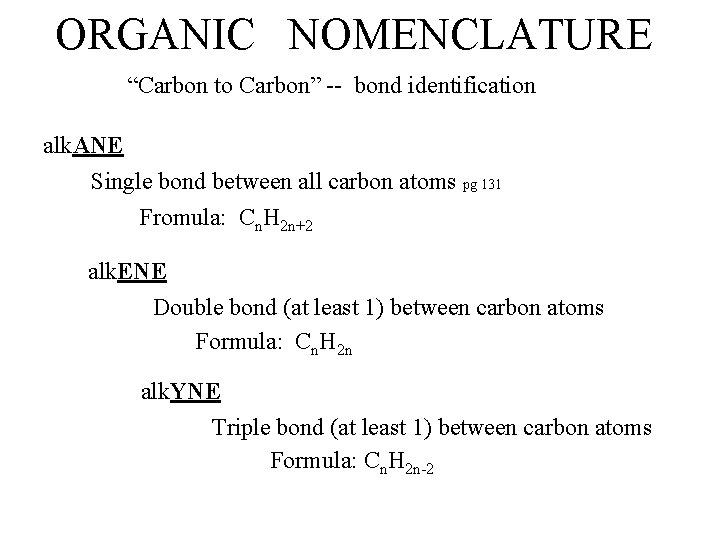
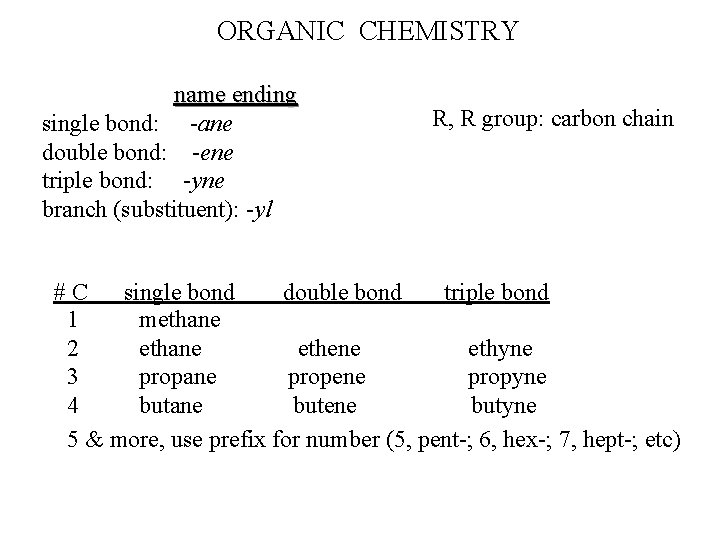
ORGANIC NOMENCLATURE “Carbon to Carbon” -- bond identification alk. ANE Single bond between all carbon atoms pg 131 Fromula: Cn. H 2 n+2 alk. ENE Double bond (at least 1) between carbon atoms Formula: Cn. H 2 n alk. YNE Triple bond (at least 1) between carbon atoms Formula: Cn. H 2 n-2

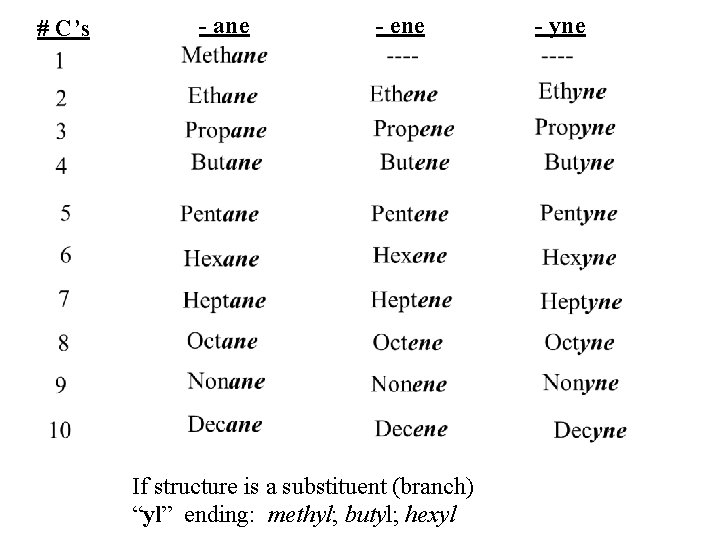
# C’s - ane - ene If structure is a substituent (branch) “yl” ending: methyl; butyl; hexyl - yne

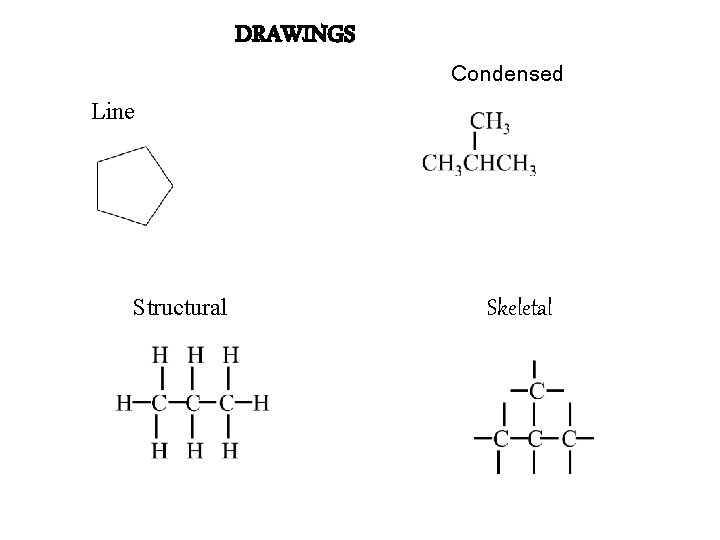
DRAWINGS Condensed Line Structural Skeletal

