Qualitative Inorganic Analysis Anions are divided into six



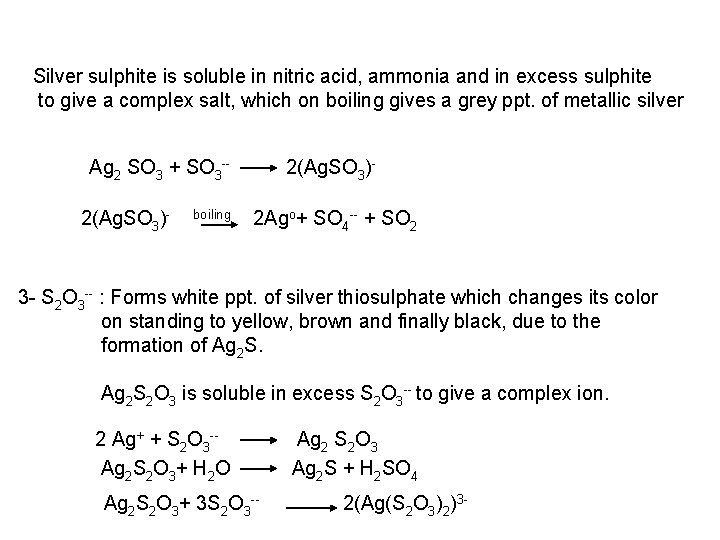


![Ag++ CI Ag. CI + 2 NH 3 [Ag(NH 3)2]CI [Ag(NH 3)2] CI + Ag++ CI Ag. CI + 2 NH 3 [Ag(NH 3)2]CI [Ag(NH 3)2] CI +](https://slidetodoc.com/presentation_image_h/0373df9f2dcb7a7b47eafbde92d6c3b6/image-34.jpg)


![2 - Ferro- and Ferricyanides: Both [Fe(CN)6]4 and [Fe(CN)6]3 react with Ag. NO 3 2 - Ferro- and Ferricyanides: Both [Fe(CN)6]4 and [Fe(CN)6]3 react with Ag. NO 3](https://slidetodoc.com/presentation_image_h/0373df9f2dcb7a7b47eafbde92d6c3b6/image-52.jpg)


![PO 43 +Mg 2++ NH 4+ Mg (NH 4) PO 4 [magnesium ammonium phosphate] PO 43 +Mg 2++ NH 4+ Mg (NH 4) PO 4 [magnesium ammonium phosphate]](https://slidetodoc.com/presentation_image_h/0373df9f2dcb7a7b47eafbde92d6c3b6/image-70.jpg)






- Slides: 88

Qualitative Inorganic Analysis Anions are divided into six groups: 1 Carbonates and Bicarbonates group 2 Sulphur containing anions 3 Halides 4 Cyanogen anions 5 Arsinic and phosphorous containing anions 6 Nitrogen containing anions

Carbonates and Bicarbonates group CO 32 HCO 3 I. General characters 1 - Parent acid: Carbonic acid (H 2 CO 3) is a very weak volatile acid (stronger than HCN and boric acid) Heating of solution of H 2 CO 3, CO 2 will evolve. H 2 CO 3 CO 2 + H 2 O Bicarbonates are considered to be the first step of ionization of carbonic acid, while in the second step carbonates are formed H 2 CO 3 H+ + HCO 3 H+ + CO 32

2 -Solubility: All carbonated with the exception of those of the alkali metals (Na+ and K+) and of ammonium are insoluble in water. All bicarbonates are soluble in water. II. General Reactions 1 - Dry Reactions a- Action of dilute HCl Decomposition with effervescence due to the evolution of CO 2 gas, for both CO 3 and HCO 3 + 2 H+ CO 2 + H 2 O Na. HCO 3+ H+ CO 2 + H 2 O + Na+ This is a type of displacement reaction in which stronger acid liberates the very weak carbonic acid, which spontaneously decomposes to CO 2 & H 2 O.

Test for CO 2 gas: The solid substance is placed in a test tube, dilute HCl is added, which immediately displaced the gas, which is evolved (upon warming) and passed into lime water or baryta water contained in another test tube. The production of a turbidity indicates the presence of carbonates or bicarbonates. CO 2 + Ca(OH)2 Ca. CO 3 + H 2 O CO 2 + Ba (OH)2 Ba. CO 3 + H 2 O With prolonged passage of CO 2, the turbidity formed due to the insoluble carbonates, slowly disappears as a result of the formation of a soluble bicarbonate. Ca. CO 3 + CO 2 + H 2 O Boiling Ca (HCO 3)2

2 - Wet Reactions In order to carry out the wet reactions, a solution of the substance in water must be done. Bicarbonates are mostly decomposed on heating with the liberation of CO 2. 2 HCO 3 + H 2 O + CO 2 . a- Reaction with Ag. NO 3 A white precipitate of silver carbonate is immediately formed. CO 3 +2 Ag+ Ag 2 CO 3 The precipitate is soluble in mineral acids (nitric acid) and in ammonia. Ag 2 CO 3 + 2 H+ 2 Ag+ + CO 2 + H 2 O Ag 2 CO 3+4 NH 3 2[Ag (NH 3)2]+ + CO 32 The precipitate becomes yellow or brown if the mixture is boiled. Ag 2 CO 3 boiling Ag 2 O +CO 2

b- Reactions with Ba. Cl 2, Ca. Cl 2 and Mg. SO 4: White precipitates of Ba. CO 3, Ca. CO 3 and Mg. CO 3 will be obtained upon the addition of these reagents to samples of carbonate solution. Ba. Cl 2 + Na. CO 3 Ba. CO 3 + 2 Na. Cl Ca++ + CO 3 Ca. CO 3 Mg++ + CO 3 Mg. CO 3 The precipitate is soluble in mineral acids For HCO 3 ; No ppt. on cold since all bicarbonates are soluble in water Ba++ +2 HCO 3 Ba(HCO 3)2 Soluble H 2 O + CO 2 + Ba. CO 3 Boiling

III. Mixture of CO 32 - & HCO-3 Both anions haves similar reactions, but CO 32 form precipitates immediately on cold upon the addition of Ca. Cl 2, Ba. Cl 2 or Mg. SO 4, while the bicarbonates of these metals are soluble. Separation: Add excess Ca. Cl 2 (Ba. Cl 2 or Mg. SO 4) to a solution of the mixture CO 32 /HCO 3 a white ppt. indicates CO 3 , centrifuge or filter Contrifugate May be HCO 3 Confirmatory test: 1) Boil 2) Add ammonia solution white ppt. Ca (HCO 3)2 + 2 NH 3 Ca. CO 3+ (NH 4)2 CO 3 White ppt. Ca. CO 32 H+ CO 2 + H 2 O

Sulphur-containing anions This group of anions, are; 1 - Sulphide (S 2 -) 2 - Sulphites (SO 32 -) 3 - Thiosulphate (S 2 O 32 -) 4 - Sulphates (SO 42 -) 5 - Perasulphate (S 2 O 82 -). I. General characters 1 - Parent Acids: a- Hydrogren sulphide or Hydrosulphuric acid (H 2 S) It is a gas with offensive rotten egg odour and poisonous. In solution it gives a weak acid, which ionizes in two steps; H 2 S H++ HS (hydrosulphide ion) HS H++ S (sulphide ion) Both HS and S ions give the same reactions.

b- Sulphurous acid: (H 2 SO 3) This acid is only known in solution (like H 2 CO 3). It has moderate strong acidity. Like H 2 CO 3 in water; present in equilibrium as follows: H 2 O + SO 2 heat H 2 SO 3 H++ HSO 3 H++ SO 3 Acid sulphite c- Thiosulphuric acid: (H 2 S 2 O 3) It is not known in the free form, and decomposes to give, H 2 O, SO 2 and S. It's more stronger than sulphurous acid in solutions. It consists of SO 32 solution and S, which upon boiling gives S 2 O 32. d- Sulphuric acid: (H 2 SO 4): It's a colourless oily liquiud (B. P. 3300 C). General properties of H 2 SO 4 1 - Acid properties; It is one of the strongest acids, ionize in dilute solutions in two steps, H 2 SO 4 H++ HSO 4 (hydrogen sulphate) HSO 4 H++ SO 4 (sulphate)

Metals can liberate hydrogen from H 2 SO 4 solution. H 2 SO 4+ Zno Zn. SO 4+ H 2 Being a strong acid can replace weak acids like, boric acids, hydrocyanic acid and volatile acids or their decomposition products due to its high B. P. 2 Na. Cl + H 2 SO 4 Na 2 SO 4+ 2 HCl 2 - Dehydrating properties; Conc. H 2 SO 4 has a great tendency to combine with water to from stable hydrates H 2 SO 4. x H 2 O. So it is used as a dehydrating agent for certain substance, and used mostly in the dissectors. It causes charring for certain organic substances as sugars due to the vigorous abstracting of water from theses substances. 3 - Oxidizing properties: It's considered to be as moderately strong oxidizing agent when heated with most reducing agents H 2 SO 4 heat H 2 O + SO 2 + [O] It is reduced to SO 2, while with active reducing agents it may be reduced to So or H 2 S.

2 -Solubility: All Na+, K+ and NH 4+ salts of sulphur containing anions are soluble in water. Sulphides : Other sulphides are in soluble except those of Ca++, Ba++, & Sr 2+ dissolve due to hydrolysis. Sulphites: Other sulphites are all in soluble. Thiosulphates: Most S 2 O 32 are soluble, Ag+, Pb++, Hg 2+ & Ba++ salts are slightly soluble. Sulphates: All sulphates are soluble except Pb++, Ba++ and Sr++. Ca++ & Mg++ salts are slightly soluble.

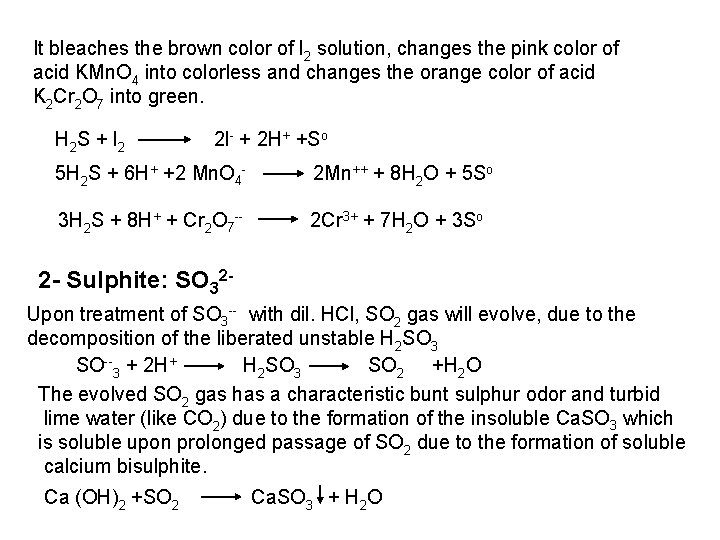
3 -Complexing agent: Thiosulphate form complex with Fe 3++ 2 S 2 O 3 (Fe(S 2 O 3)2) purple color 4 -Reducing agent: Sulphides, sulphites and thiosulphates are reducing agents. They reduce solutions of I 2, KMn. O 4 and K 2 Cr 2 O 7 with varying activities in acidified solutions. H+ I 2+S 2 lodine (brown) 2 I +So Colourless 2 KMn. O 4+ 5 S 2 + 16 H+ 2 Mn+++ 5 SO 4 + 8 H 2 O +2 K+

I 2+SO 32 +H 2 O SO 42 +2 I +2 H+ 2 Mn. O 4 + 5 SO 3 + 6 H+ Cr 2 O 7 + 3 SO 32 + 8 H+ I 2+2 S 2 O 3 H+ + Fe 3++2 S 2 O 32 H 2 Mn+++ 5 SO 4 + 3 H 2 O 2 Cr 3++ 3 SO 4 +4 H 2 O S 4 O 62 +2 I Tetrathionate S 4 O 62 +Fe 2+ 8 Mn. O 4 + 5 S 2 O 3 + 14 H+ 4 Cr 2 O 72 + 3 S 2 O 32 + 26 H+ 8 Mn+++10 SO 4 +7 H 2 O 8 Cr 3++6 SO 4 + 13 H 2 O

II. General Reactions 1 - Dry Reactions a- Action of dilute HCl 1. Sulphide; S 2 H 2 S gas; evolved upon adding dil. HCl to a solid sample. The gas evolved has its characteristic rotten egg odour, and could be identified by 1 blackening of filter paper moistened with lead acetate sol. S + 2 H+ H 2 S+Pb++ H 2 S Pb. S black 2 alternatively, a filter paper moistened with cadmium acetate solution, turns yellow H 2 S + Cd++ Cd. S Yellow H 2 S has reducing character, It reacts with l 2 solution, acid KMn. O 4, acid K 2 Cr 2 O 7

It bleaches the brown color of l 2 solution, changes the pink color of acid KMn. O 4 into colorless and changes the orange color of acid K 2 Cr 2 O 7 into green. H 2 S + l 2 2 l + 2 H+ +So 5 H 2 S + 6 H+ +2 Mn. O 4 2 Mn++ + 8 H 2 O + 5 So 3 H 2 S + 8 H+ + Cr 2 O 7 2 Cr 3+ + 7 H 2 O + 3 So 2 - Sulphite: SO 32 Upon treatment of SO 3 with dil. HCl, SO 2 gas will evolve, due to the decomposition of the liberated unstable H 2 SO 3 + 2 H+ H 2 SO 3 SO 2 +H 2 O The evolved SO 2 gas has a characteristic bunt sulphur odor and turbid lime water (like CO 2) due to the formation of the insoluble Ca. SO 3 which is soluble upon prolonged passage of SO 2 due to the formation of soluble calcium bisulphite. Ca (OH)2 +SO 2 Ca. SO 3 + H 2 O

Ca. SO 3 + SO 2 + H 2 O Ca(HSO 3)2. SO 2 like H 2 S has reducing character, bleaches the brown color of iodine, reacts with acid KMn. O 4 and acid K 2 Cr 2 O 7. l 2 + SO 2 + H 2 O SO 3 + 2 H++ 2 l 2 Mn. O 4 + 5 SO 2 + 6 H+ 2 Mn++ + 5 SO 3 + 3 H 2 O Cr 2 O 72 +3 SO 2 + 8 H+ 2 Cr 3++ 3 SO 3 + 4 H 2 O 3 - Thiosulphate; S 2 O 32 No immediate change on cold, but on warming with dil. HCl or standing, the solution become turbid due to the liberated yellow colloidal sulphur with evolution of SO 2 gas. This is due to the decomposition of the produced unstable thiosulphuric acid. S 2 O 3 + 2 H+ H 2 S 2 O 3 H 2 O + SO 2 + So Thiosulphate has the same action of sulphite with HCl in addition to formation of yellow colloidal precipitate.

4 - Sulphate: SO 42 No reaction with dil. HCl. 2 - Wet Reactions a- Reaction with Ba. Cl 2: Add Ba. Cl 2 reagent to neutral sample solution: 1 S 2 : No visible reaction 2 SO 32 : White ppt. of Ba. SO 3 is formed which is soluble in dil. HCl. Ba+++ SO 32 Ba. SO 3 3 S 2 O 3 : No ppt. in dilute solution, but a ppt. is formed from very concentrated solution. 4 SO 4 : A white ppt. of Ba. SO 4 is formed which is insoluble in dil. HCl, even upon boiling. Ba+++ SO 4 Ba. SO 4 White

b- Reaction with Ag. NO 3: Add Ag. NO 3 reagent to the neutral sample solution 1 S 2 : a black ppt. of Ag 2 S is formed which is soluble in hot dil. HNO 3, insoluble in ammonia and KCN solution 2 Ag++ S Ag 2 S black 2 SO 32 : A white crystalline ppt. of Ag 2 SO 3 is formed, which on boiling with water undergoes self oxidation reduction with the production of grey ppt. of metallic silver. 2 Ag++ SO 32 2 Ag 2 SO 3 boil Ag 2 SO 3 White 2 Ago + Ag 2 SO 4 + SO 2
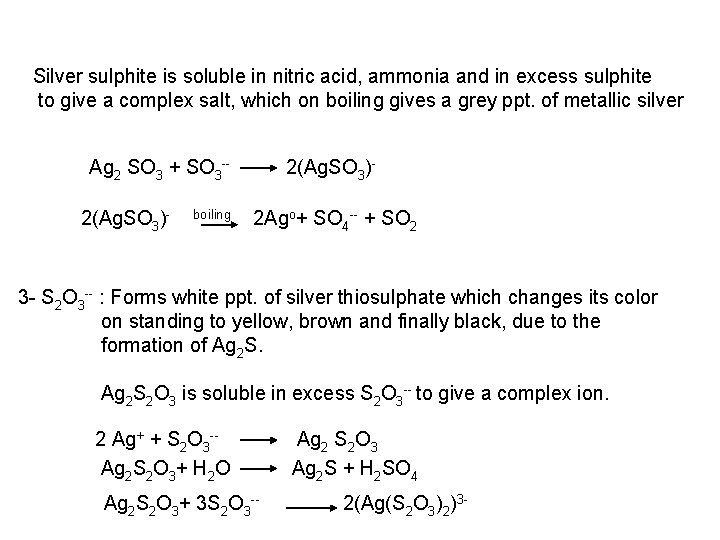
Silver sulphite is soluble in nitric acid, ammonia and in excess sulphite to give a complex salt, which on boiling gives a grey ppt. of metallic silver Ag 2 SO 3 + SO 3 2(Ag. SO 3) boiling 2(Ag. SO 3) 2 Ago+ SO 4 + SO 2 3 S 2 O 3 : Forms white ppt. of silver thiosulphate which changes its color on standing to yellow, brown and finally black, due to the formation of Ag 2 S 2 O 3 is soluble in excess S 2 O 3 to give a complex ion. 2 Ag+ + S 2 O 3 Ag 2 S 2 O 3+ H 2 O Ag 2 S 2 O 3+ 3 S 2 O 3 Ag 2 S 2 O 3 Ag 2 S + H 2 SO 4 2(Ag(S 2 O 3)2)3

4 SO 42 : No ppt. in dil solution, but a ppt. may be formed in a very concentrated solution. c- Reaction with Fe. Cl 3: Add Fe. Cl 3 reagent to the neutral sample solution 1 S 2 : a black ppt. of Fe 2 S 3 is formed which is soluble in dil. HNO 3 2 Fe 3++ 3 S Fe 2 S 3 black 2 SO 3 : A drak red color of ferric sulphite is produced on cold. 2 Fe 3++ SO 3 Fe 2(SO 3)3 3 S 2 O 32 : A purple color of complex ferric thiosulphate is produced which disappears on boiling as tetrathionate and Fe 2+ are formed from the oxidation of S 2 O 32 with Fe 3+, even on cold Fe 3++ 2 S 2 O 32 2 S 2 O 3 + 2 Fe 3+ (Fe(S 2 O 3)2) 2 Fe+++ S 4 O 6 4 SO 42 : do not react with Fe. Cl 3.

d- Reaction with lead acetate: Adding lead acetate reagent to the neutral sample solution. 1 S : A black ppt. of Pb. S is produced Pb+++ S Pb. S 2 SO 32 : A with ppt. of lead sulphite which is soluble in cold HNO 3. On boiling oxidation to Pb. SO 4 which is a white ppt. occurs. SO 3 + Pb++ Pb. SO 3 3 S 2 O 3 : A white ppt. of lead thiosulphate is formed which is soluble in cold HNO 3, on boiling a black ppt. of Pb. S is formed. Pb+++S 2 O 3 Pb. S 2 O 3 4 SO 4 : A white ppt. lead suphate, which is insoluble in cold dil. mineral acids, but soluble in ammonium acetate and hydroxide solutions (Na+ and K+)

Pb+++ SO 42 Pb. SO 4+ 4 CH 3 COO Pb. SO 4+ 3 OH (Pb (CH 3 COO)4)2 + SO 42 HPb. O 2 + H 2 O +SO 42 Plumbites III. Special Tests 1. Sulphide; S 2 Cadmium carbonate test : The sulphide solution is shaken with Cd. CO 3 powder, a canary yellow ppt. of Cd. S is produced. S + Cd. CO 3 Cd. S + CO 32 This test could be used for the identification and separation of S 2 when present in a mixture with other sulphur containing anions, or those anions which do not react with Cd. CO 3.

