Chemical Reactions Catalysts CHEMICAL REACTIONS a process that



- Slides: 11

Chemical Reactions & Catalysts

CHEMICAL REACTIONS • a process that changes one set of chemicals into another set of chemicals • REACTANTS – elements or compounds that enter into a chemical reaction • PRODUCTS – elements or compounds that are produced in a chemical reaction • Chemical reactions always involve the breaking of bonds in reactants and the formation of new bonds in products.

• In a reaction, energy is either: • TAKEN IN (ENDOTHERMIC) or • GIVEN OFF (EXOTHERMIC) • Can you think of an everyday example of each type of reaction?

Enzymes and Enzyme Action • catalyst: inorganic or organic substance which speeds up the rate of a chemical reaction without entering the reaction itself • enzymes: organic catalysts made of protein • most enzyme names end in -ase • enzymes lower the energy needed to start a chemical reaction. (activation energy) • begin to be destroyed above 45 o. C. (above this temperature all proteins begin to be destroyed)

It is thought that, in order for an enzyme to affect the rate of a reaction, the following events must take place. 1. The enzyme must form a temporary association with the substance or substances whose reaction rate it affects. These substances are known as substrates. 2. The association between enzyme and substrate is thought to form a close physical association between the molecules and is called the enzyme-substrate complex. 3. While the enzyme-substrate complex is formed, enzyme action takes place. 4. Upon completion of the reaction, the enzyme and product(s) separate. The enzyme molecule is now available to form additional complexes.

How do enzymes work? • substrate: molecules upon which an enzyme acts • the enzyme is shaped so that it can only lock up with a specific substrate molecule enzyme substrate -------> product

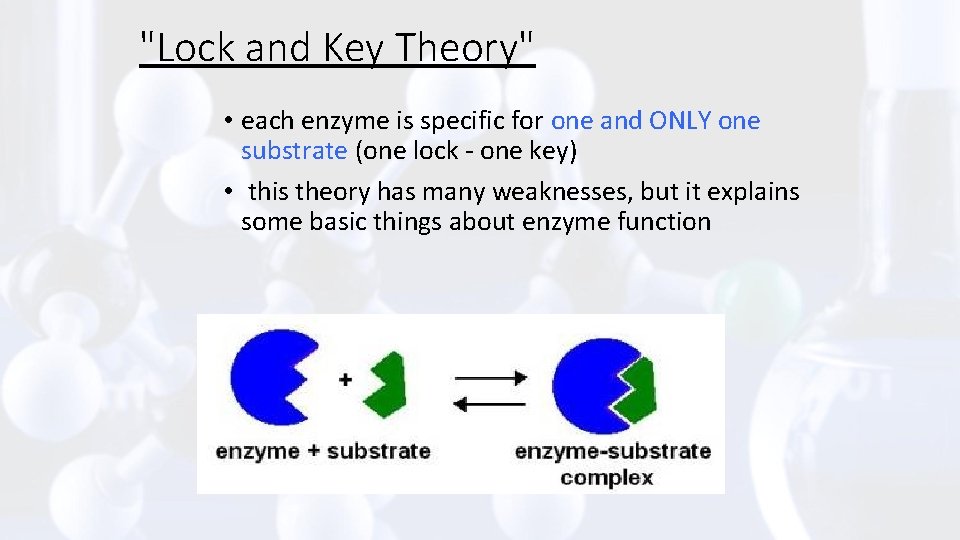
"Lock and Key Theory" • each enzyme is specific for one and ONLY one substrate (one lock - one key) • this theory has many weaknesses, but it explains some basic things about enzyme function