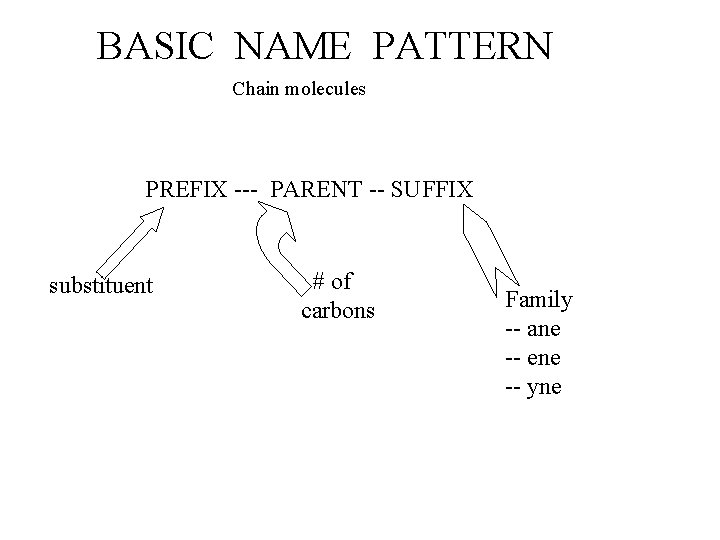
BASIC NAME PATTERN Chain molecules PREFIX --- PARENT -- SUFFIX substituent # of carbons Family -- ane -- ene -- yne

ORGANIC CHEMISTRY name ending single bond: -ane double bond: -ene triple bond: -yne branch (substituent): -yl R, R group: carbon chain #C single bond double bond triple bond 1 methane 2 ethane ethene ethyne 3 propane propene propyne 4 butane butene butyne 5 & more, use prefix for number (5, pent-; 6, hex-; 7, hept-; etc)

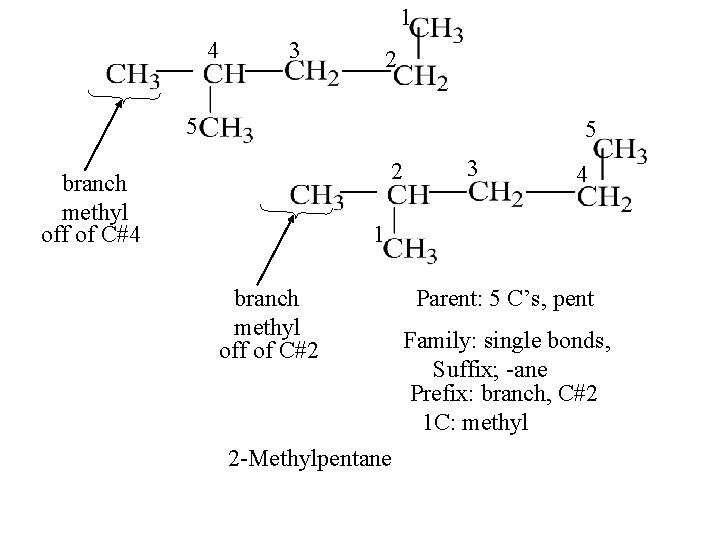
NAMING HYDROCARBONS 1. Find longest continuous carbon chain -- Parent 2. Count from end closest to 1 st branch -- Prefix 3. Family, base bond type -- Suffix Prefix - Parent - Suffix “-” dashes bet. # & name “, ” comma bet. numbers & name

1 4 3 2 5 5 2 branch methyl off of C#4 3 4 1 branch methyl off of C#2 2 -Methylpentane Parent: 5 C’s, pent Family: single bonds, Suffix; -ane Prefix: branch, C#2 1 C: methyl

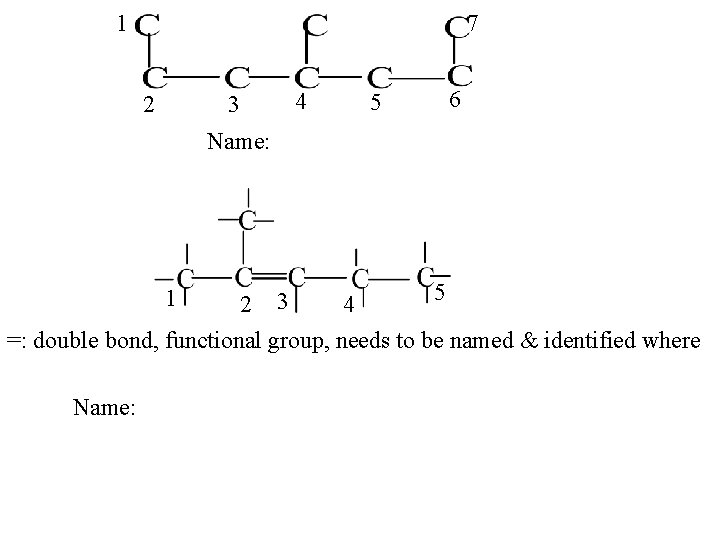
1 7 2 4 3 5 6 Name: 5 4 =: double bond, functional group, needs to be named & identified where 1 Name: 2 3