2 - Sulphite: SO 32 - Zinc nitroprusside test : Add to cold saturated Zn. SO 4 solution, equal volume of K 4[Fe (CN)6] solution, add few drops of 1% sodium nitroprusside solution. This solution is added to the SO 32 solution, a salmon colored ppt. of zinc nitroprusside is formed Zn (Fe(CN)5 NO). The latter reacts with moist SO 2 to give a red ppt. of Na 5[Fe(CN)5 SO 3] 3 - Thiosulphate; S 2 O 32 - Formation of thiocyanate : By boiling with KCN solution (poison), in the presence of Na. OH, Cool, acidify and add Fe. CI 3, a blood red color of ferric thiocyanate complex is produced. S 2 O 3 + CN Fe 3++ SCN OH boil Cool SCN + SO 3 Fe(SCN)2+

4 - Sulphate: SO 42 Hepar’s test Sulpate is reduced by carbon to sulphide by heating on a piece of charcoal in the presence of Na 2 CO 3 in the reducing zone of the flame MSO 4+ Na 2 CO 3 Na 2 SO 4+ C Fusion Na 2 SO 4+ MCO 3 Na 2 S + 4 CO Transfer the fusion product to a silver coin and moisten with a little water, a brownish black stain of Ag 2 S results. S + 2 H 2 O 2 OH + H 2 S + 2 Ag Ag 2 S +H 2

IV. Analysis of Mixtures 1 - Mixture of S 2 -, SO 32 -, S 2 O 32 - and SO 42 - : Separation is carried first shaking the mixture solution with Cd. CO 3 powder. The centrifugate is allowed to react with Ba. Cl 2 solution which will precipitate Ba. SO 4 and Ba. SO 3 leaving S 2 O 32 as soluble centifugate. The precipitated Ba. SO 4 and Ba. SO 3 can be separated by the solubility of Ba. SO 3 in excess dil. HCI. S 2 , SO 32 , S 2 O 32 , & SO 42 Solution + Cd. CO 3 Yellow ppt. S 2 Centrifugate + Ba. CI 2 White ppt. Ba. SO 3+Ba. SO 4 HCl White PPt SO 42 Centrifugate SO 32 confirm by reducing character Centrifugate S 2 O 32 Heat HCl SO 2 + So

2 - Mixture of CO 32 - and SO 32 - or S 2 O 32 This type of mixtures are considered to be difficult, due to the interference occur upon the addition of dil. HCI which liberates CO 2 and SO 2 gases which turbides lime water and disappears on prolonged passage. SO 2 can be detected by its reducing characters as discussed before, but CO 2 has non reducing characters. Therefore SO 32 or S 2 O 32 ions must be firstly oxidized into SO 42 by an oxidizing agent such as H 2 O 2, K 2 Cr 2 O 7 or KMn. O 4 and dil. H 2 SO 4 and warm, CO 2 will only evolve which can be test with lime water. 3 - Mixture of H 2 S and SO 2 gases: In order to differentiate between these two gases which evolve upon the addition of dil. HCI to sulphides, sulphites and thiosulphates and having similar reducing properties. A paper moistened with lead acetate solution changes into black when exposed to H 2 S gas, SO 2 can cause turbidity to lime water

Halides This group of anions, are; 1 - Fluoride (F-) 2 - Chloride (Cl-) 3 - Bromide (Br-) 4 - Iodide (I-) Fluorides, chlorides, bromides and iodides are known as halogens. They are characterized by their higher electronegativity As the ionic size increases, the tendency to loose electrons increases and therefore iodide ion is firstly and easily oxidized into free I 2 by loosing readily an electron followed by Br when present in a mixture. However it's difficult to oxidize F into F 2, hence F ions are highly stable to held strongly a proton. Therefore the order of stronger halogen acid is from HI HBr HCl HF.

I. General characters 1 - Parent Acids: a- Hydrofluoric acid; HF : It's coloress fuming highly corrosive and itching liquid (B. P. 19. 4 o. C). Soluble in water producing the weakest acidic solution in the halogen acid series. b- Hydrochloric acid : HCl Colorless gas with irritating odor, fumes in moist air, extremely soluble in water to form acidic solution. Concentrated HCI contains 37% of HCI gas. c- Hydrobromic acids : HBr Colorless gas with irritating odor, fumes in moist air and is extremely soluble in water forming very strongly acidic solution. On standing the solution becomes yellow due to the oxidation to bromine. d- Hydroiodic acid: HI Colorless gas with irritating odor, fumes strongly in moist air, soluble in water forming the strongest acidic solution of the haloacid series. the solution is colorless, becomes brown on standing due to the liberated iodine.

2 -Solubility: All the salts of CI , Br and I are soluble except Ag+, Hg 22+, & Cu+ salts, their lead salts are slightly soluble in cold water, soluble in hot water. The alkali metal salts of fluorides, ammonium and silver salts are soluble, other salts are insoluble or sparingly soluble. 3 -Reducing agent: Cl has very weak reducing character. Br and I have reducing character, they can react with oxidizing agent like chlorine water to give Br 2 or I 2. I has strong reducing power than Br so it react with Fe. Cl 3, H 2 O 2 and nitrite solutions. II. General Reactions 1 - Dry Reactions a- Action of dilute HCl Hydrochloric acid shows no reaction upon treatment of the solid sample with it even on heating. This reaction can differentiate carbonate and sulphur group from halides.

b- Action of concentrated H 2 SO 4: Decomposition of the halides occurs upon the addition of the strong non volatile concentrated H 2 SO 4 to the solid sample, this occurs in the cold, completely on warming with the evolution of HX which can be recognized by a) the fumes evolved. b) Confirmatory chemical test 2 X + H 2 SO 4 = 2 HX + SO 42 X = may be CI , Br and F 1 - For Fluoride: Fluoride gives a characteristic reaction when treated with conc. H 2 SO 4. Hydrofluoric acid is produced which is colorless and fumes with moist air. due to the corrosive and itching action of the gas on the glass in presence of H 2 O, the test tube or the glass rod subjected to the evolved HF gas acquire oily appearance due to the formation of silicic acid and hydrofluorosilicic acid. This test is considered to be specific for fluoride anion, even in the presence of other halides. 2 F + H 2 SO 4 4 HF + Si. O 2 Si. F 4+ 2 H 2 O glass H 2 Si. O 3+ 2 H 2 Si. F 6 silicic acid hydrofluoro silicic acid 2 H F + SO 4 3 Si. F 4+ 3 H 2 O

2 - For chloride : HCI gas is evolved upon treatment with conc. H 2 SO 4 which can be identified by : 2 CI + H 2 SO 4 2 HCI + SO 4 1 Formation of white fumes with moist air due the formation of droplets of hydrochloric acid. 2 Pungent irritating odor. 3 Changing a blue moistened litmus paper into red. 4 Formation of white fumes of NH 4 CI when a glass rod moistened with ammonium hydroxide solution is exposed to the evolved gas. NH 4 OH + HCI NH 4 CI + H 2 O 3 - For Bromide: A mixture of HBr and Br 2 may be formed which have characteristic brown color especially on warming. At the same time sulphuric acid will be reduced into SO 2, H 2 S or S 2 Br + H 2 SO 4 2 HBr + H 2 SO 4 2 HBr + SO 4 Br 2 + SO 2+ 2 H 2 O

4 - For iodide: Since HI is the most active reducing agent, so it is readily oxidized to iodine which appears as violet fumes. I 2 can be detected by exposing the evolved gas to paper moistened with starch solution, it changes into blue. 2 I + H 2 SO 4 2 HI + H 2 SO 4 6 HI + H 2 SO 4 8 HI + H 2 SO 4 2 HI + SO 42 I 2 + SO 2 + 2 H 2 O 3 I 2 + S + 4 H 2 O 4 I 2 + H 2 S + 4 H 2 O c- Action of concentrated H 2 SO 4 and Mn. O 2: If the solid halide is mixed with an equal quantity of precipitated manganese dioxide, concentrated H 2 SO 4 added and the mixture gently warmed. Chlorine, bromine and iodine are evolved from CI , Br and I but F liberates HF since it has no reducing properties. 2 X + 4 H++ Mn. O 2 X = may be CI , Br and I Mn+++ 2 H 2 O +X 2