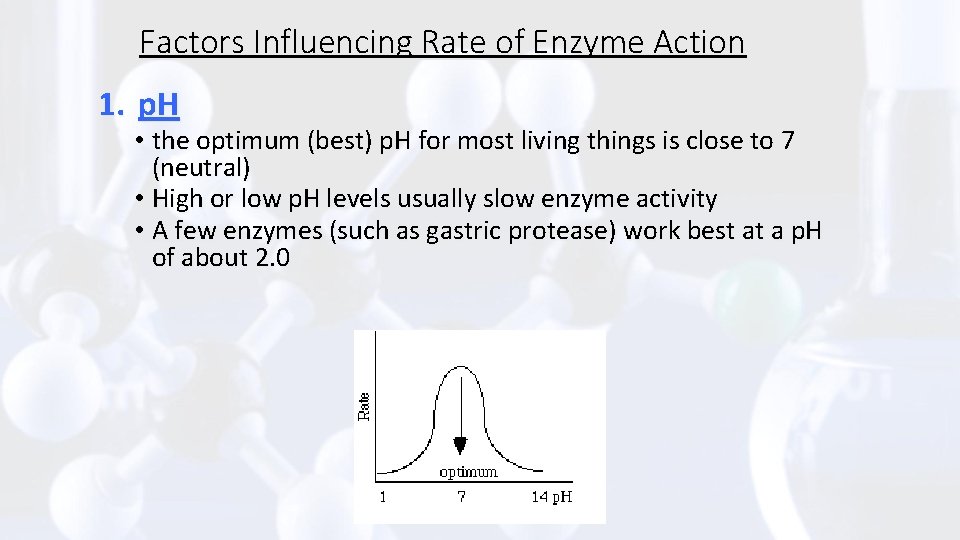
Factors Influencing Rate of Enzyme Action 1. p. H • the optimum (best) p. H for most living things is close to 7 (neutral) • High or low p. H levels usually slow enzyme activity • A few enzymes (such as gastric protease) work best at a p. H of about 2. 0

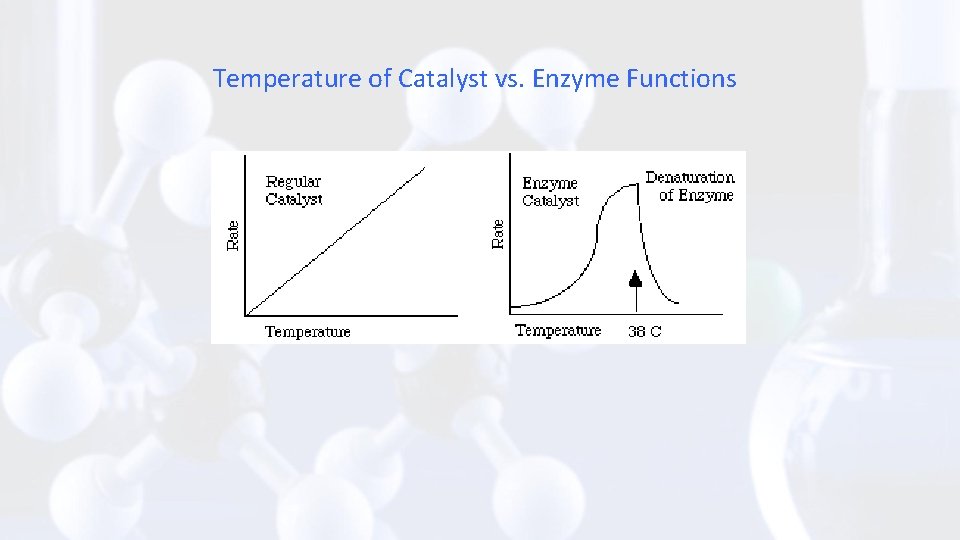
2. Temperature - strongly influences enzyme activity • optimum temperature for maximum enzyme function is usually about 35 -40 °C. • reactions proceed slowly below optimal temperatures • above 45 °C most enzymes are denatured (change in their shape so the enzyme active site no longer fits with the substrate and the enzyme can't function)

Temperature of Catalyst vs. Enzyme Functions

3. Concentrations of Enzyme and Substrate • ** When there is a fixed amount of enzyme and an excess of substrate molecules -- the rate of reaction will increase to a point and then level off.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Relational escalation catalysts
Relational escalation catalysts Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block av độ 2
Block av độ 2 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng





