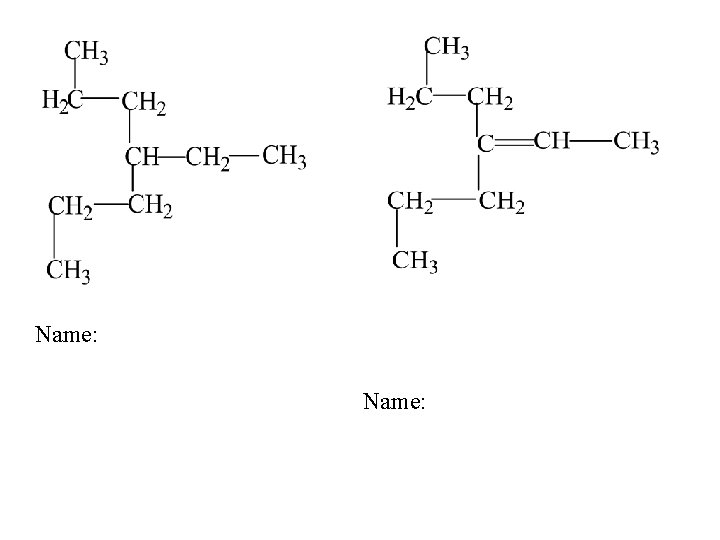
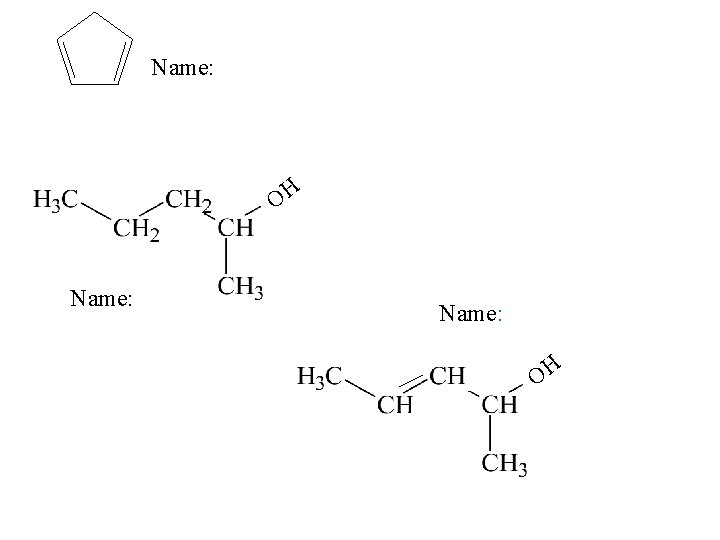
Name:

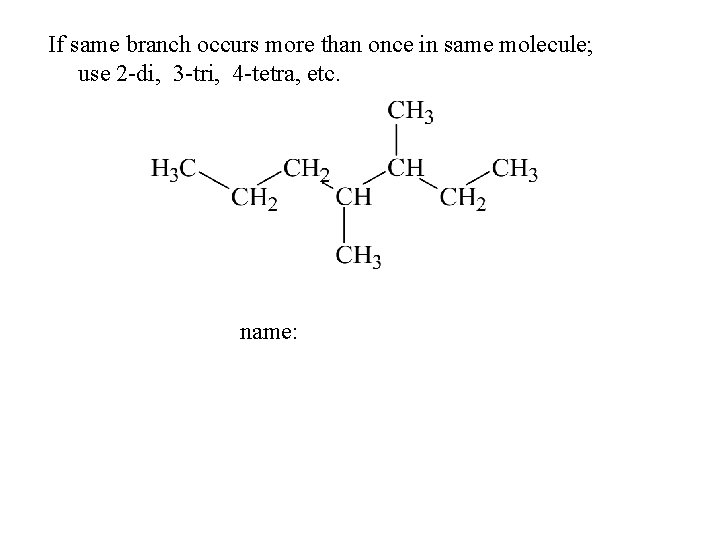
If same branch occurs more than once in same molecule; use 2 -di, 3 -tri, 4 -tetra, etc. name:

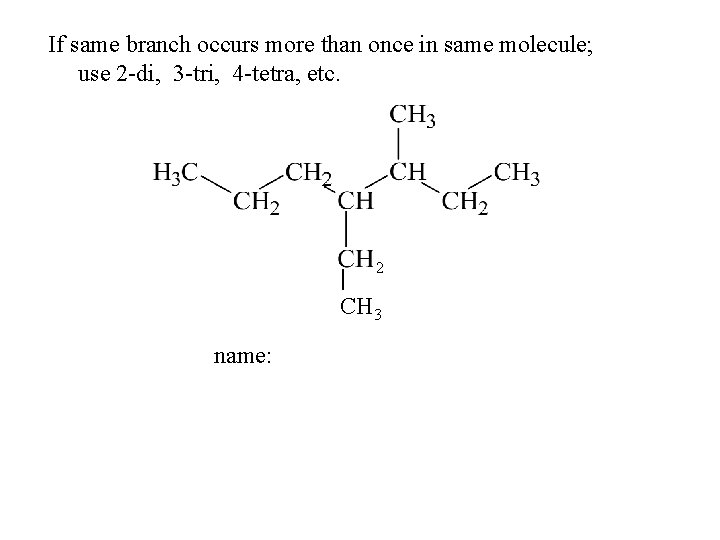
If same branch occurs more than once in same molecule; use 2 -di, 3 -tri, 4 -tetra, etc. | 2 CH 3 name:

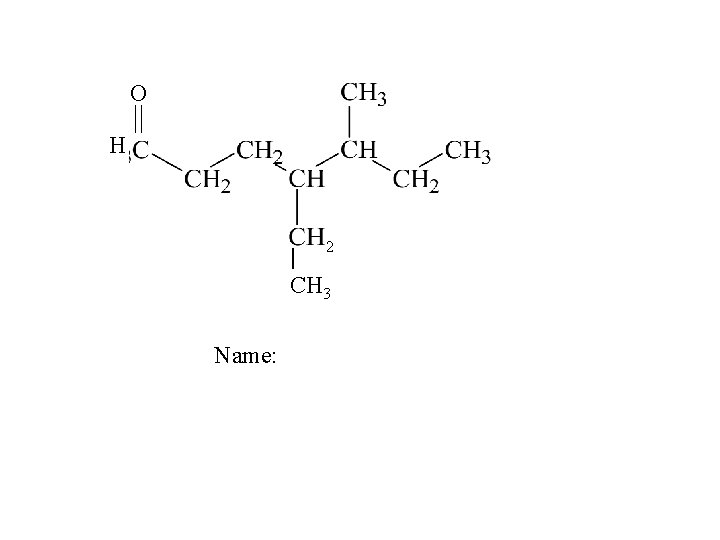
O H 2 | CH 3 Name:

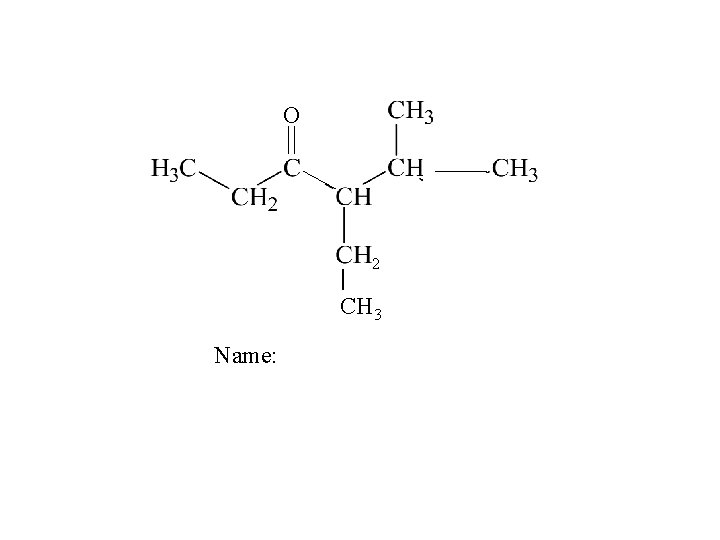
O 2 | CH 3 Name:

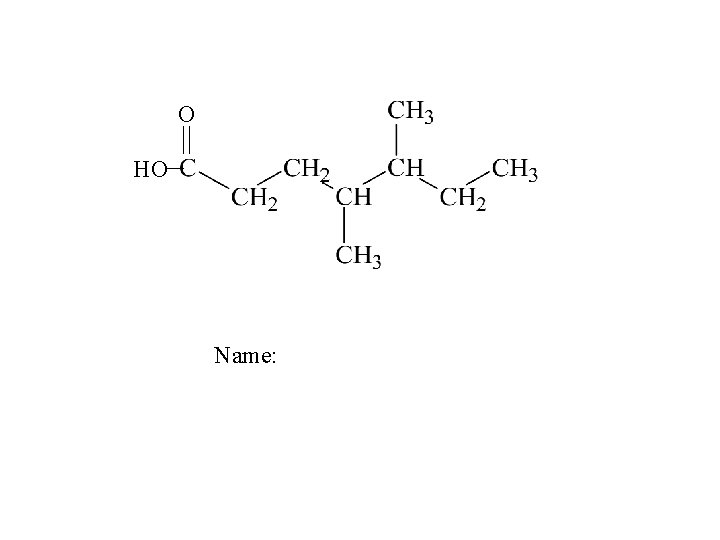
O HO Name:

Name: OH

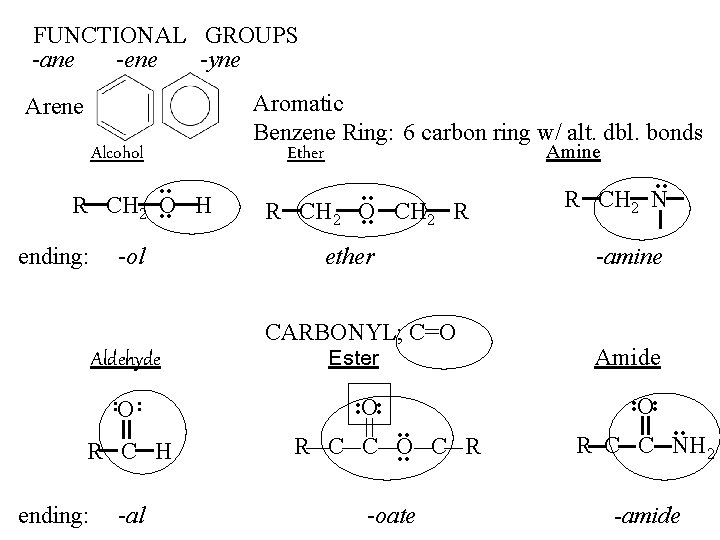
FUNCTIONAL GROUPS -ane -ene -yne Arene Alcohol . . R CH 2. . O H ending: -ol Aldehyde. . O Aromatic Benzene Ring: 6 carbon ring w/ alt. dbl. bonds Ether Amine . . R CH 2 O. . CH 2 R ether CARBONYL; C=O Ester . . O. . R CH 2 N -amine Amide . . O. . R C H . . R C C O. . C R R C C NH 2 -al -oate -amide ending:

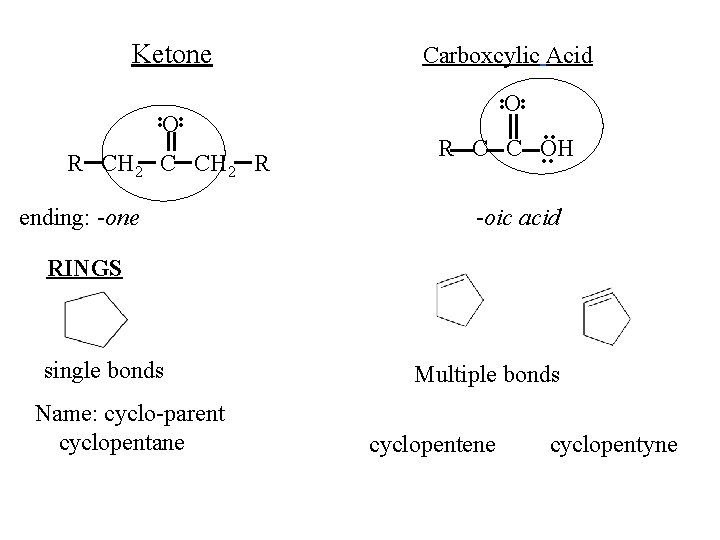
Ketone. . O. . R CH 2 C CH 2 R ending: -one Carboxcylic Acid . . O. . R C C OH. . -oic acid RINGS single bonds Name: cyclo-parent cyclopentane Multiple bonds cyclopentene cyclopentyne