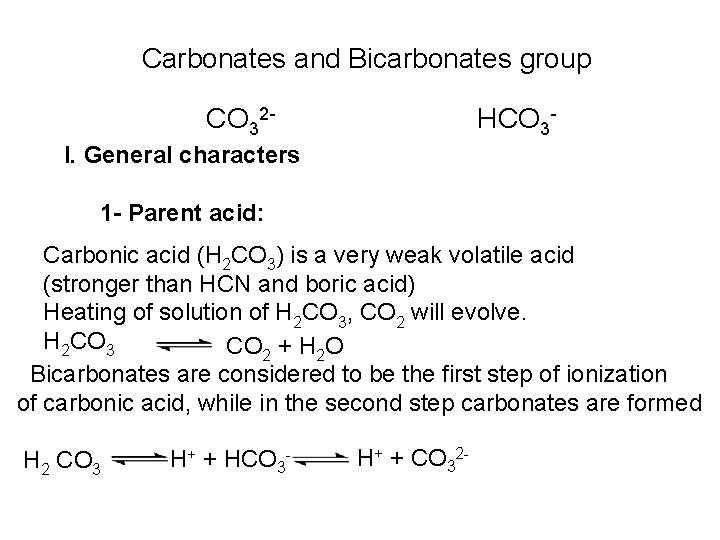
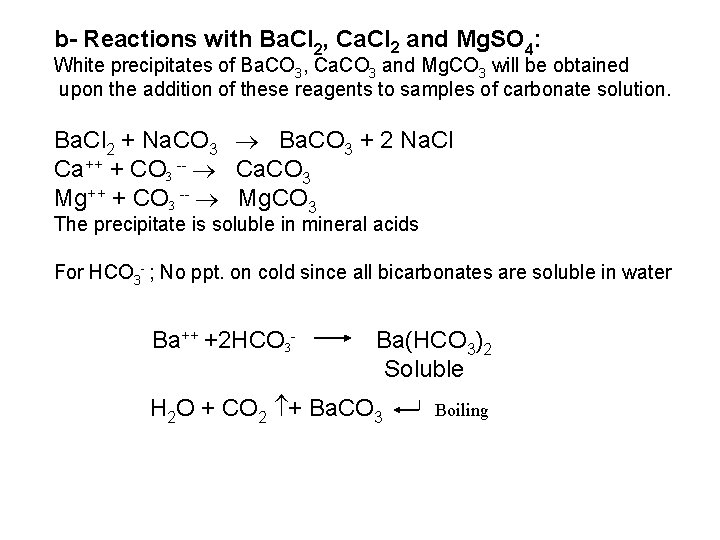
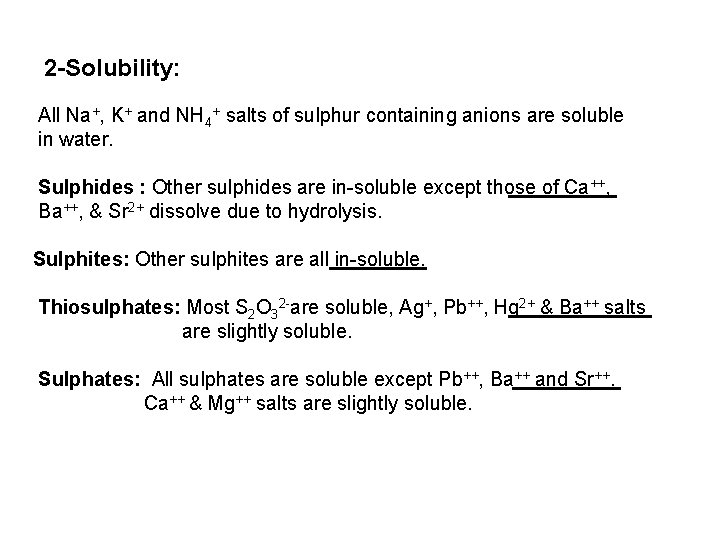
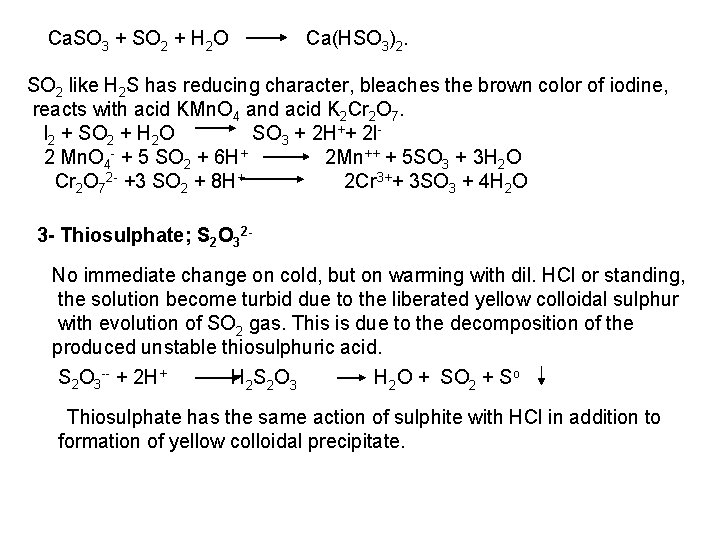
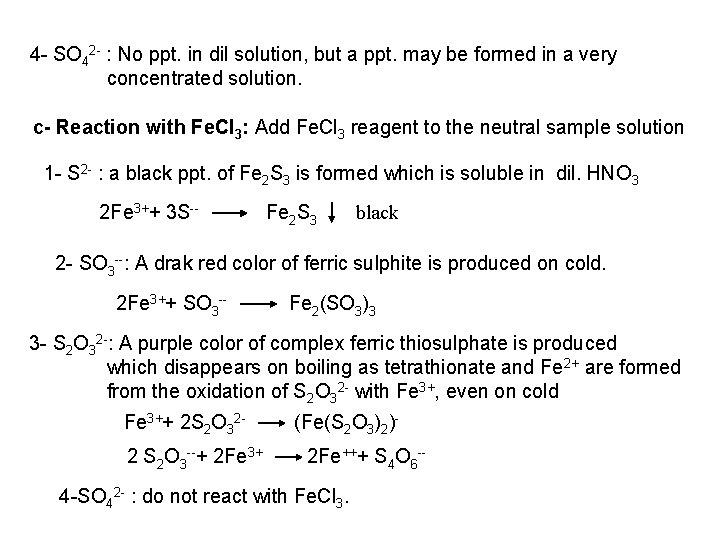
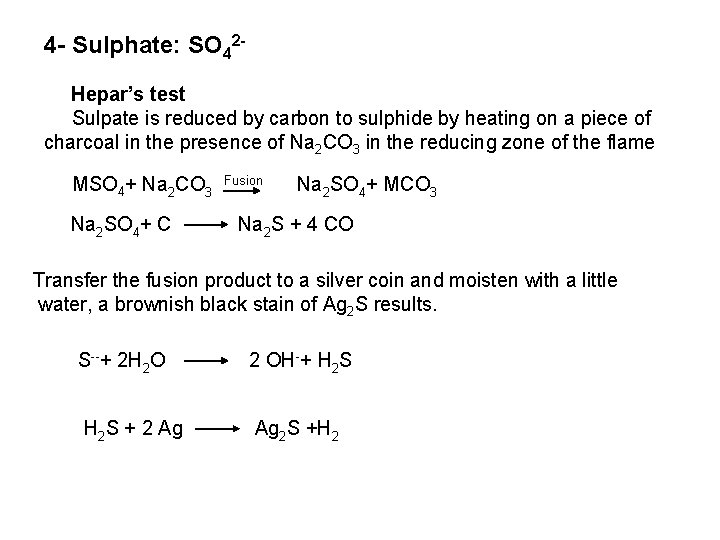
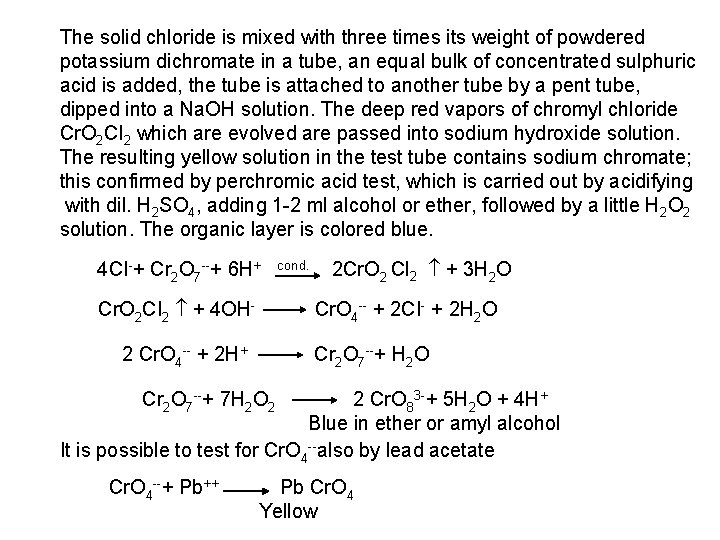
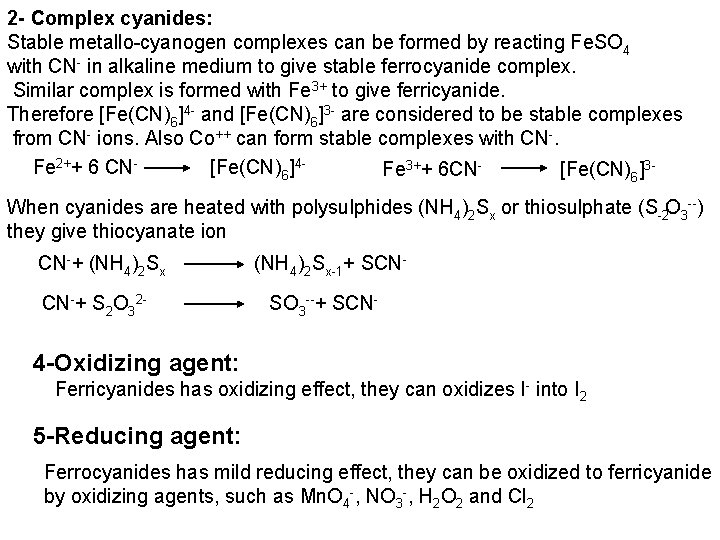
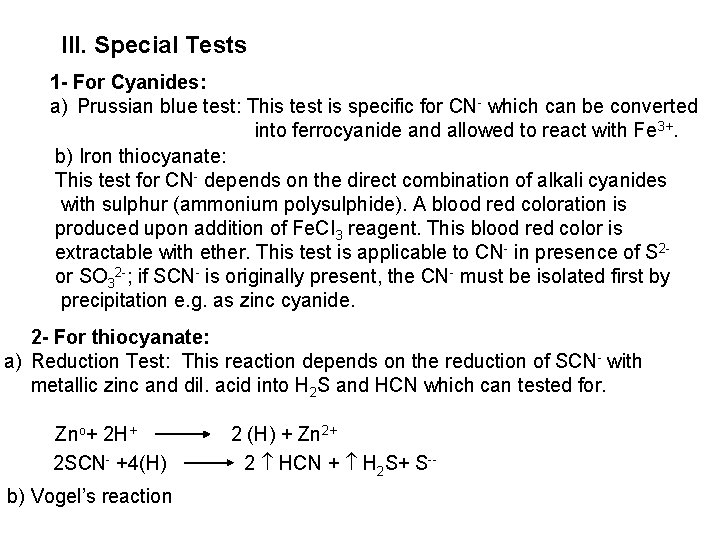
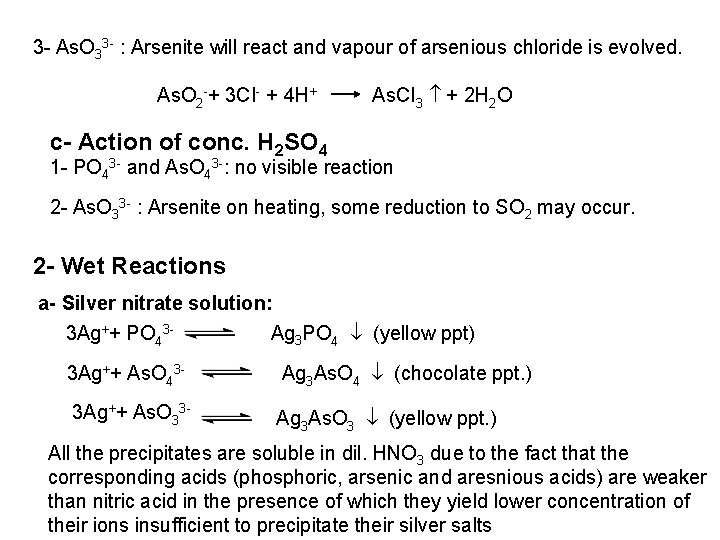
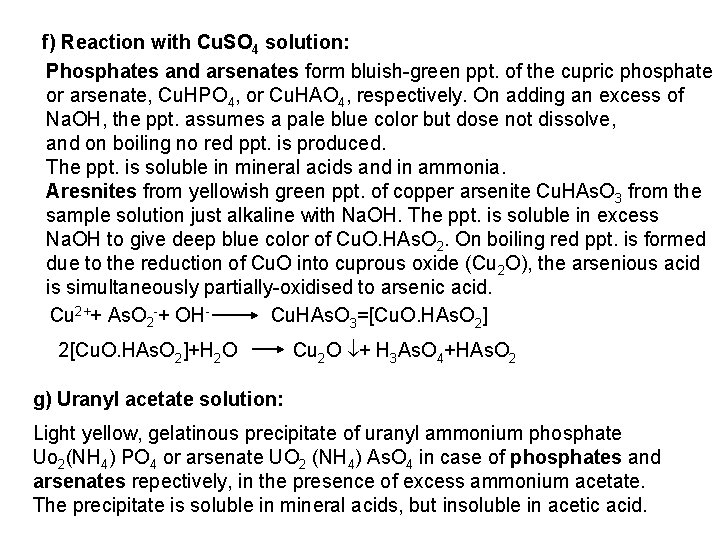
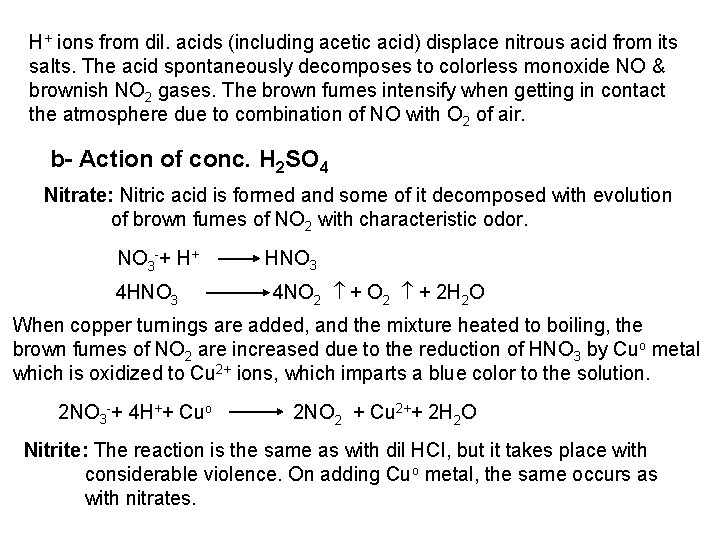
The free halogen, (X 2) could be detected by: 1 Bleaching of a moistened colored litmus paper. 2 Suffocating, and irritating odor. 3 Characteristic color of Br 2 (brown), I 2 (violet) and CI 2 gas (greenish tint). 4 I 2 changes starch paper into blue, Br 2 turns it orange. 5 CI 2 and Br 2 change a starch – KI into blue due to the oxidation of I to I 2 produce a blue adsorption complex. CI 2+ 2 KI 2 KCI + I 2 Br 2+ 2 KI 2 KBr + I 2 2 - Wet Reactions a- Reaction with Ag. NO 3: To 1 ml of the salt solution add Ag. NO 3 reagent. 1 Fluoride: No precipitate, since Ag. F is soluble in water. 2 Chloride: A white curdy ppt. of Ag. CI which is insoluble in nitric acid, soluble in KCN and Na 2 S 2 O 3 as other silver halides. The precipitated Ag. CI is soluble in dil. ammonia solution to give the ammine complex.
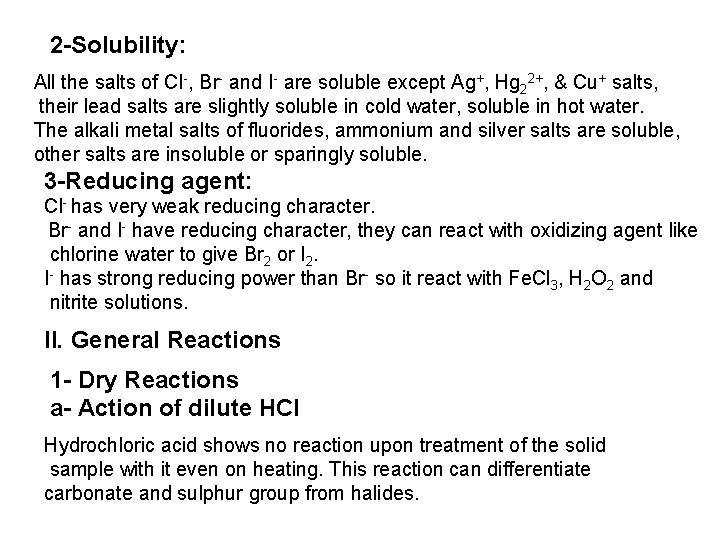
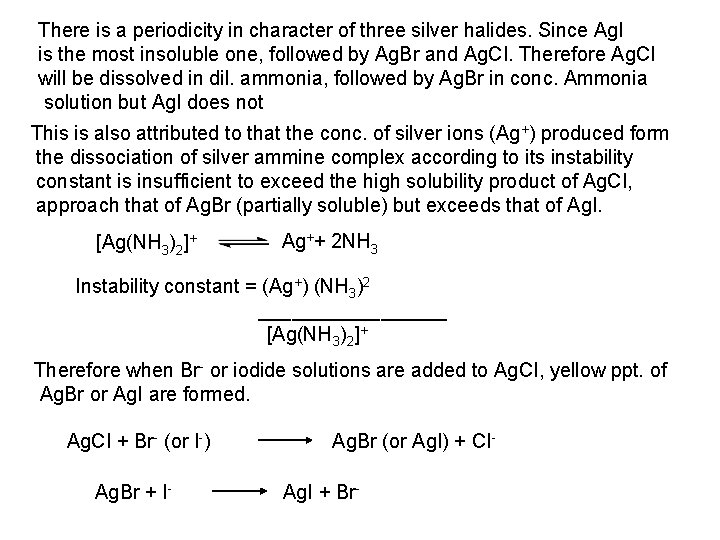
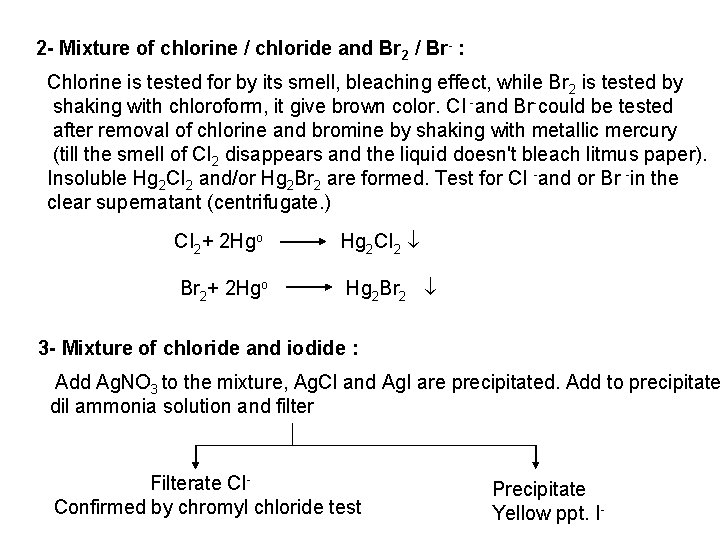
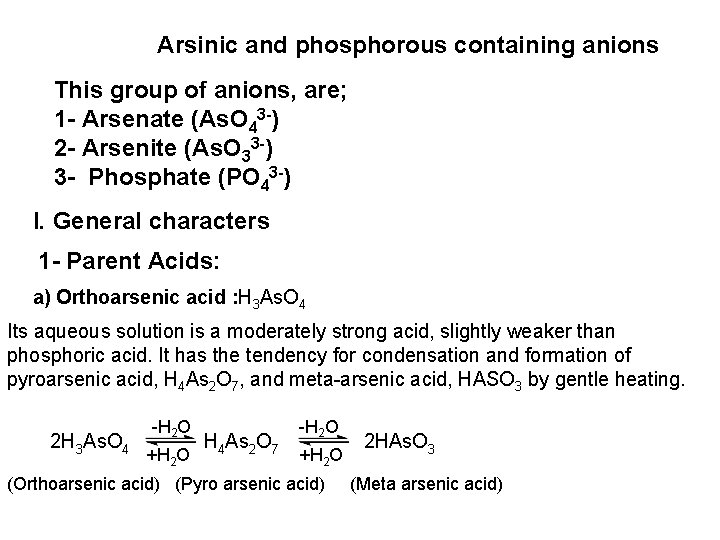
![Ag CI Ag CI 2 NH 3 AgNH 32CI AgNH 32 CI Ag++ CI Ag. CI + 2 NH 3 [Ag(NH 3)2]CI [Ag(NH 3)2] CI +](https://slidetodoc.com/presentation_image_h/0373df9f2dcb7a7b47eafbde92d6c3b6/image-34.jpg)
Ag++ CI Ag. CI + 2 NH 3 [Ag(NH 3)2]CI [Ag(NH 3)2] CI + 2 H+ Silver ammine chloride 2 NH 4++ Ag. CI is reprecipitated upon treatment of the ammine complex with acid. Ag. X + 2 CN [Ag (CN)2] +X Ag. X + 2 S 2 O 3 Soluble complex [Ag(S 2 O 3)2]3 +X 3 Bromide: A curdy, pale yellow precipitate of Ag. Br, sparingly soluble in dilute, but readily soluble in conc. ammonia solution Ag++ Br Ag. Br + 2 NH 3 Ag. Br [Ag(NH 3)2]++ Br 4 Iodide: A curdy yellow ppt. of Ag. I is formed which is insoluble in dil. ammonia but very slightly soluble in conc. ammonia solution. Ag++ I Ag. I

There is a periodicity in character of three silver halides. Since Ag. I is the most insoluble one, followed by Ag. Br and Ag. CI. Therefore Ag. CI will be dissolved in dil. ammonia, followed by Ag. Br in conc. Ammonia solution but Ag. I does not This is also attributed to that the conc. of silver ions (Ag+) produced form the dissociation of silver ammine complex according to its instability constant is insufficient to exceed the high solubility product of Ag. CI, approach that of Ag. Br (partially soluble) but exceeds that of Ag. I. [Ag(NH 3)2]+ Ag++ 2 NH 3 Instability constant = (Ag+) (NH 3)2 _________ [Ag(NH 3)2]+ Therefore when Br or iodide solutions are added to Ag. CI, yellow ppt. of Ag. Br or Ag. I are formed. Ag. CI + Br (or I ) Ag. Br + I Ag. Br (or Ag. I) + CI Ag. I + Br

b- Reaction with Ba. CI 2 solution: Only fluoride gives a white gelatinous ppt. when Ba. CI 2 reagent is added to sample solution. Ba+++ 2 F Ba. F 2 The white gelatinous Ba. F 2 ppt. is partially soluble in dil. HCI or HNO 3 No ppt. is formed in case of other halides. c- Reaction with Fe. CI 3: Add few drops of Fe. CI 3 reagent to concentrated sample solution. 1 F : a white crystalline ppt. of the complex salt, which is sparingly soluble in water Fe 3++ 6 F‑ [Fe. F 6]3 2 CI and Br : do not react with Fe. CI 3 3 lodide reacts with Fe. CI 3, due to its strong reducing action with the liberation of I 2. d- Reaction with lead acetate Precipitates of Pbx 2 are formed in cold solution when lead acetate reagent is added to sample solutions.

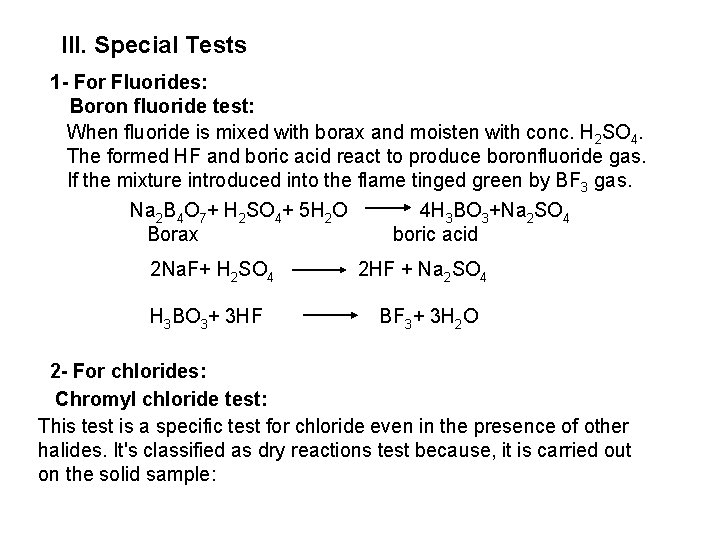
F , Cl and Br form a white ppt with lead acetate, sparingly soluble in cold more soluble in hot water, crystallize on cooling Pb+++ 2 F Pb+++ 2 CI Pb+++ 2 Br Pb. F 2 Pb. CI 2 Pb. Br 2 Iodide forms a bright yellow ppt of Pb. I 2 which is soluble in hot water and crystallizes on cooling as golden spangles. e- Chlorine water test: Chloride and Fluoride do not react with chlorine water. Chlorine water oxidizes I and Br into I 2 and Br 2 which can be extracted with chloroform or carbon tetrachloride as violet color or brown or yellow color of I 2 and Br 2, respectively. Iodide react first with chlorine water before bromide as it has more reducing character.

Chlorine water reagent is added drop wise to a solution of iodide or bromide as excess chlorine water converts Br 2 into yellow bromine monochloride or into colorless hypobromous acid or bromic acid and the organic layer turns pale yellow or colorless. Also, excess chlorine water oxidized I 2 to colorless iodic acid. 2 Br + CI 2 Br 2+ 2 CI Br 2+ CI 2 2 Br. CI (yellow) bromine monochloride Br 2+ CI 2 (excess) + 2 H 2 O 2 HOBr+2 HCI hypobromous acid Br 2+ 5 CI 2 (excess) + 6 H 2 O 2 HBr. O 3+10 HCI bromic acid 2 I + CI 2 I 2+ 2 CI I 2+ 5 CI 2 (excess) + 6 H 2 O 2 HIO 3+10 HCI iodic acid Colorless

III. Special Tests 1 - For Fluorides: Boron fluoride test: When fluoride is mixed with borax and moisten with conc. H 2 SO 4. The formed HF and boric acid react to produce boronfluoride gas. If the mixture introduced into the flame tinged green by BF 3 gas. Na 2 B 4 O 7+ H 2 SO 4+ 5 H 2 O 4 H 3 BO 3+Na 2 SO 4 Borax boric acid 2 Na. F+ H 2 SO 4 H 3 BO 3+ 3 HF 2 HF + Na 2 SO 4 BF 3+ 3 H 2 O 2 - For chlorides: Chromyl chloride test: This test is a specific test for chloride even in the presence of other halides. It's classified as dry reactions test because, it is carried out on the solid sample:

The solid chloride is mixed with three times its weight of powdered potassium dichromate in a tube, an equal bulk of concentrated sulphuric acid is added, the tube is attached to another tube by a pent tube, dipped into a Na. OH solution. The deep red vapors of chromyl chloride Cr. O 2 CI 2 which are evolved are passed into sodium hydroxide solution. The resulting yellow solution in the test tube contains sodium chromate; this confirmed by perchromic acid test, which is carried out by acidifying with dil. H 2 SO 4, adding 1 2 ml alcohol or ether, followed by a little H 2 O 2 solution. The organic layer is colored blue. 4 CI + Cr 2 O 7 + 6 H+ Cr. O 2 CI 2 + 4 OH cond. 2 Cr. O 2 Cl 2 + 3 H 2 O Cr. O 4 + 2 CI + 2 H 2 O 2 Cr. O 4 + 2 H+ Cr 2 O 7 + H 2 O Cr 2 O 7 + 7 H 2 O 2 2 Cr. O 83 + 5 H 2 O + 4 H+ Blue in ether or amyl alcohol It is possible to test for Cr. O 4 also by lead acetate Cr. O 4 + Pb++ Pb Cr. O 4 Yellow

N. B. 1 Some CI 2 may also be liberated owing to the reacting. 6 CI + Cr 2 O 7 + 14 H+ 3 CI 2+ 2 Cr 3++ 7 H 2 O and this decreases the sensitivity of the test. 2 Fluorides give rise to the volatile Cr. O 2 F 2 which is decomposed by water, and hence should be absent or removed. 3 Nitrites and nitrates interfere, as nitrosyl chloride may be formed. 4 Bromides and iodides give rise to the free halogens, which yield colorless or pale yellow solution with Na. OH. 6 Br + Cr 2 O 7 + 14 H+ 2 Cr 3++ 3 Br 2+ 7 H 2 O 6 I + Cr 2 O 7 + 14 H+ 2 Cr 3++ 3 I 2+ 7 H 2 O Br 2+ 2 OH I 2+ 2 OH OBr + H 2 O (hypobromide) OI + H 2 O (hypoiodide)

3 - For iodides: A) lodide is readily oxidized in acid solution (dil. H 2 SO 4) with nitrite solution or H 2 O 2 into free l 2 2 I + 2 NO 2 + 4 H+ 2 I + H 2 O 2+ 2 H+ I 2+ 2 NO + 2 H 2 O I 2+ 2 H 2 O B) I reacts with Cu++ forming a whit ppt. of Cu 2 I 2, the I being oxidized to free I 2. Thus a white ppt. in brown solution is formed on treating I with Cu. SO 4 solution. 2 Cu+++ 4 I Cu 2 I 2 +I 2 C) I reacts with mercuric chloride solution mercuric iodide Hg. I 2 will be precipitated as yellow scarlet red ppt. which dissolves in excess iodide forming soluble colorless complex. Hg. CI 2+ 2 I Hg. I 2+ 2 I Hg. I 2 + 2 CI Scarlet red (Hg. I 4)2 Soluble complex Nessler's reagent

IV. Analysis of Mixtures 1 - Mixture of F-, Cl-, Br- and I- : a) The F is separated by treating the mixture solution acidified with CH 3 COOH with Ba(NO 3)2 or Ca (NO 3)2 Centrifuge White PPt. Ba. F 2 Confirmed by Conc. H 2 SO 4 test Centrifugate CI ‑, Br and I b) for the centrifugate ( Cl , Br and I ), carry out chlorine water test for both I and Br – ( or get rid of I by oxidation to I 2 using H 2 O 2 or nitrite and extract I 2 by chloroform then test for Br in aqueous solution c) For CI , carry out chromyl test on a solid sample.

2 - Mixture of chlorine / chloride and Br 2 / Br- : Chlorine is tested for by its smell, bleaching effect, while Br 2 is tested by shaking with chloroform, it give brown color. CI and Br could be tested after removal of chlorine and bromine by shaking with metallic mercury (till the smell of CI 2 disappears and the liquid doesn't bleach litmus paper). Insoluble Hg 2 CI 2 and/or Hg 2 Br 2 are formed. Test for CI and or Br in the clear supernatant (centrifugate. ) CI 2+ 2 Hgo Hg 2 CI 2 Br 2+ 2 Hgo Hg 2 Br 2 3 - Mixture of chloride and iodide : Add Ag. NO 3 to the mixture, Ag. Cl and Ag. I are precipitated. Add to precipitate dil ammonia solution and filter Filterate Cl Confirmed by chromyl chloride test Precipitate Yellow ppt. I

Cyanogen anions This group of anions, are; 1 - Cyanide (CN-) 2 - Thiocyanate (SCN-) 3 - Ferrocyanide [Fe(CN)6]44 - Ferricyanide [Fe(CN)6]3 All cyanide containing anions are highly poisonous. In all experiments in which the gas is likely to be evolved or those in which cyanides are heated, should be carried out cautiously in the fume cupboard. I. General characters 1 - Parent Acids: a) Hydrocyanic acid : HCN It's very poisonous. It's colorless volatile liquid (B. P. 26. 5 o. C). It has an odor of bitter almonds. It is not stable in solution due the formation of ammonium formate. Any dil. mineral acid can replace HCN in its solution.

On passing CO 2 to CN solution HCN is produced with HCO 3. CN + CO 2+ H 2 O HCN + HCO 3 b) Thiocyanic acid: HSCN It is colorless toxic liquid (B. P. 85 o. C) with unpleasant odor. It is as strong as HCI but unstable. It is soluble in ether after the addition of HCI to an aqueous solution of SCN. On standing its aqueous solution is decomposed to HCN and yellow solid polymer. 3 HCNS HCN + H 2 N 2 C 2 S 3 c) Ferrocyanic acid : H 4 [Fe. CN)6] It's white crystalline solid. Its aqueous solution is strongly acidic. The first two protons are nearly completely ionized. d) Ferricyanic acid: H 3 [Fe(CN)6] It's browinish crystalline solid, soluble in water to give strongly acids solution. The three protons are nearly completely ionized.

2 -Solubility: CN-: All cyanides are water insoluble except alkali metals (Na+, K+), ammonium salt, alkaline earth metals ( Ba 2+, Sr 2+ and Ca 2+) and mercuric cyanide. SCN-: All thiocyanates are water soluble except Ag. SCN, Hg 2(SCN)2 & Cu 2 (SCN)2. Pb (SCN)2 as Pb. CI 2 is sparingly soluble in cold water, but soluble in hot water. Ferro and Ferricyanides: All are insoluble in water except those of alkali metals, ammonium salt and alkaline earth metals. 3 -Complexing agent: Cyanide ion has strong tendency to the formation of complexes which may be double cyanides or complex cyanides. 1 - Argentocyanide complexes: Double cyanides When a ppt. is formed upon reacting CN with Ag+, at first white turbidity is formed which is Ag. CN. According to the medium, if CN ions are present in excess a soluble complex is formed. Ag. CN + CN (Ag (CN)2)

2 - Complex cyanides: Stable metallo cyanogen complexes can be formed by reacting Fe. SO 4 with CN in alkaline medium to give stable ferrocyanide complex. Similar complex is formed with Fe 3+ to give ferricyanide. Therefore [Fe(CN)6]4 and [Fe(CN)6]3 are considered to be stable complexes from CN ions. Also Co++ can form stable complexes with CN. Fe 2++ 6 CN [Fe(CN)6]4 Fe 3++ 6 CN [Fe(CN)6]3 When cyanides are heated with polysulphides (NH 4)2 Sx or thiosulphate (S 2 O 3 ) they give thiocyanate ion CN + (NH 4)2 Sx CN + S 2 O 32 (NH 4)2 Sx 1+ SCN SO 3 + SCN 4 -Oxidizing agent: Ferricyanides has oxidizing effect, they can oxidizes I into I 2 5 -Reducing agent: Ferrocyanides has mild reducing effect, they can be oxidized to ferricyanide by oxidizing agents, such as Mn. O 4 , NO 3 , H 2 O 2 and Cl 2

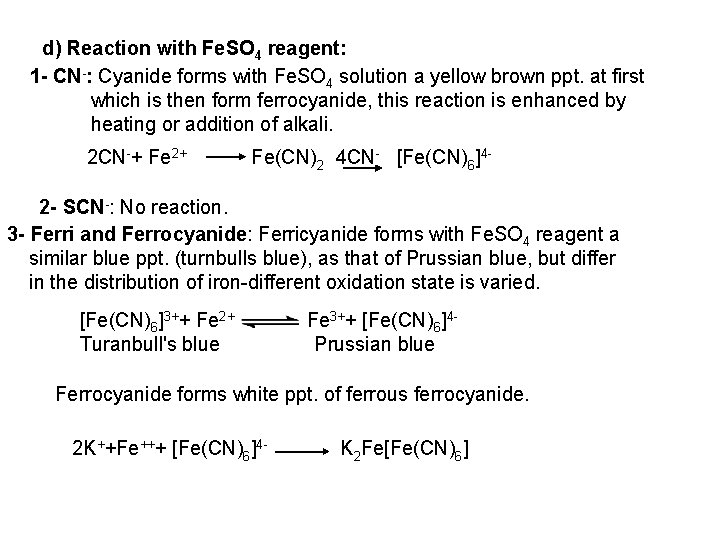
II. General Reactions 1 - Dry Reactions a- Action of dilute HCl a) CN-: HCN gas evolved with characteristic bitter almond odor and can be tested by: 1 Converting HCN evolved into SCN , by exposing the evolved HCN gas to a paper moistened with ammonium polysulphide. The resulted SCN can be tested by adding dil. HCI and a drop of Fe. CI 3 solution, a blood red color is produced. 2 By passing the evolved gas into Ag. NO 3 solution, a white ppt. of Ag. CN is formed insoluble in dil. HNO 3, soluble in ammonia solution. HCN + Ag. NO 3 Ag. CN + HNO 3 Ag. CN + 2 NH 3 (Ag(NH 3)2)CN 3 Prussian blue test: The evolved HCN gas is passed into Na. OH solution, add drops of Fe. SO 4 solution, heat to boiling, the HCN is converted into ferrocyanide which can be tested by adding drops of Fe. Cl 3 solution to produce a prussian blue ppt.

b) SCN-: No reaction as SCN is as strong as HCl c) Ferrocyanide and Ferricyanide: With cold dil. HCI, no gases, but may be precipitation of hydro ferrocyanic and hydroferricyanic acid occur. (Fe(CN)6)4 + 4 H+ H 4(Fe(CN)6)3 + 3 H+ H 3(Fe(CN)6) b- Action of conc. H 2 SO 4: a) CN- ; All cyanides are decomposed on heating. CN +2 H++ H 2 O NH 4+ +CO b) CNS-: Decomposition with evolution of carbonyl sulphide, which burns with a blue flame. SCN + 4 H++ 2 SO 4 + H 2 O NH 4++ 2 HSO 4 +COS Carbonyl Sulphide