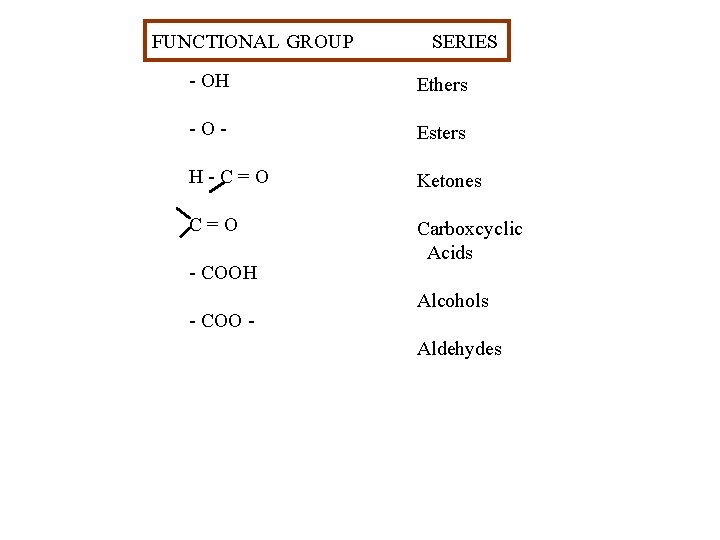
FUNCTIONAL GROUP SERIES - OH Ethers -O- Esters H-C=O Ketones C=O Carboxcyclic Acids - COOH - COO - Alcohols Aldehydes

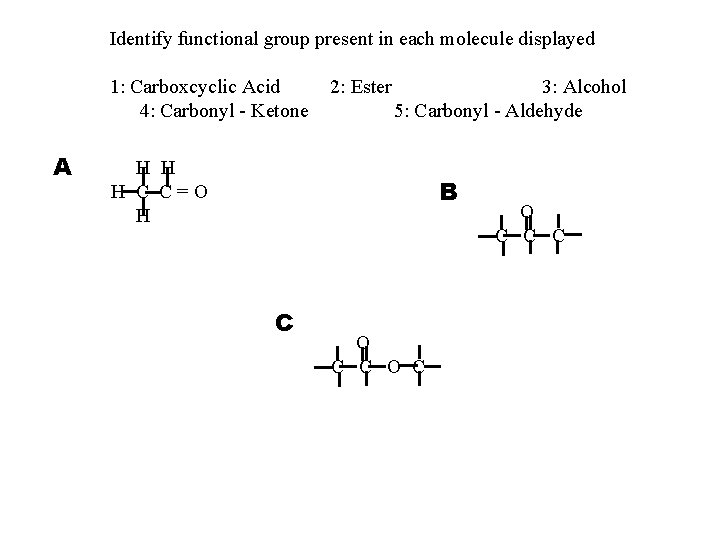
Identify functional group present in each molecule displayed 1: Carboxcyclic Acid 4: Carbonyl - Ketone A 2: Ester 3: Alcohol 5: Carbonyl - Aldehyde H H H C C=O H B C O C O C C C

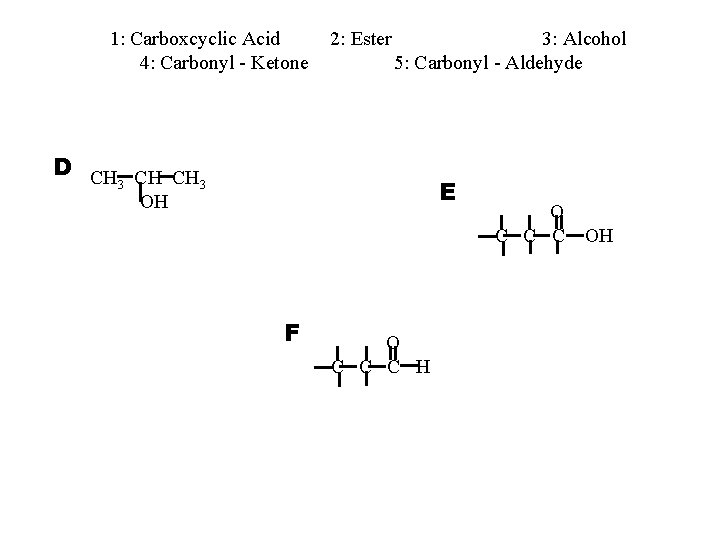
1: Carboxcyclic Acid 4: Carbonyl - Ketone 2: Ester 3: Alcohol 5: Carbonyl - Aldehyde D CH CH CH 3 3 E OH F O C C C H O C C C OH

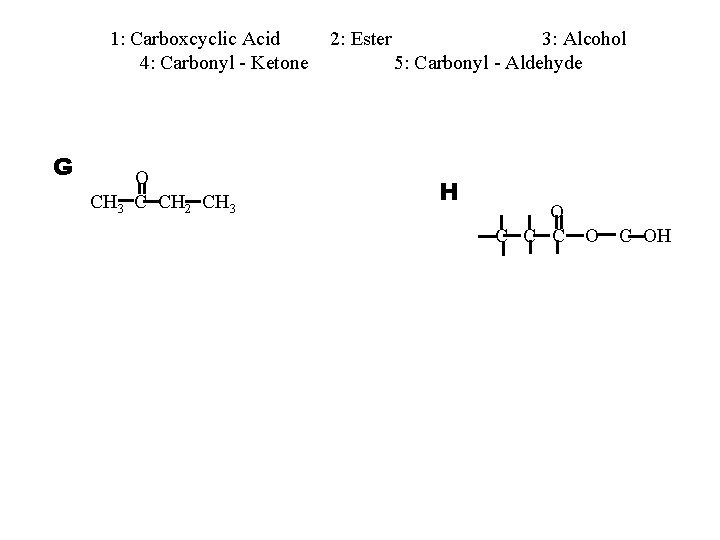
1: Carboxcyclic Acid 4: Carbonyl - Ketone G O CH 3 C CH 2 CH 3 2: Ester 3: Alcohol 5: Carbonyl - Aldehyde H O C C C OH