c) Ferrocyanide and Ferricyanide: On heating with conc. H 2 SO 4, CO will be evolve which burns with a blue flame. SO 2 is produced in case of ferrocyanide. (Fe(CN)6)4 + 6 H 2 O +22 H++ 10 SO 42 2 Fe 2++ 4 H++ SO 4 Fe 2++6 NH 4++ 10 HSO 4 + 6 CO SO 2+ 2 H 2 O + 2 Fe 3+ (Fe(CN)6)3 + 6 H 2 O + 22 H++ 10 SO 42 Fe 3++ 6 NH 4++ 10 HSO 4 + 6 CO 2 - Wet Reactions a- Silver nitrate solution: 1 - CN- & SCN- : form white ppts. of silver cyanide and silver thiocyanate. Ag. CN is soluble in excess CN , ammonia solution, but insoluble in dil. HNO 3 Ag++ SCN Ag++ CN Ag. SCN Ag. CN CN (Ag(CN)2 ) H+ HCN+ Ag. CN
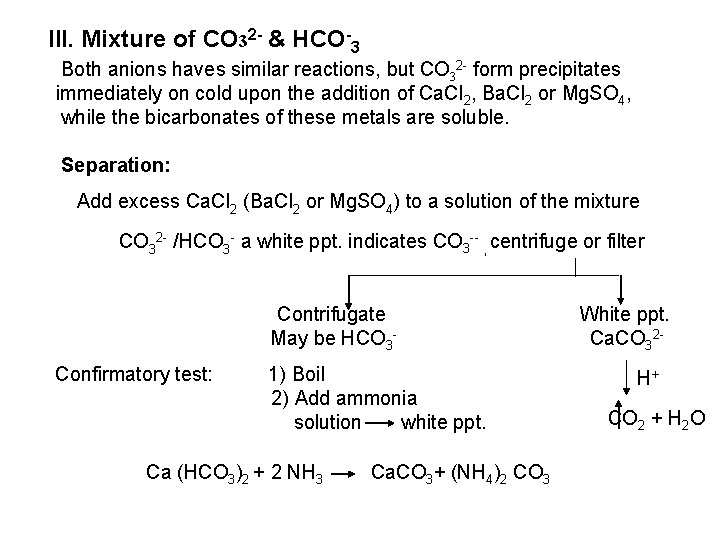
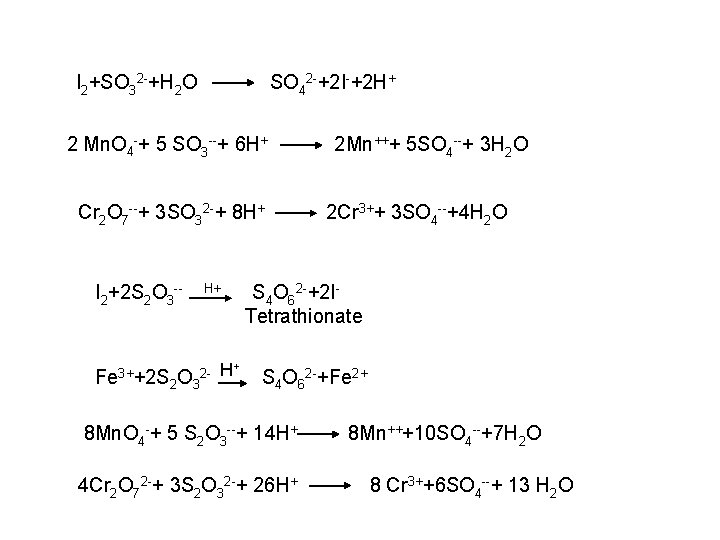
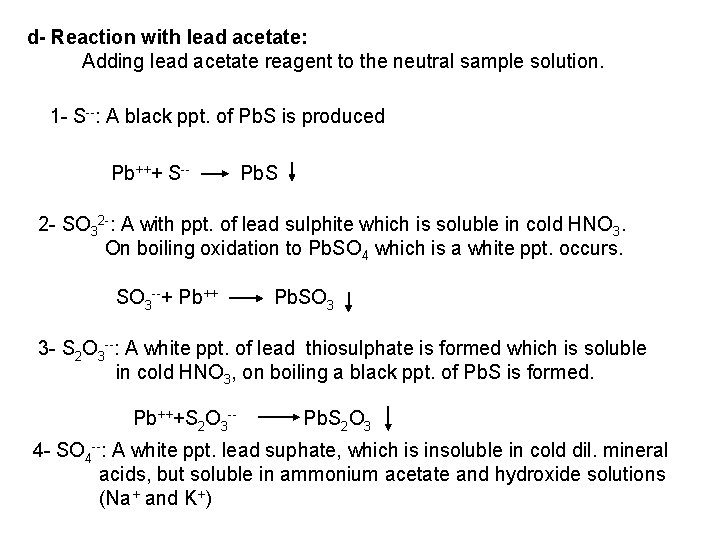
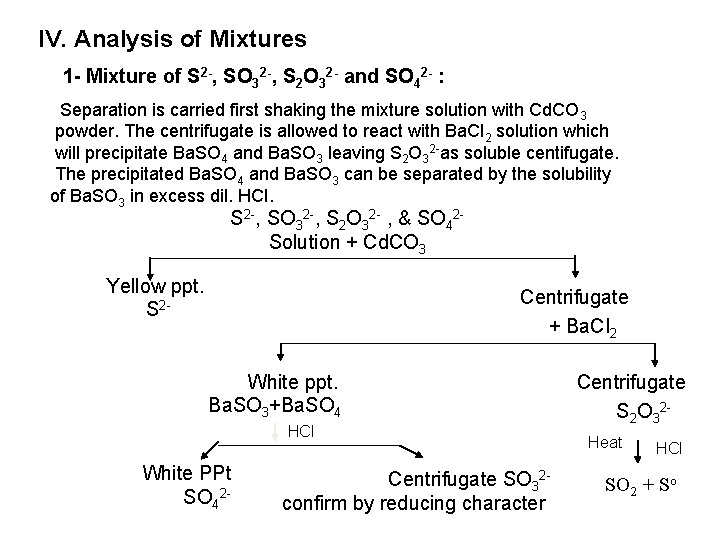
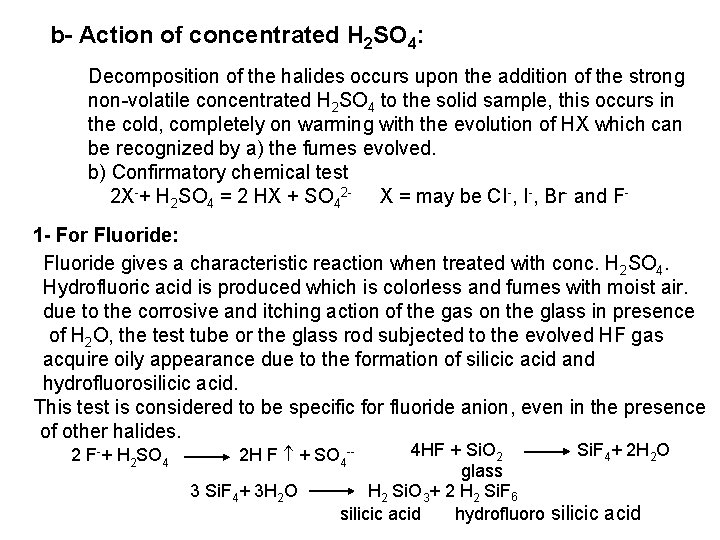
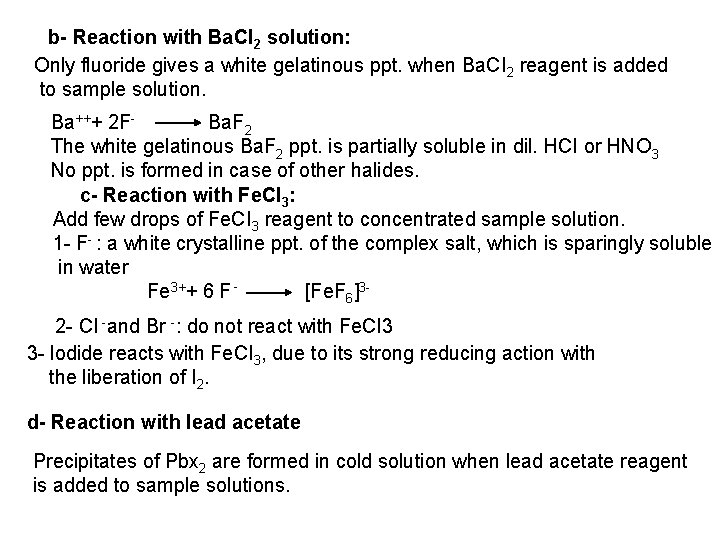
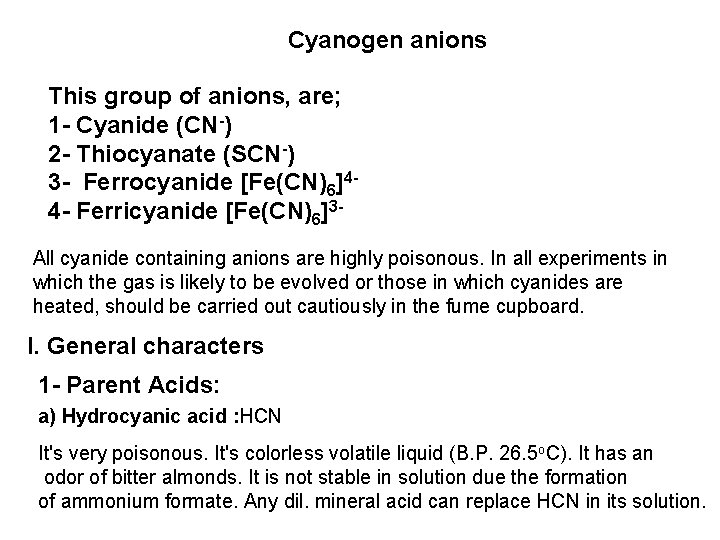
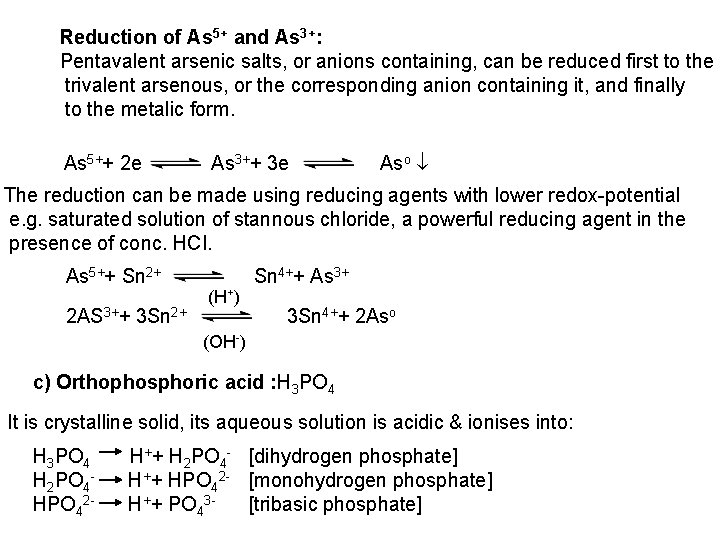
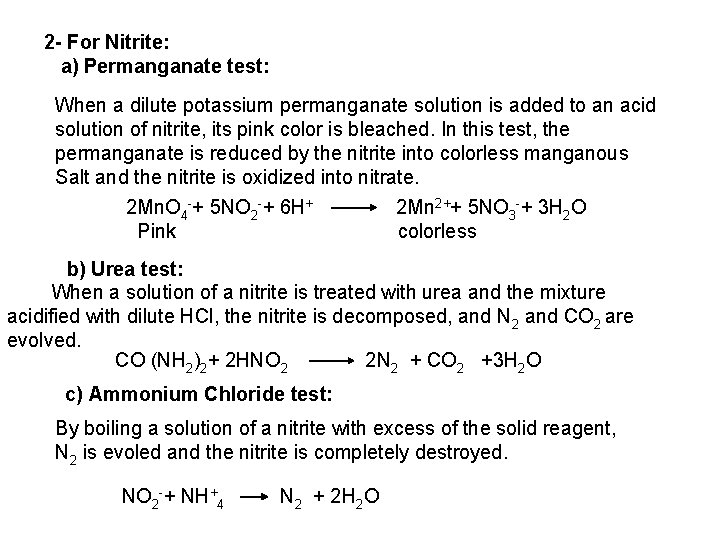
![2 Ferro and Ferricyanides Both FeCN64 and FeCN63 react with Ag NO 3 2 - Ferro- and Ferricyanides: Both [Fe(CN)6]4 and [Fe(CN)6]3 react with Ag. NO 3](https://slidetodoc.com/presentation_image_h/0373df9f2dcb7a7b47eafbde92d6c3b6/image-52.jpg)
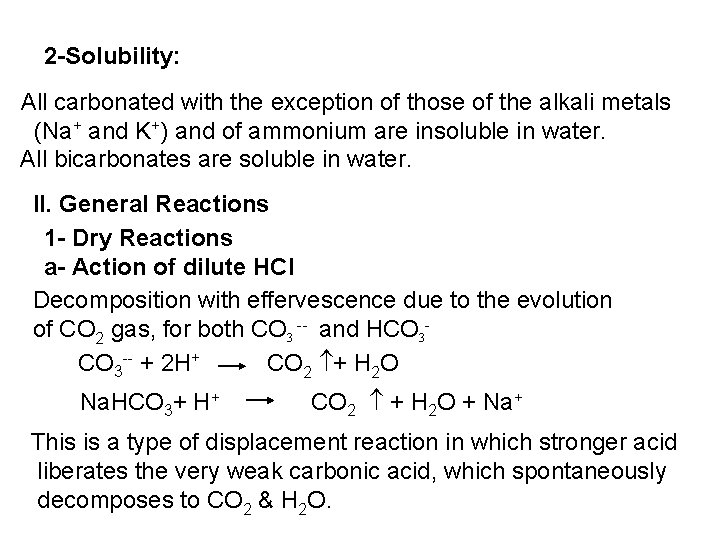
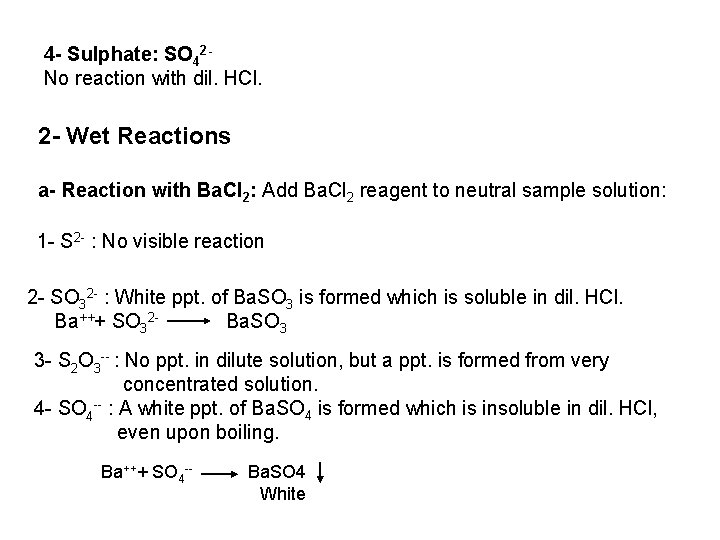
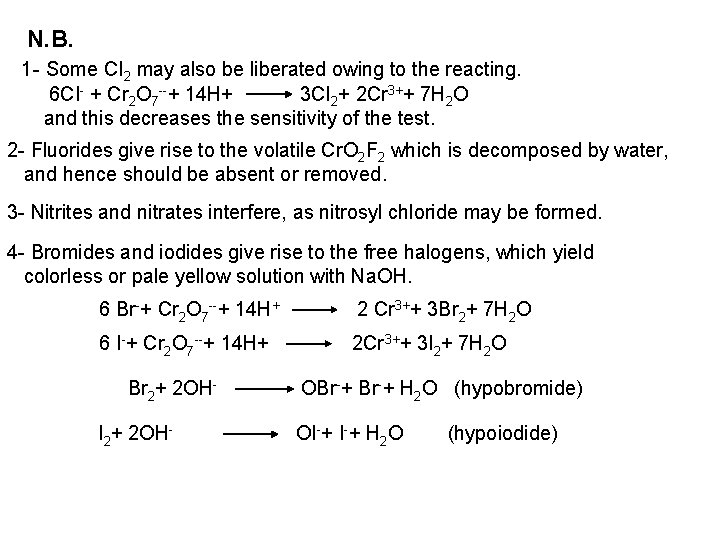
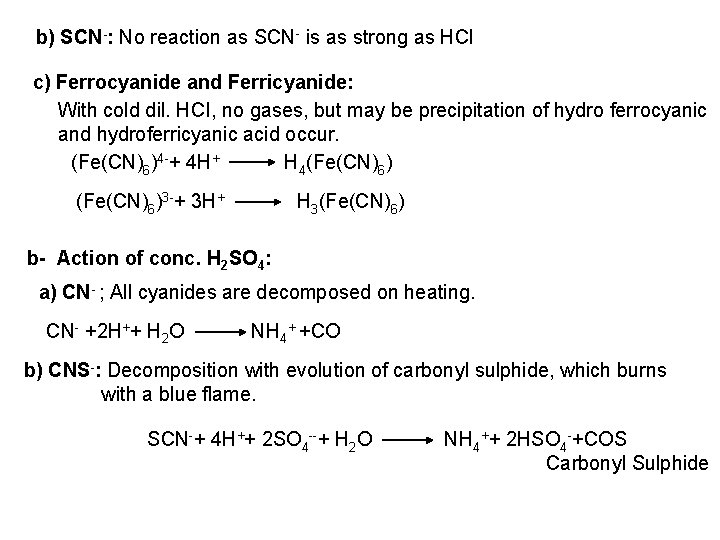
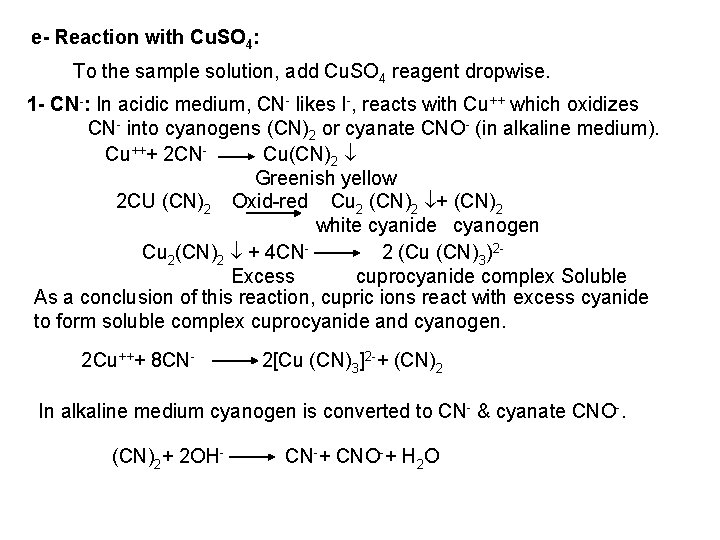
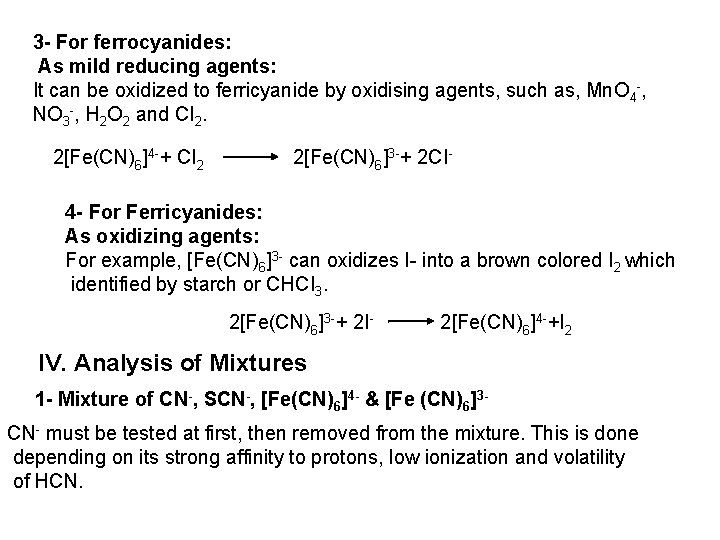
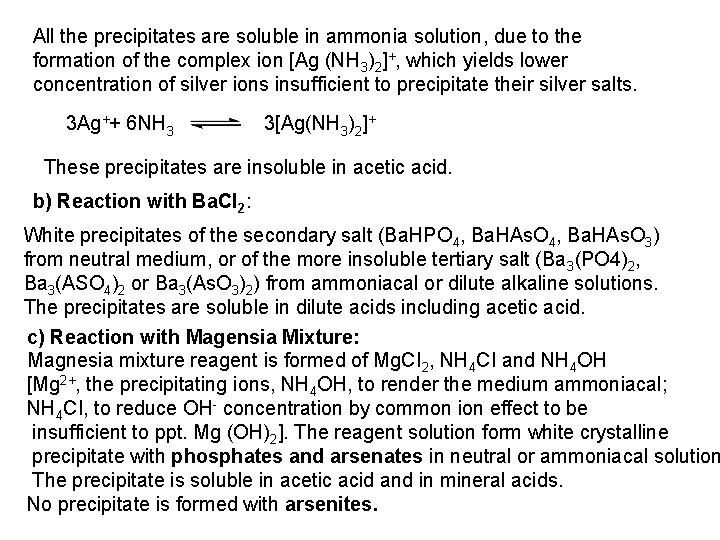
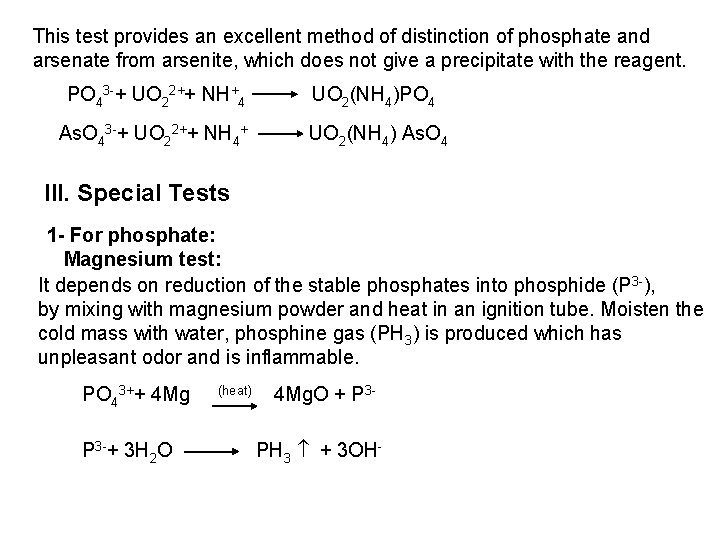
2 - Ferro- and Ferricyanides: Both [Fe(CN)6]4 and [Fe(CN)6]3 react with Ag. NO 3 solution with the formation of a white ppt. and orange red ppt. , respectively 4 Ag++ [Fe(CN)6]4 Ag 4[Fe(CN)6] Insoluble in dil. ammonia Insoluble in dil. HNO 3 3 Ag++ [Fe(CN)6]3 Ag 3[Fe(CN)6] Orange red ppt. Insoluble in dil. HNO 3 Soluble in dil. ammonia The solubility of silver ferricyanide ppt. can be used for the separation of ferrocyanide and ferricyanide when present in a mixture. Oxidation of the white ppt. of Ag 4 [Fe(CN)6] by warming with few drops of conc. HNO 3, leads to orange red ppt. of Ag 3 [Fe(CN)6] which becomes soluble in dil. ammonia solution. b) Reaction with Ba. CI 2: No observed reaction

c) Reaction with Fe. CI 3: This reaction is very important, since it is diffrantiating reaction. The diluted sample solution is added to a 1 ml of Fe. CI 3 reagent. 1 - CN-: iron (III) cyanide will be formed form dil. solution as a ppt. which is dissolved in excess cyanide forming ferricyanide. Fe 3++ 3 CN Fe (CN)3 3 CN [Fe(CN)6]3 Ferricyanide 2 - SCN-: This reaction is specific for iron(III) and SCN in the absence of other interfering ions. A cold acidic solution of SCN is treated with Fe. CI 3 reagent, a blood red color is produced which is extractable with ether. The formed color is subjected to have the following structures: Fe 3++ SCN [Fe(SCN)]++ or Fe(SCN)3 or [Fe(SCN)6]3 In order to increase the sensitivity of the test the following precautions must be done: 1. Ensure the presence of iron in the Fe 3+ state.

2 Acidification of the medium (dil. HCI is preferable). 3 Cooling of the solution befor testing. 4 Removal of intreferring ions by precipitation or complexation. F , PO 43 , oxalate and tartrate bleach the colour, therefore it must be absent F for e. g, reacts with iron to form stable complex. 6 F + Fe 3+ (Fe. F 6)3 other ions which react with SCN e. g, Hg 2+ which form unionized Hg (SCN)2 which is colorless. Iodides also interferes by being oxidized by Fe 3+ into the brown colour I 2. 2 I + 2 Fe 3+ H+ I 2+ 2 Fe 2+ 3 - Ferro and Ferricyanides: A Prussian blue characteristic ppt. is formed form acidic solution of [Fe(CN)6]4 , which is insoluble in dil. HCI, but soluble in alkali hydroxide. 3[Fe(CN)6)4 + 4 Fe 3+ Fe 4[Fe(CN)6]3 Prussian blue In case of Ferricyanide, a brown color is formed of the non ionised ferricyanide Fe 3++ [Fe(CN)6]3 Fe[Fe(CN)6] Brown color This test can be used to differentiate between ferro and ferricyanide

d) Reaction with Fe. SO 4 reagent: 1 - CN-: Cyanide forms with Fe. SO 4 solution a yellow brown ppt. at first which is then form ferrocyanide, this reaction is enhanced by heating or addition of alkali. 2 CN + Fe 2+ Fe(CN)2 4 CN [Fe(CN)6]4 2 - SCN-: No reaction. 3 - Ferri and Ferrocyanide: Ferricyanide forms with Fe. SO 4 reagent a similar blue ppt. (turnbulls blue), as that of Prussian blue, but differ in the distribution of iron different oxidation state is varied. [Fe(CN)6]3++ Fe 2+ Turanbull's blue Fe 3++ [Fe(CN)6]4 Prussian blue Ferrocyanide forms white ppt. of ferrous ferrocyanide. 2 K++Fe+++ [Fe(CN)6]4 K 2 Fe[Fe(CN)6]

e- Reaction with Cu. SO 4: To the sample solution, add Cu. SO 4 reagent dropwise. 1 - CN-: In acidic medium, CN likes I , reacts with Cu++ which oxidizes CN into cyanogens (CN)2 or cyanate CNO (in alkaline medium). Cu+++ 2 CN Cu(CN)2 Greenish yellow 2 CU (CN)2 Oxid red Cu 2 (CN)2 + (CN)2 white cyanide cyanogen Cu 2(CN)2 + 4 CN 2 (Cu (CN)3)2 Excess cuprocyanide complex Soluble As a conclusion of this reaction, cupric ions react with excess cyanide to form soluble complex cuprocyanide and cyanogen. 2 Cu+++ 8 CN 2[Cu (CN)3]2 + (CN)2 In alkaline medium cyanogen is converted to CN & cyanate CNO. (CN)2+ 2 OH CN + CNO + H 2 O

2 - SCN-: Thiocyanate reacts with Cu. SO 4 reagent, to form a green color which changes into a black ppt Cu (SCN)2 with excess Cu. SO 4 reagent Cu (SCN)2 decomposes gradually to white cuprous thiocyanate Cu 2(SCN)2 and separation of thioyanogen as a gummy mass Cu+++ SCN Cu (SCN)2 2 Cu (SCN)2 Cu 2 (SCN)2+ (SCN)2 decomposition white gummy mass unstable 3 - Ferro and Ferricyanides: Both ferro and ferricyanides form brown and green ppts. of copper ferro and copper ferricyanides, respectively. Both ppts. are insol. in dil. acids [Fe(CN)6]4 + 2 Cu++ 2 [Fe(CN)6]3 + 3 Cu++ Cu 2[Fe(CN)6] Brown Cu 3[Fe(CN)6]2 green

f- Reaction with Cobalt Nitrate: To the sample solution, add excess Co(NO 3)2 reagent. 1 - CN-: A buff ppt. , of cabaltous cyanide dihydrate is formed, which is soluble in excess CN to form soluble complex, cobaltocyanide Co 2++ 2 CN + 2 H 2 O Co (CN)2. 2 H 2 O 4 CN [Co (CN) 6]4 soluble complex. 2 - SCN-: Vogel's Reaction The reaction of Co++ with SCN to produce a characteristic blue color extractable with ether or amyl alcohol; known as vogel's reaction. Other cyanogen anions form precipitates with Co (NO 3)2 reagent. Co 2++ 4 SCN [Co (SCN)4]2 Extractable with ether (blue) 3 - Ferro and Ferricyanide: Both form greyish green and red ppts. of cobalt ferrocyanide and cobalt ferricyanide. 2 Co 2++ [Fe(CN)6]4 Co 2[Fe(CN)6] greyish green 3 Co 2++ 2[Fe(CN)6]3 Co 3[Fe(CN)6]2 red ppt.

III. Special Tests 1 - For Cyanides: a) Prussian blue test: This test is specific for CN which can be converted into ferrocyanide and allowed to react with Fe 3+. b) Iron thiocyanate: This test for CN depends on the direct combination of alkali cyanides with sulphur (ammonium polysulphide). A blood red coloration is produced upon addition of Fe. CI 3 reagent. This blood red color is extractable with ether. This test is applicable to CN in presence of S 2 or SO 32 ; if SCN is originally present, the CN must be isolated first by precipitation e. g. as zinc cyanide. 2 - For thiocyanate: a) Reduction Test: This reaction depends on the reduction of SCN with metallic zinc and dil. acid into H 2 S and HCN which can tested for. Zno+ 2 H+ 2 SCN +4(H) b) Vogel’s reaction 2 (H) + Zn 2+ 2 HCN + H 2 S+ S

3 - For ferrocyanides: As mild reducing agents: It can be oxidized to ferricyanide by oxidising agents, such as, Mn. O 4 , NO 3 , H 2 O 2 and CI 2. 2[Fe(CN)6]4 + CI 2 2[Fe(CN)6]3 + 2 CI 4 - For Ferricyanides: As oxidizing agents: For example, [Fe(CN)6]3 can oxidizes I into a brown colored I 2 which identified by starch or CHCI 3. 2[Fe(CN)6]3 + 2 I 2[Fe(CN)6]4 +I 2 IV. Analysis of Mixtures 1 - Mixture of CN-, SCN-, [Fe(CN)6]4 - & [Fe (CN)6]3 CN must be tested at first, then removed from the mixture. This is done depending on its strong affinity to protons, low ionization and volatility of HCN.

The following procedure could be applied. a Passing CO 2 in the mixture solution using acetic acid or Na. HCO 3 and heat, until no more HCN evolved which can be confirmed by: i Passing in Ag. NO 3 solution acidified dil. HNO 3 which gives a white ppt. ii Passing in Na. OH, adding Fe. SO 4 solution heating, followed by HCI then Fe. CI 3 solution (Prussian blue). b To the remaining solution, after removal of CN , acidify with dil. HCI, cool and add Fe. CI 3 solution and centrifuge Deep blue ppt. Centrifugate [Fe (CN)6]4 blood red color extractable with ether SCN brown solution Sn. CI 2 blue ppt. [Fe (CN)6]3

2 - Mixture of SCN-, CI-, Br- and ISCN is tested for by reacting with Fe. CI 3, to give blood red color which is extractable with ether and removed. In presence of I , I 2 is also formed which can be extracted with CHCI 3 (Violet color). The blue complex formed with Co 2+ can also be used to detect and remove SCN by extraction with ether or amyl alcohol. The halides are tested for in the usual way after the removal of SCN , since it interferes with their precipitation. After testing for SCN , it is removed by igniting the mixture till no more blackening or no odor of burnt sulphur is observed. The residue will contain only CI , Br , I , and test for CI by chromyl chloride test for I and Br , carry out chlorine water test.

Arsinic and phosphorous containing anions This group of anions, are; 1 - Arsenate (As. O 43 -) 2 - Arsenite (As. O 33 -) 3 - Phosphate (PO 43 -) I. General characters 1 - Parent Acids: a) Orthoarsenic acid : H 3 As. O 4 Its aqueous solution is a moderately strong acid, slightly weaker than phosphoric acid. It has the tendency for condensation and formation of pyroarsenic acid, H 4 As 2 O 7, and meta arsenic acid, HASO 3 by gentle heating. 2 H 3 As. O 4 H 2 O +H 2 O H 4 As 2 O 7 H 2 O +H 2 O (Orthoarsenic acid) (Pyro arsenic acid) 2 HAs. O 3 (Meta arsenic acid)

Arsenic acid and arsenate ion are mild oxidizing agents. Three series of arsenates exist, the primary arsenate H 2 As. O 4 , the secondary arsenate (HAs. O 42 ) and the tertiary arsenate (As. O 43 ). b) Arseneous acid : H 3 As. O 3 It exist in aqueous solutions, cannot be isolated as such because of thermal decomposition to the anhydride, As 2 O 3, sometimes written as As 4 O 6. The oxide is slightly soluble in water yielding ortho arsenious acid and meta arsenious acid. As 4 O 6+ 6 H 2 O 4 H 3 As. O 3 4 HAs. O 2+ 4 H 2 O (ortho arsenious acid) (meta arsenious acid) Two series of salts of arsenites exist, orthoarsenites H 2 As. O 3 , meta arsenites As. O 2 , both respond similarly to different reactions. **[Arsin containing acids and salts are highly poisonous]**

Reduction of As 5+ and As 3+: Pentavalent arsenic salts, or anions containing, can be reduced first to the trivalent arsenous, or the corresponding anion containing it, and finally to the metalic form. As 5++ 2 e As 3++ 3 e Aso The reduction can be made using reducing agents with lower redox potential e. g. saturated solution of stannous chloride, a powerful reducing agent in the presence of conc. HCI. As 5++ Sn 2+ 2 AS 3++ 3 Sn 2+ (H+) Sn 4++ As 3+ 3 Sn 4++ 2 Aso (OH ) c) Orthophosphoric acid : H 3 PO 4 It is crystalline solid, its aqueous solution is acidic & ionises into: H 3 PO 4 H 2 PO 4 HPO 42 H++ H 2 PO 4 [dihydrogen phosphate] H++ HPO 42 [monohydrogen phosphate] H++ PO 43 [tribasic phosphate]

The intermolecular loss of water from two molecules of orthophosphoric acid, will give pyrophosphoric acid (H 4 P 2 O 7) and metaphosphoric acids (HPO 3). Orthophosphoric acid forms three series of salts in which one, two or three hydrogens are replaced by metals, for example, Na. H 2 PO 4, Na 2 HPO 4 and Na 3 PO 4. these salts are known respectively as primary, secondary and tertiary orthophosphates. The aqueous solution of the primary salt is acid, that of the secondary is slightly alkaline while in the case of the tertiary salt, the solution is strongly alkaline. 2 -Solubility: All their salts are insoluble in water except those of Na+, K+ and NH 4+ beside the alkali dihydrogen salts as Ba(H 2 As. O 4)2 3 - Redox-reaction with I 2/I-: Aresnate has oxidizing effect and aresnite has reducing effect Arsenate (As. O 43 ) ions oxidises iodide into iodine; but the redox reaction is reversible due to the narrow difference in Eo values of the two redox systems.

As. O 43 +2 H++ 2 I H+ As. O 33 + H 2 O +I 2 Na. HCO 3 Arsenate oxidise iodide into iodine in acid medium, while arsenite (mild reducing agent) reduces iodine into iodide in alkaline medium. II. General Reactions 1 - Dry Reactions a- Action of dilute HCl No visible reaction, since phosphates, arsenates and arsenite acid are non volatile. b- Action of conc. HCl 1 PO 43 : no visible reaction 2 As. O 43 : On hot arsenate ion oxidises HCI into free CI 2, while it will be reduced to arsenite 2 CI + As. O 43 + 4 H+ CI 2 +As. O 2 + 2 H 2 O

3 As. O 33 : Arsenite will react and vapour of arsenious chloride is evolved. As. O 2 + 3 CI + 4 H+ As. CI 3 + 2 H 2 O c- Action of conc. H 2 SO 4 1 PO 43 and As. O 43 : no visible reaction 2 As. O 33 : Arsenite on heating, some reduction to SO 2 may occur. 2 - Wet Reactions a- Silver nitrate solution: Ag 3 PO 4 (yellow ppt) 3 Ag++ PO 43 3 Ag++ As. O 43 Ag 3 As. O 4 (chocolate ppt. ) 3 Ag++ As. O 33 Ag 3 As. O 3 (yellow ppt. ) All the precipitates are soluble in dil. HNO 3 due to the fact that the corresponding acids (phosphoric, arsenic and aresnious acids) are weaker than nitric acid in the presence of which they yield lower concentration of their ions insufficient to precipitate their silver salts