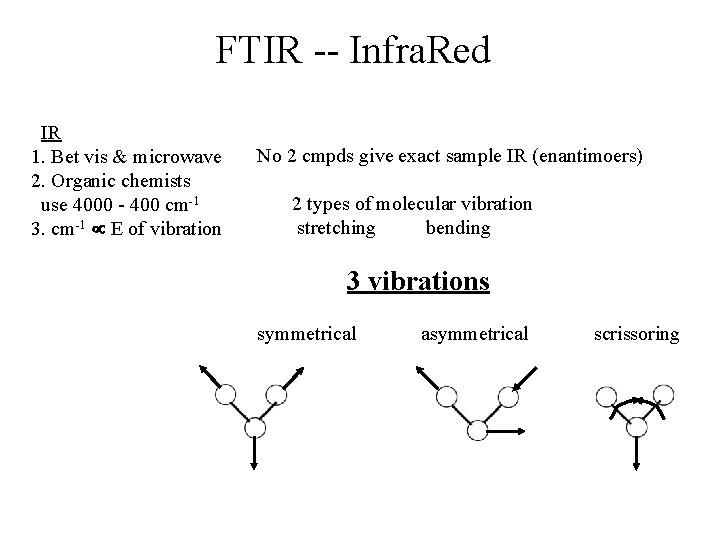
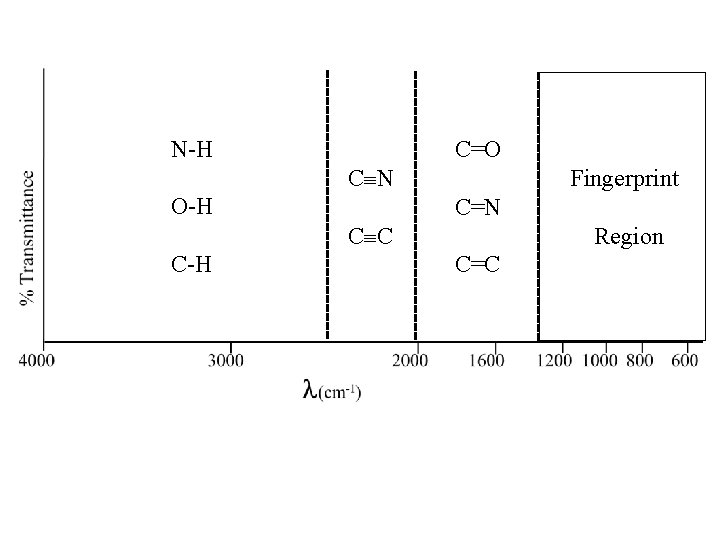
FTIR -- Infra. Red IR 1. Bet vis & microwave 2. Organic chemists use 4000 - 400 cm-1 3. cm-1 E of vibration No 2 cmpds give exact sample IR (enantimoers) 2 types of molecular vibration stretching bending 3 vibrations symmetrical asymmetrical scrissoring

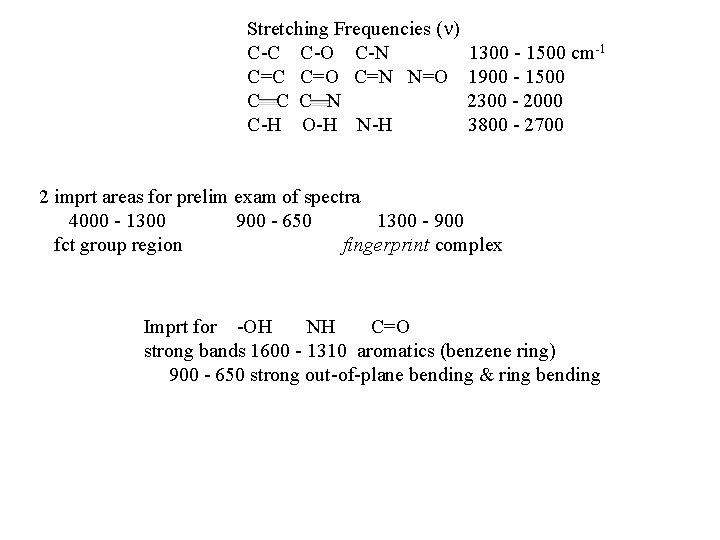
Stretching Frequencies ( ) C-C C-O C-N C=C C=O C=N N=O C C C N C-H O-H N-H 1300 - 1500 cm-1 1900 - 1500 2300 - 2000 3800 - 2700 2 imprt areas for prelim exam of spectra 4000 - 1300 900 - 650 1300 - 900 fct group region fingerprint complex Imprt for -OH NH C=O strong bands 1600 - 1310 aromatics (benzene ring) 900 - 650 strong out-of-plane bending & ring bending

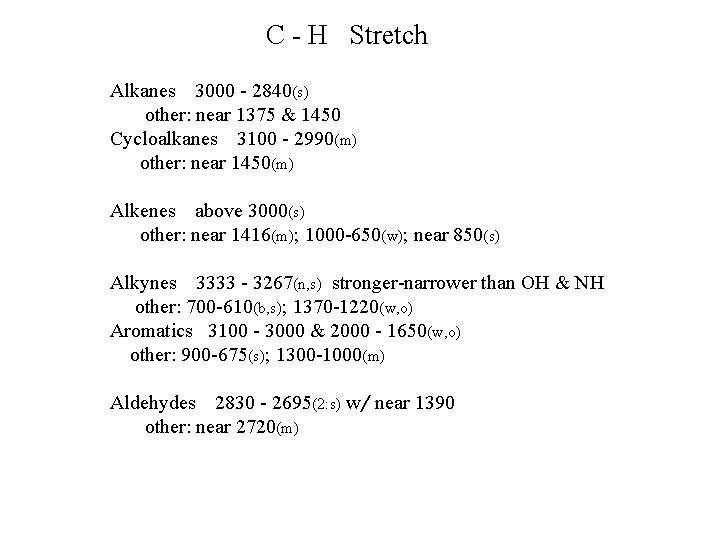
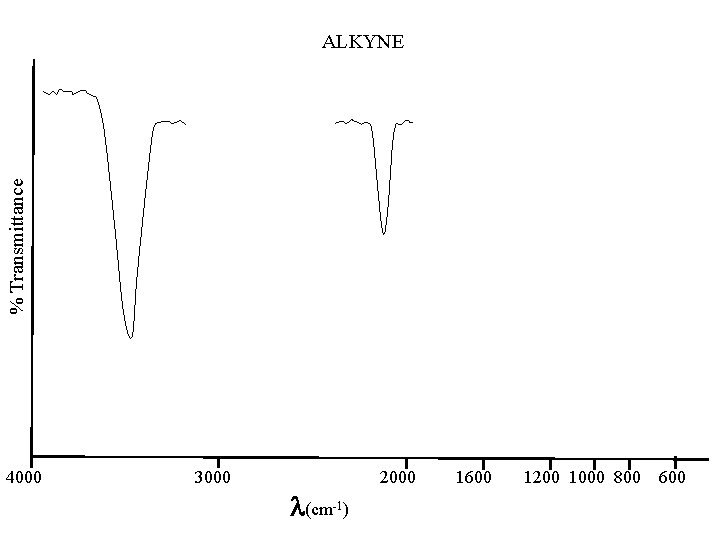
C - H Stretch Alkanes 3000 - 2840(s) other: near 1375 & 1450 Cycloalkanes 3100 - 2990(m) other: near 1450(m) Alkenes above 3000(s) other: near 1416(m); 1000 -650(w); near 850(s) Alkynes 3333 - 3267(n, s) stronger-narrower than OH & NH other: 700 -610(b, s); 1370 -1220(w, o) Aromatics 3100 - 3000 & 2000 - 1650(w, o) other: 900 -675(s); 1300 -1000(m) Aldehydes 2830 - 2695(2: s) w/ near 1390 other: near 2720(m)

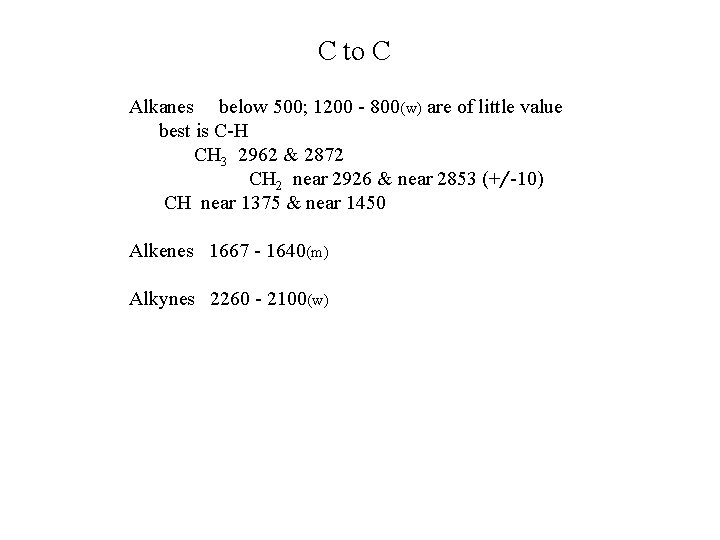
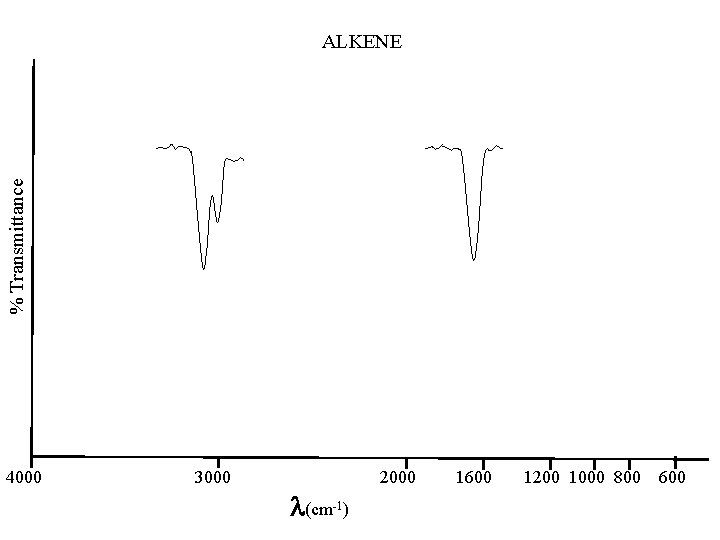
C to C Alkanes below 500; 1200 - 800(w) are of little value best is C-H CH 3 2962 & 2872 CH 2 near 2926 & near 2853 (+/-10) CH near 1375 & near 1450 Alkenes 1667 - 1640(m) Alkynes 2260 - 2100(w)

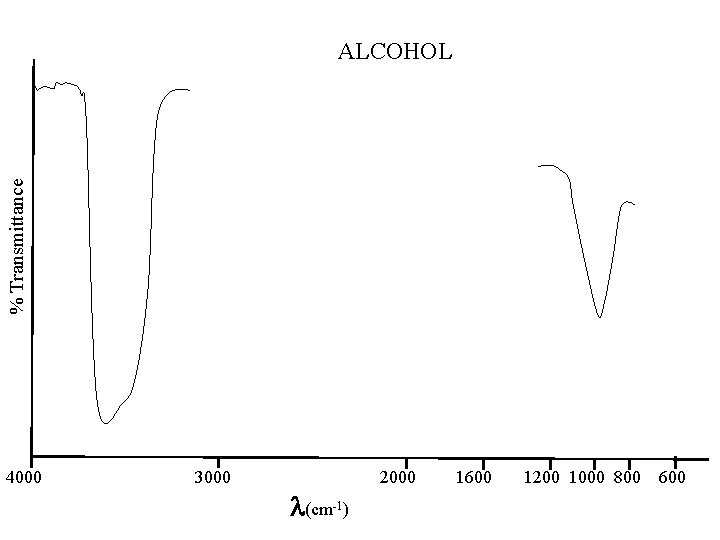
ALCOHOLS -- PHENOLS Alkenes - Phenols -OH & C-O OH 3650 - 3584(b, s); 1420 - 1330(m) CO 1260 - 1000(s) Ethers C-O-C similar to C-C-C but more intense 1150 - 1085(s) ETHERS Ethers C-O-C similar to C-C-C but more intense 1150 - 1085(s)