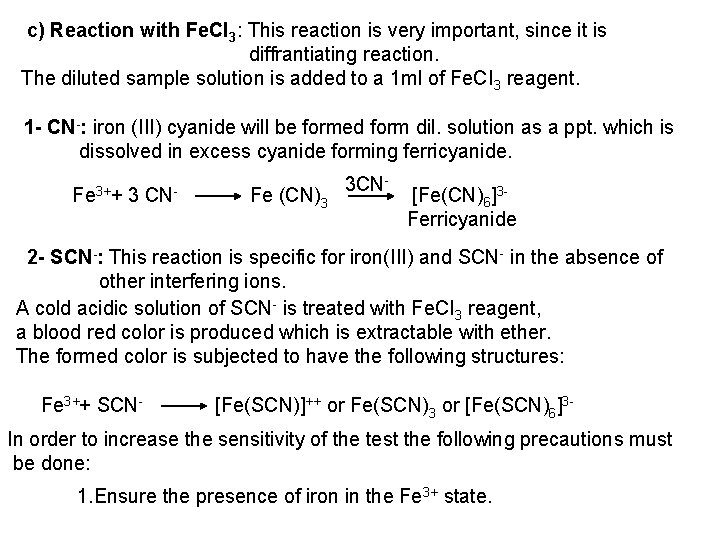
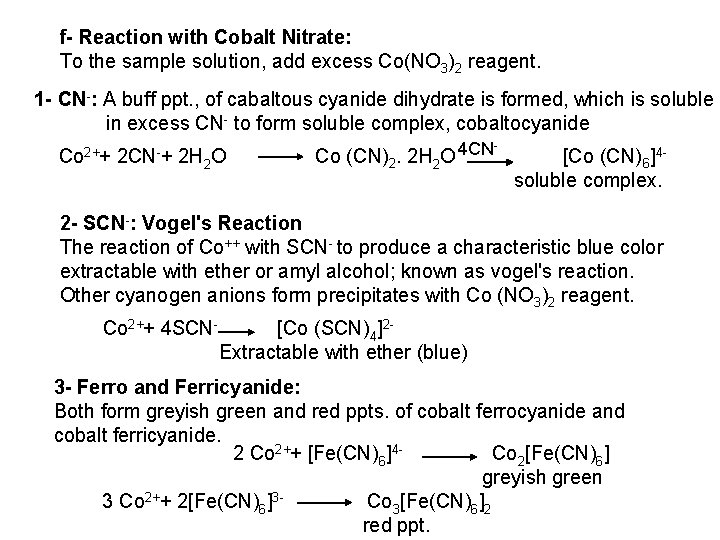
All the precipitates are soluble in ammonia solution, due to the formation of the complex ion [Ag (NH 3)2]+, which yields lower concentration of silver ions insufficient to precipitate their silver salts. 3 Ag++ 6 NH 3 3[Ag(NH 3)2]+ These precipitates are insoluble in acetic acid. b) Reaction with Ba. CI 2: White precipitates of the secondary salt (Ba. HPO 4, Ba. HAs. O 3) from neutral medium, or of the more insoluble tertiary salt (Ba 3(PO 4)2, Ba 3(ASO 4)2 or Ba 3(As. O 3)2) from ammoniacal or dilute alkaline solutions. The precipitates are soluble in dilute acids including acetic acid. c) Reaction with Magensia Mixture: Magnesia mixture reagent is formed of Mg. CI 2, NH 4 CI and NH 4 OH [Mg 2+, the precipitating ions, NH 4 OH, to render the medium ammoniacal; NH 4 CI, to reduce OH concentration by common ion effect to be insufficient to ppt. Mg (OH)2]. The reagent solution form white crystalline precipitate with phosphates and arsenates in neutral or ammoniacal solution The precipitate is soluble in acetic acid and in mineral acids. No precipitate is formed with arsenites.
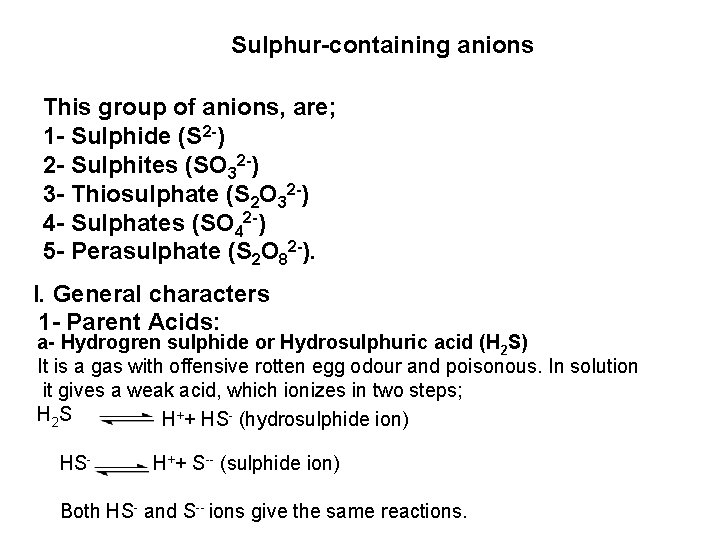
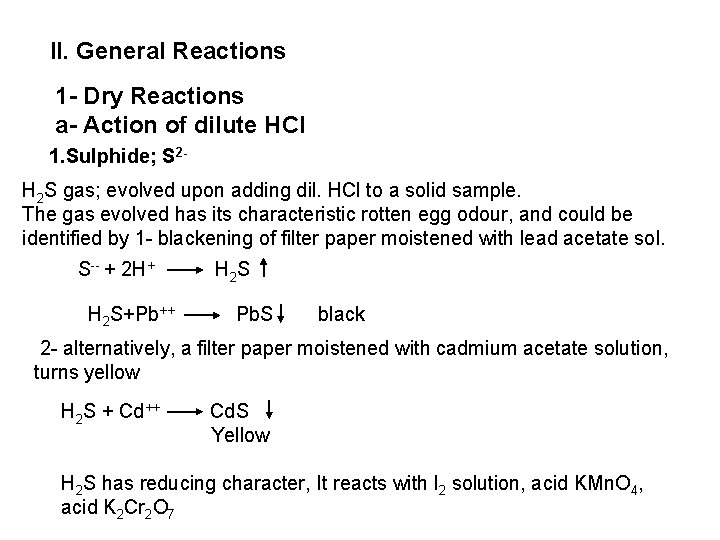
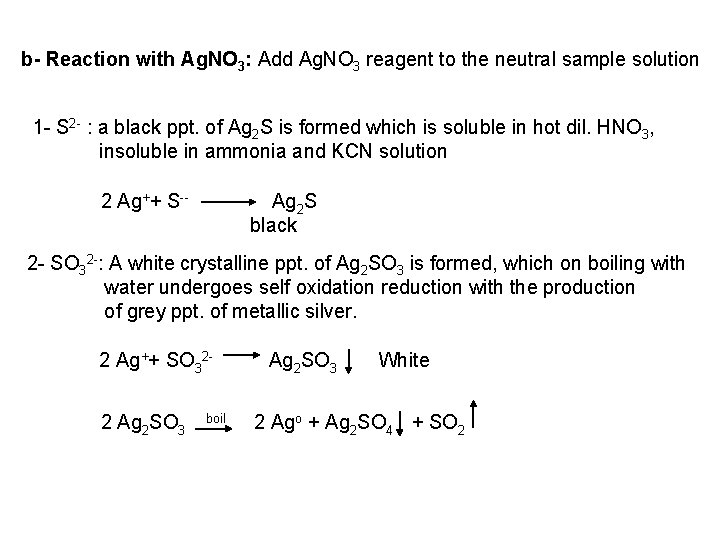
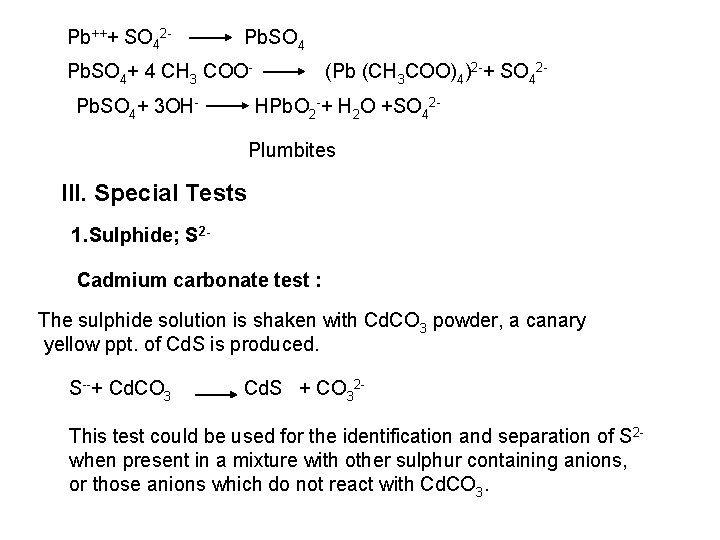
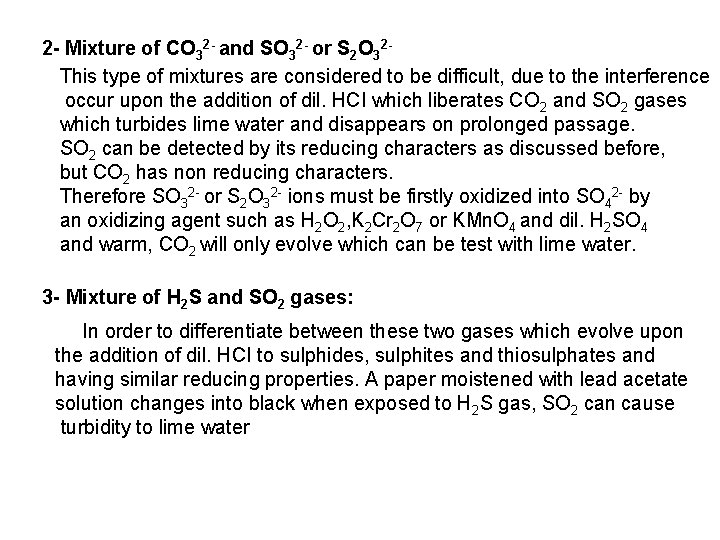
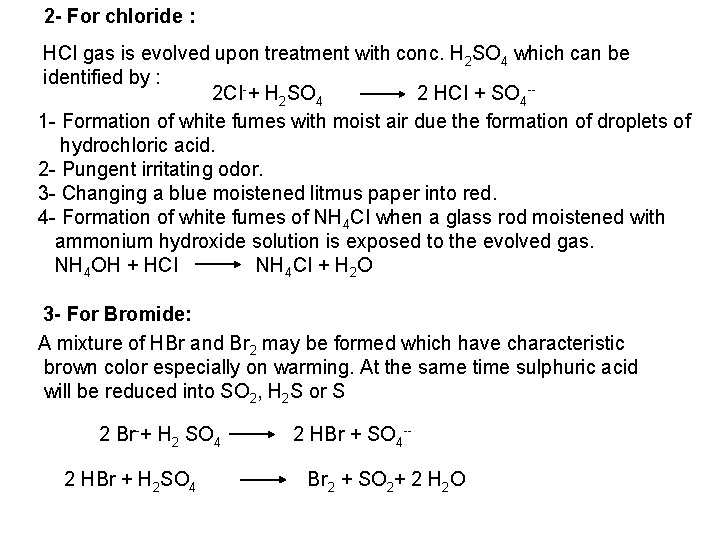
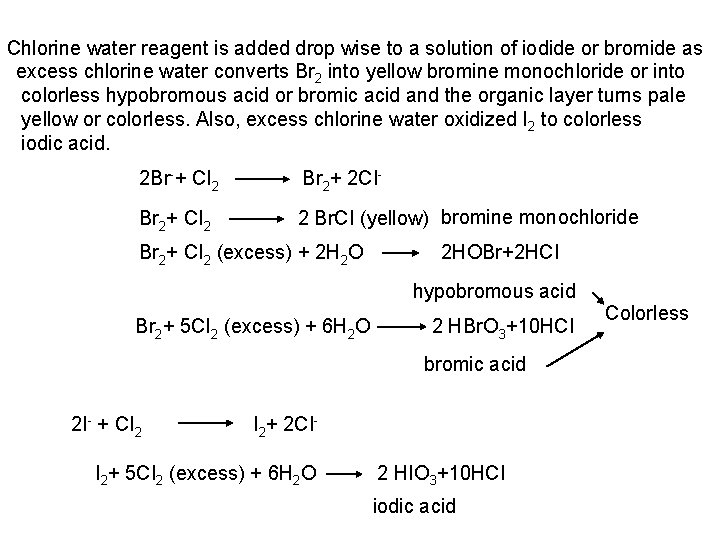
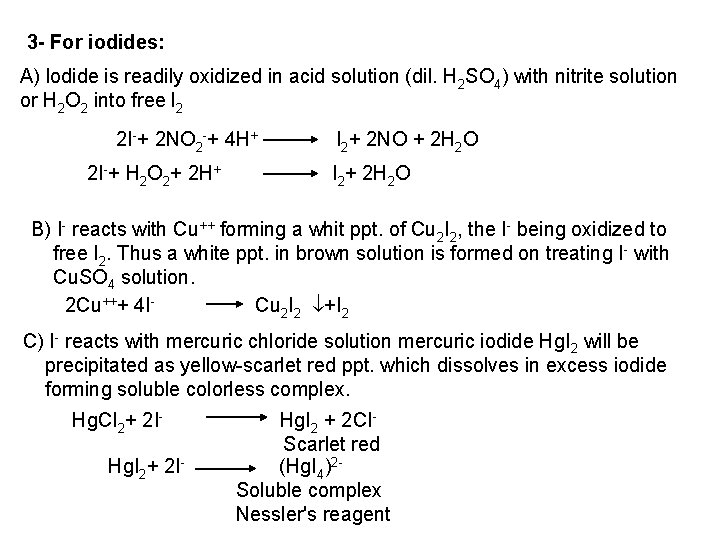
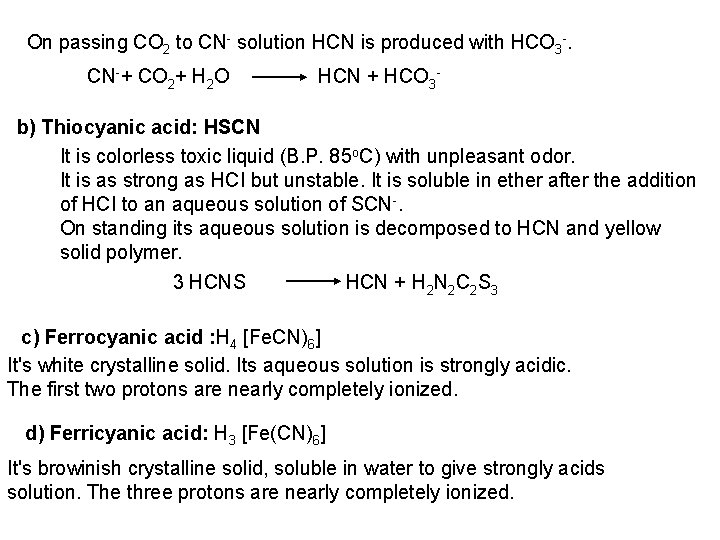
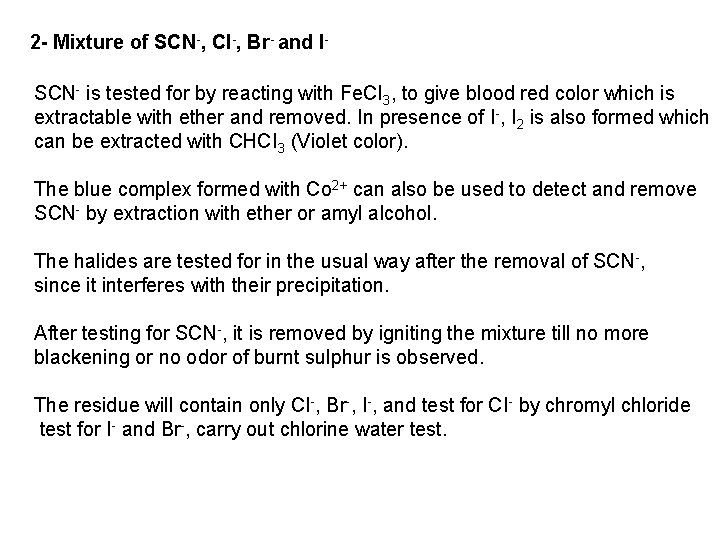
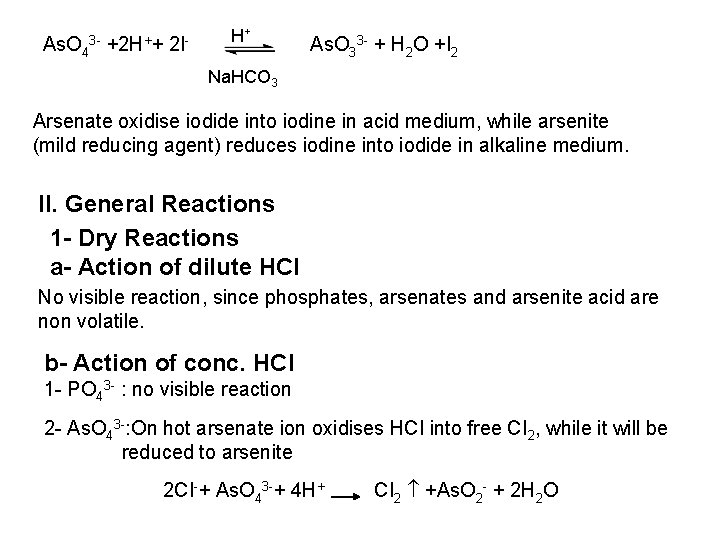
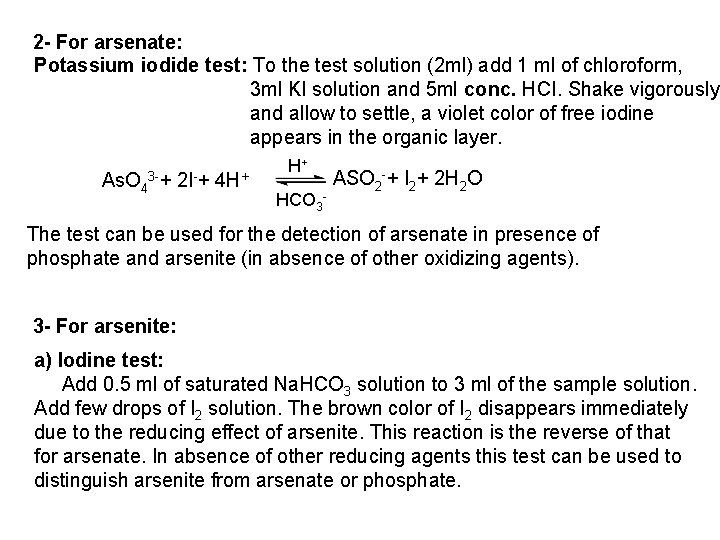
![PO 43 Mg 2 NH 4 Mg NH 4 PO 4 magnesium ammonium phosphate PO 43 +Mg 2++ NH 4+ Mg (NH 4) PO 4 [magnesium ammonium phosphate]](https://slidetodoc.com/presentation_image_h/0373df9f2dcb7a7b47eafbde92d6c3b6/image-70.jpg)
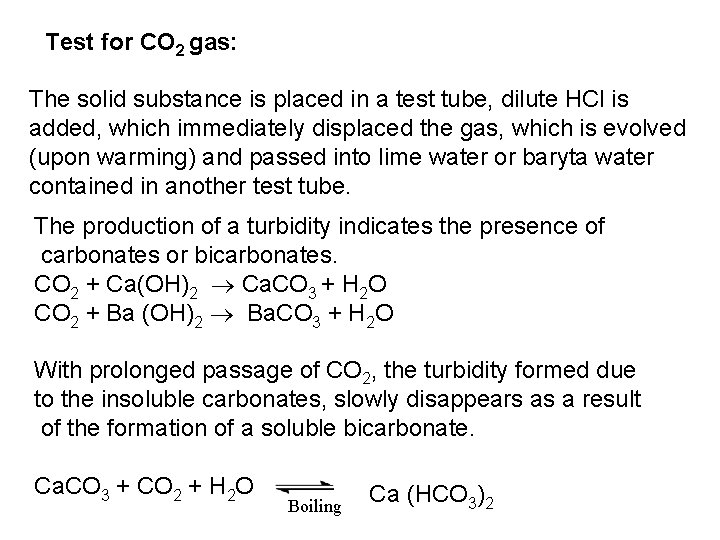
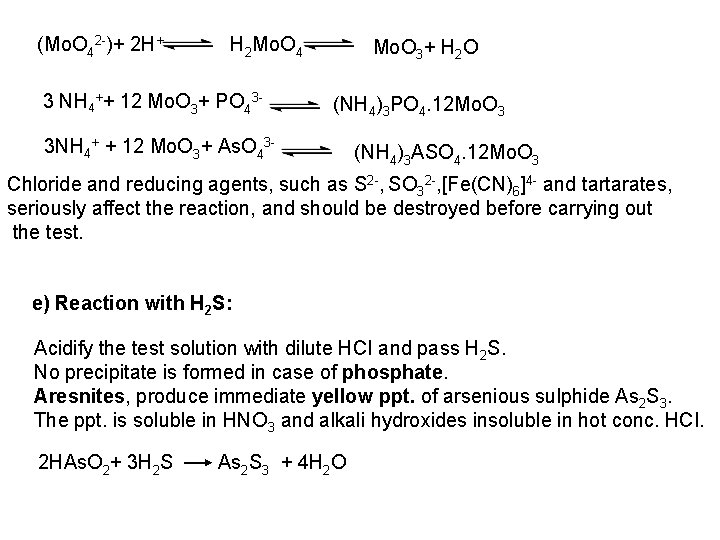
PO 43 +Mg 2++ NH 4+ Mg (NH 4) PO 4 [magnesium ammonium phosphate] As. O 43 + Mg 2++ NH 4+ Mg(NH 4)As. O 4 [magnesium ammonium arsenate] If the white precipitates are treated with Ag. NO 3 (in acetic acid medium), that of the phosphate will be transformed into yellow ppt. while that of the arsenate into chocolate ppt. due to the transformation to the less soluble Ag 3 PO 4 and Ag 3 As. O 4 respectively. d) Reaction with ammonium molybdate: The addition of a large excess (2 3 ml) of this reagent in conc. HNO 3 to a small volume (0. 5 ml) of the test solution acidified with HNO 3 and heat gradually, produces a canary yellow crystalline precipitates of ammonium phosphomolybdate (NH 4)3 PO 4. 12 Mo. O 3 (on warming to 40 o. C) and of ammonium arsnomolybdate (NH 4)3 As. O 4. 12 Mo. O 3 (on boiling) in case of phosphates and arsenates respectively. No precipitate is formed with arsenites. The precipitates are soluble in ammonia or alkali hydroxides, in excess phosphates or arsenates respectively and on boiling with ammonium acetate solution, insoluble in HNO 3. Mo. O 3 produced from the action of acid on ammonium molybdate.

(Mo. O 42 )+ 2 H+ H 2 Mo. O 4 3 NH 4++ 12 Mo. O 3+ PO 43 Mo. O 3+ H 2 O (NH 4)3 PO 4. 12 Mo. O 3 3 NH 4+ + 12 Mo. O 3+ As. O 43 (NH 4)3 ASO 4. 12 Mo. O 3 Chloride and reducing agents, such as S 2 , SO 32 , [Fe(CN)6]4 and tartarates, seriously affect the reaction, and should be destroyed before carrying out the test. e) Reaction with H 2 S: Acidify the test solution with dilute HCI and pass H 2 S. No precipitate is formed in case of phosphate. Aresnites, produce immediate yellow ppt. of arsenious sulphide As 2 S 3. The ppt. is soluble in HNO 3 and alkali hydroxides insoluble in hot conc. HCI. 2 HAs. O 2+ 3 H 2 S As 2 S 3 + 4 H 2 O

Arsenates, not produce any immediate visible change, but after prolonged passage of H 2 S, yellow ppt. of AS 2 S 3 is produced. It is evident that the first action of H 2 S is to reduce the arsenate into arsenite through the formation of thioarsenate ion H 2 As. O 3 S which decomposes slowly arsenious acid and suphhur. H 2 As. O 4 + H 2 S H 2 As. O 3 S + H 2 O H 2 As. O 3 S + H+ HAs. O 2+ H 2 O +S 2 HAs. O 2+ 3 H 2 S As 2 S 3 + 4 H 2 O If the acid concentration is high and the strean of H 2 S is rapid, no preliminary reduction to arsenite occurs and arsenic pentasulphide precipitate (As 2 S 5) is produced. 2 H 2 As. O 4 + 5 H 2 S +2 H+ As 2 S 5 +8 H 2 O However, if the solution is heated under the same conditions, mixture of As 2 S 3 and As 2 S 5 is formed.

f) Reaction with Cu. SO 4 solution: Phosphates and arsenates form bluish green ppt. of the cupric phosphate or arsenate, Cu. HPO 4, or Cu. HAO 4, respectively. On adding an excess of Na. OH, the ppt. assumes a pale blue color but dose not dissolve, and on boiling no red ppt. is produced. The ppt. is soluble in mineral acids and in ammonia. Aresnites from yellowish green ppt. of copper arsenite Cu. HAs. O 3 from the sample solution just alkaline with Na. OH. The ppt. is soluble in excess Na. OH to give deep blue color of Cu. O. HAs. O 2. On boiling red ppt. is formed due to the reduction of Cu. O into cuprous oxide (Cu 2 O), the arsenious acid is simultaneously partially oxidised to arsenic acid. Cu 2++ As. O 2 + OH Cu. HAs. O 3=[Cu. O. HAs. O 2] 2[Cu. O. HAs. O 2]+H 2 O Cu 2 O + H 3 As. O 4+HAs. O 2 g) Uranyl acetate solution: Light yellow, gelatinous precipitate of uranyl ammonium phosphate Uo 2(NH 4) PO 4 or arsenate UO 2 (NH 4) As. O 4 in case of phosphates and arsenates repectively, in the presence of excess ammonium acetate. The precipitate is soluble in mineral acids, but insoluble in acetic acid.

This test provides an excellent method of distinction of phosphate and arsenate from arsenite, which does not give a precipitate with the reagent. PO 43 + UO 22++ NH+4 UO 2(NH 4)PO 4 As. O 43 + UO 22++ NH 4+ UO 2(NH 4) As. O 4 III. Special Tests 1 - For phosphate: Magnesium test: It depends on reduction of the stable phosphates into phosphide (P 3 ), by mixing with magnesium powder and heat in an ignition tube. Moisten the cold mass with water, phosphine gas (PH 3) is produced which has unpleasant odor and is inflammable. PO 43++ 4 Mg P 3 + 3 H 2 O (heat) 4 Mg. O + P 3 PH 3 + 3 OH

2 - For arsenate: Potassium iodide test: To the test solution (2 ml) add 1 ml of chloroform, 3 ml KI solution and 5 ml conc. HCI. Shake vigorously and allow to settle, a violet color of free iodine appears in the organic layer. As. O 43 + 2 I + 4 H+ H+ HCO 3 ASO 2 + I 2+ 2 H 2 O The test can be used for the detection of arsenate in presence of phosphate and arsenite (in absence of other oxidizing agents). 3 - For arsenite: a) Iodine test: Add 0. 5 ml of saturated Na. HCO 3 solution to 3 ml of the sample solution. Add few drops of I 2 solution. The brown color of I 2 disappears immediately due to the reducing effect of arsenite. This reaction is the reverse of that for arsenate. In absence of other reducing agents this test can be used to distinguish arsenite from arsenate or phosphate.

b) Bettendorf's test: A few drops of the test solution are added to 4 ml of conc. HCI, and 1 ml of saturated stannous chloride solution is added. The solution is gently warmed; it becomes drak brown and finally black ppt. of arsenic is formed. Strong reducing agents as Sn. CI 2 reduce arsenite in presence of conc. HCI to elemental arsenic. 3 Sn 2++ 8 H++ 2 As. O 2 (heat) 2 As +3 Sn 4++ 4 H 2 O This test is also positive with arsenates, being first reduced into arsenites. However, the test can be made use of to establish the presence of arsenic containing anions. c) Marsh's reaction: [ for small amounts of arsenic. ] In acidic solution arsenic (III) and (V) compounds are reduced by hydrogen to the poisonous hydrogen arsenide gas (H 3 As) with garlic like odor which when heated dissociates to elementary arsenic and hydrogen: As. O 33 + 3 Zno+ 9 H+ 2 H 3 As (heat) H 3 As + 3 Zn 2++ 3 H 2 O 2 Aso + 3 H 2

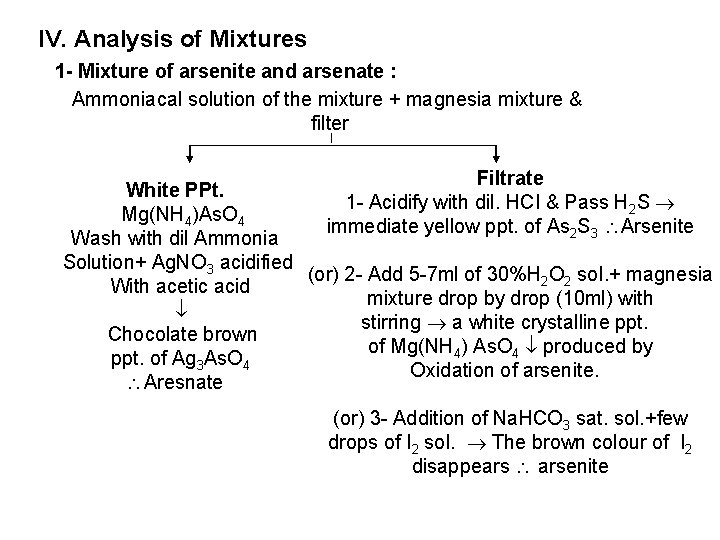
IV. Analysis of Mixtures 1 - Mixture of arsenite and arsenate : Ammoniacal solution of the mixture + magnesia mixture & filter Filtrate White PPt. 1 Acidify with dil. HCI & Pass H 2 S Mg(NH 4)As. O 4 immediate yellow ppt. of As 2 S 3 Arsenite Wash with dil Ammonia Solution+ Ag. NO 3 acidified (or) 2 Add 5 7 ml of 30%H 2 O 2 so. I. + magnesia With acetic acid mixture drop by drop (10 ml) with stirring a white crystalline ppt. Chocolate brown of Mg(NH 4) As. O 4 produced by ppt. of Ag 3 As. O 4 Oxidation of arsenite. Aresnate (or) 3 Addition of Na. HCO 3 sat. sol. +few drops of I 2 sol. The brown colour of I 2 disappears arsenite

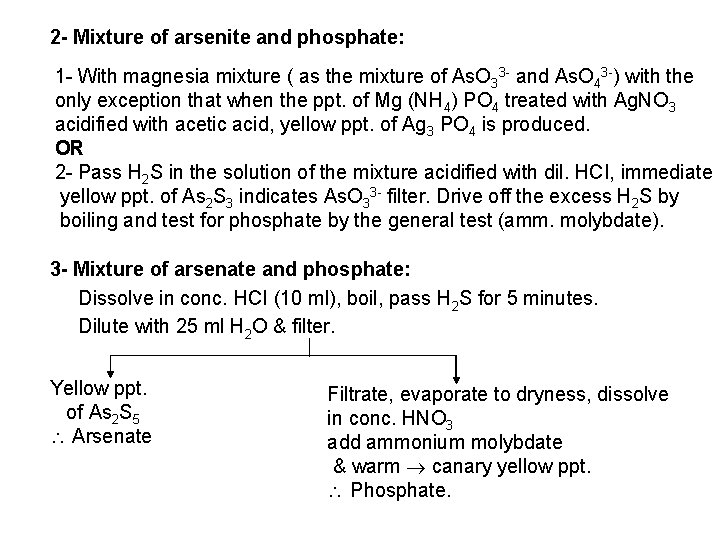
2 - Mixture of arsenite and phosphate: 1 With magnesia mixture ( as the mixture of As. O 33 and As. O 43 ) with the only exception that when the ppt. of Mg (NH 4) PO 4 treated with Ag. NO 3 acidified with acetic acid, yellow ppt. of Ag 3 PO 4 is produced. OR 2 Pass H 2 S in the solution of the mixture acidified with dil. HCI, immediate yellow ppt. of As 2 S 3 indicates As. O 33 filter. Drive off the excess H 2 S by boiling and test for phosphate by the general test (amm. molybdate). 3 - Mixture of arsenate and phosphate: Dissolve in conc. HCI (10 ml), boil, pass H 2 S for 5 minutes. Dilute with 25 ml H 2 O & filter. Yellow ppt. of As 2 S 5 Arsenate Filtrate, evaporate to dryness, dissolve in conc. HNO 3 add ammonium molybdate & warm canary yellow ppt. Phosphate.

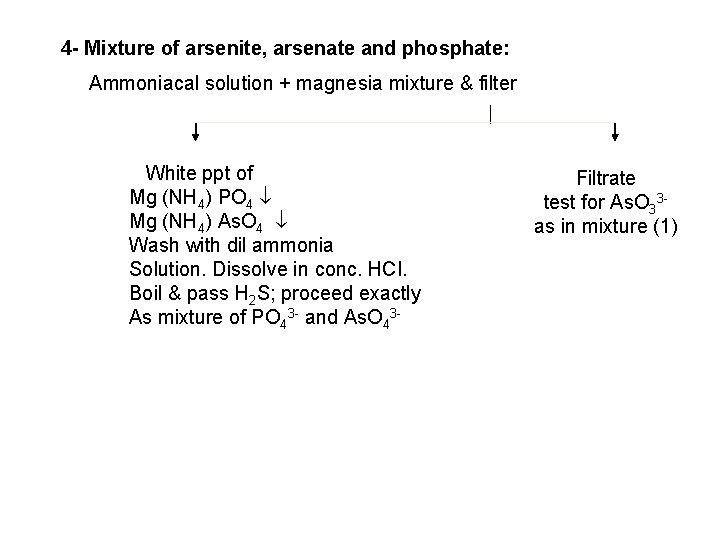
4 - Mixture of arsenite, arsenate and phosphate: Ammoniacal solution + magnesia mixture & filter White ppt of Mg (NH 4) PO 4 Mg (NH 4) As. O 4 Wash with dil ammonia Solution. Dissolve in conc. HCI. Boil & pass H 2 S; proceed exactly As mixture of PO 43 and As. O 43 Filtrate test for As. O 33 as in mixture (1)

Nitrogen- containing anions This group of anions, are; 1 - Nitrate (NO 3 -) 2 - Nitrite (NO 2 -) I. General characters 1 - Parent Acids: a) Nitric acid : HNO 3 Colorless liquid (B. P. 83 OC), decomposes on aging to nitrogen dioxide (NO 2). Its solution in water are strongly acidic. 4 HNO 3 4 NO 2 +2 H 2 O b) Nitrous acid : HNO 2 The pure acid has never been isolated, due to its thermal instability. 2 HNO 2 NO + NO 2 + H 2 O However addition of a strong acid to a solid nitrite or its solution in the cold yields a transient pale blue liquid (due to the presence of free HNO 2 acid or its anhydride, N 2 O 3) and the evolution of brown fumes of NO 2.

2 -Solubility: All nitrates are soluble in water. Also all nitrites are soluble in water except Ag. NO 2 which is slightly soluble. 3 - Redox-reaction : The nitrate ion contains in its highest oxidation state of + 5, thus reacts only as oxidizing agent, while nitrite ion contains nitrogen which has oxidation number + 3, it can therefore act either as a reducing or as oxidizing agent. II. General Reactions 1 - Dry Reactions a- Action of dilute HCl No reaction case of nitrates, with nitrites, brown fumes of nitrogen dioxide NO 2 evolve and a transient pale blue liquid. 2 NO 2 + 2 H+ 2 NO + O 2 2 HNO 2 2 NO 2 NO + NO 2 +H 2 O

H+ ions from dil. acids (including acetic acid) displace nitrous acid from its salts. The acid spontaneously decomposes to colorless monoxide NO & brownish NO 2 gases. The brown fumes intensify when getting in contact the atmosphere due to combination of NO with O 2 of air. b- Action of conc. H 2 SO 4 Nitrate: Nitric acid is formed and some of it decomposed with evolution of brown fumes of NO 2 with characteristic odor. NO 3 + H+ 4 HNO 3 4 NO 2 + 2 H 2 O When copper turnings are added, and the mixture heated to boiling, the brown fumes of NO 2 are increased due to the reduction of HNO 3 by Cuo metal which is oxidized to Cu 2+ ions, which imparts a blue color to the solution. 2 NO 3 + 4 H++ Cuo 2 NO 2 + Cu 2++ 2 H 2 O Nitrite: The reaction is the same as with dil HCI, but it takes place with considerable violence. On adding Cuo metal, the same occurs as with nitrates.

2 - Wet Reactions a) Reaction with Ag 2 SO 4 solution: Nitrate: No ppt. Nitrite: White crystalline ppt. of Ag. NO 2 form concentrated solutions. NO 2 + Ag. NO 2 b) Reaction with Ba. CI 2 solution: No precipitate is formed with either NO 3 nor NO 2 c) Reaction with KI solution: Acidify the test solution (3 ml) dil. H 2 SO 4, then add Kl solution and few drops starch solution. Nitrate: No reaction. Nitrite: I 2 is liberated imparting blue color to the starch. 2 NO 2 + 2 I + 4 H+ 2 NO + I 2+ 2 H 2 O

d) Reaction with Fe SO 4 solution. (Brown Ring Test): Acidify the test solution (5 ml) with dil. H 2 SO 4, add (1 ml) freshly prepared Fe. SO 4 solution. Nitrate: No visible change in case of using only dil. H 2 SO 4, but on adding conc. H 2 SO 4 cautiously down the sides of the test tube, a brown ring is formed at the interface. Nitrite: Brown colour in the whole solution if Fe. SO 4 solution is not cautiously added or a brown ring at the junction of the two liquids, if cautiously added. Fe. SO 4 reduces nitrate or nitrite ions to nitrogen monoxide, NO; nitrate ion is not reduced except in solutions containing a high H+ ion concentration, that is conc. H 2 SO 4. The excess Fe 2+ ions then combines with the NO produced to form the unstable brownish black complex ion [Fe (NO)]2+, readily decomposed by heat. 3 Fe 2++ NO 3 + 4 H+ Fe 2++ NO 2 + 2 H+ Fe 2++ NO 3 Fe 3++ NO + 2 H 2 O Fe 3++ NO + H 2 O [Fe (NO)]2+

This test differentiates NO 3 ion from NO 2 ion, since the latter gives the brown ring in presence of dil. H 2 SO 4 or even acetic acid, while NO 3 ion dose not form the ring except in presence of conc. H 2 SO 4. (NO 2 , I and Br ions will interfere) III. Special Tests 1 - For Nitrate: Ammonia test: If solution of NO 3 is boiled with Zno or Alo metals and Na. OH solution, NH 3 will be evolved which can identified by its odor or with red litmus paper (nitrites interfere). NO 3 + 4 Zno+ 7 OH 3 NH 3 + 4[Zn. O 2]2 + 2 H 2 O zincate ions 3 NO 3 + 8 Alo+ 5 OH + 2 H 2 O 3 NH 3 + 8 [Al. O 2] In acidic solution ( CH 3 COOH), nitrate can be reduced with Zno to nitrite.

2 - For Nitrite: a) Permanganate test: When a dilute potassium permanganate solution is added to an acid solution of nitrite, its pink color is bleached. In this test, the permanganate is reduced by the nitrite into colorless manganous Salt and the nitrite is oxidized into nitrate. 2 Mn. O 4 + 5 NO 2 + 6 H+ Pink 2 Mn 2++ 5 NO 3 + 3 H 2 O colorless b) Urea test: When a solution of a nitrite is treated with urea and the mixture acidified with dilute HCl, the nitrite is decomposed, and N 2 and CO 2 are evolved. CO (NH 2)2+ 2 HNO 2 2 N 2 + CO 2 +3 H 2 O c) Ammonium Chloride test: By boiling a solution of a nitrite with excess of the solid reagent, N 2 is evoled and the nitrite is completely destroyed. NO 2 + NH+4 N 2 + 2 H 2 O

d) Thiourea test: When a dil. acetic acid solution of a nitrite is treated with a little thiourea, N 2 is evolved and thiocyanic acid is produced. The latter may be identified by the red color produced with dil. HCl and Fe. Cl 3 solution. CS (NH 2)2+ HNO 2 N 2+ H++ CNS + 2 H 2 O N. B: Thiocyanates and iodides interfere, and if present must be removed either with Ag 2 SO 4 (solid) or dil Ag. NO 3 solution. IV. Analysis of Mixtures 1 - Mixture of Nitrate and Nitrite : Nitrite can be tested for in presence of nitrate (by treatment with dil HCI, KMn. O 4, Fe. SO 4 in dil. H 2 SO 4); and by the special tests for nitrite. Nitrate cannot be tested for in presence of nitrite, since nitrite gives all the reactions of nitrate (conc. H 2 SO 4, brown ring test and ammonia test). Therefore nitrite be removed before testing for nitrate by: 1 Decomposition of NO 2 through its brown complex with Fe. SO 4 formed in dil. H 2 SO 4 or acetic acid by heat and shaking. [Fe (NO)]2+ heat NO + Fe 2+

2 Decomposition of NO 2 through its reduction to nitrogen by boiling with NH 4 CI or warming with urea and few drops of dilute H 2 SO 4 or warming with little sulphamic acid. HO. SO 2. NH 2+ HNO 2 N 2 + H 2 SO 4+ H 2 O 2 - Mixture of Nitrate and Bromide or / and lodide: Br and I can be detected in presence of NO 3 by chlorine water test. NO 3 can be detected in presence of Br and I by the ammonia test. On the other, the brown ring test for nitrates cannot be applied in the presence of Br and I , since the liberation of free halogen with conc. H 2 SO 4 will obscure the brown ring due to NO 3 Therefore Br and I must be first removed by either: 1 Addition of saturated solution of silver sulphate, where Ag. Br and Ag. I are precipitated and then filtered off, the excess Ag+ is precipitated with Na 2 CO 3 OR 2 Adding potassium persulphate and dil. H 2 SO 4 and warming to about 80 o. C. The halogen is removed by boiling or extraction with organic solvent. S 2 O 82 + 2 Br Br 2+ 2 SO 42 S 2 O 82 + 2 I I 2 + 2 SO 42 The Halide free solution is tested for NO 3 by the brown ring test