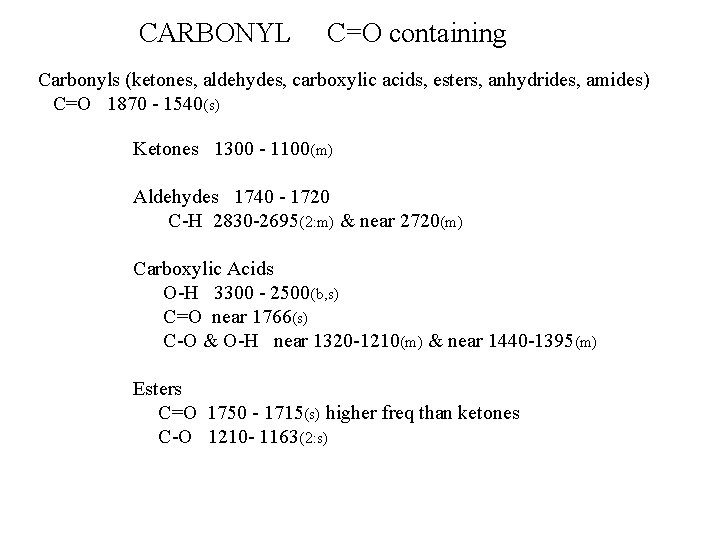
CARBONYL C=O containing Carbonyls (ketones, aldehydes, carboxylic acids, esters, anhydrides, amides) C=O 1870 - 1540(s) Ketones 1300 - 1100(m) Aldehydes 1740 - 1720 C-H 2830 -2695(2: m) & near 2720(m) Carboxylic Acids O-H 3300 - 2500(b, s) C=O near 1766(s) C-O & O-H near 1320 -1210(m) & near 1440 -1395(m) Esters C=O 1750 - 1715(s) higher freq than ketones C-O 1210 - 1163(2: s)

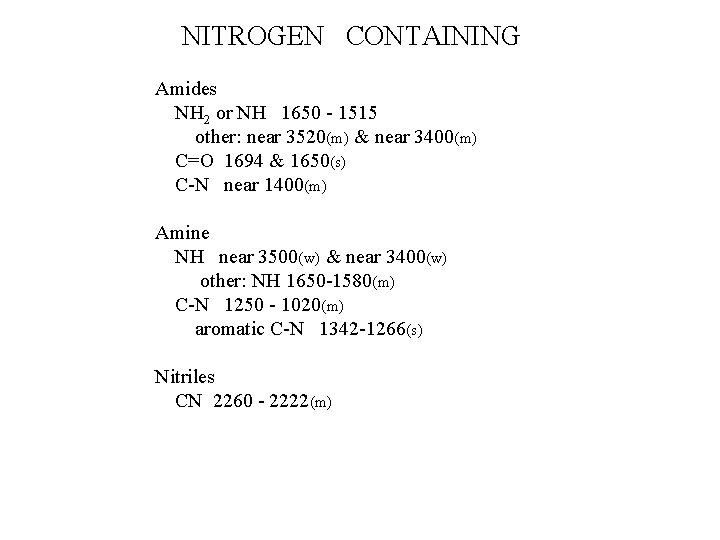
NITROGEN CONTAINING Amides NH 2 or NH 1650 - 1515 other: near 3520(m) & near 3400(m) C=O 1694 & 1650(s) C-N near 1400(m) Amine NH near 3500(w) & near 3400(w) other: NH 1650 -1580(m) C-N 1250 - 1020(m) aromatic C-N 1342 -1266(s) Nitriles CN 2260 - 2222(m)

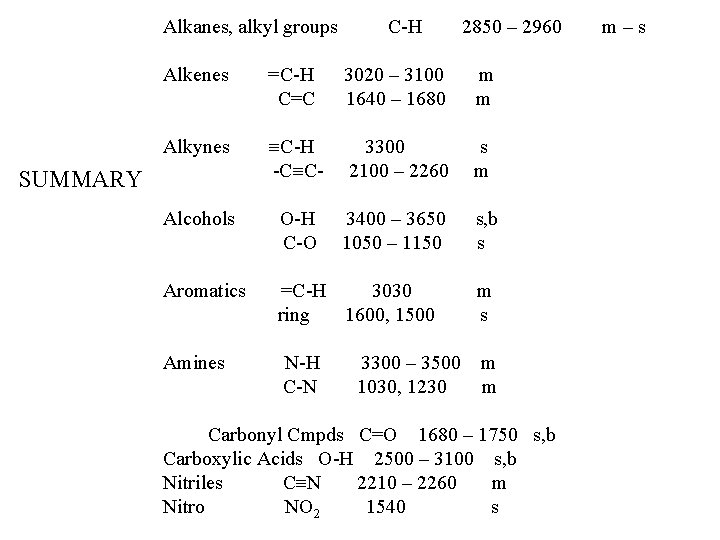
Alkanes, alkyl groups C-H 2850 – 2960 Alkenes =C-H C=C 3020 – 3100 1640 – 1680 m m Alkynes C-H -C C- 3300 2100 – 2260 s m Alcohols O-H C-O 3400 – 3650 1050 – 1150 s, b s Aromatics =C-H 3030 ring 1600, 1500 m s Amines N-H C-N m m SUMMARY 3300 – 3500 1030, 1230 Carbonyl Cmpds C=O 1680 – 1750 s, b Carboxylic Acids O-H 2500 – 3100 s, b Nitriles C N 2210 – 2260 m Nitro NO 2 1540 s m–s

N-H C=O C N O-H Fingerprint C=N C C C-H Region C=C

% Transmittance ALKENE 4000 3000 (cm-1) 2000 1600 1200 1000 800 600

% Transmittance ALKYNE 4000 3000 (cm-1) 2000 1600 1200 1000 800 600

% Transmittance ALCOHOL 4000 3000 (cm-1) 2000 1600 1200 1000 800 600

Website for spectral information of molecules http: //riodb 01. ibase. aist. go. jp/sdbs/cgi-bin/cre_index. cgi? lang=eng