Qualitative Analysis of Anions THEORY There are two


- Slides: 13

Qualitative Analysis of Anions

THEORY: There are two types of analysis methods, which are quantitative and qualitative analysis. • Qualitative Analysis which is a method of that determination of elements and the compounds in a sample. • Qualitative Analysis which is a method of that determination of relative amounts of elements and compounds in a sample.

THEORY: • What is the meaning of anion? Anion is a compound that has negative charge. It is obtained from ionization acids and salts. For example;

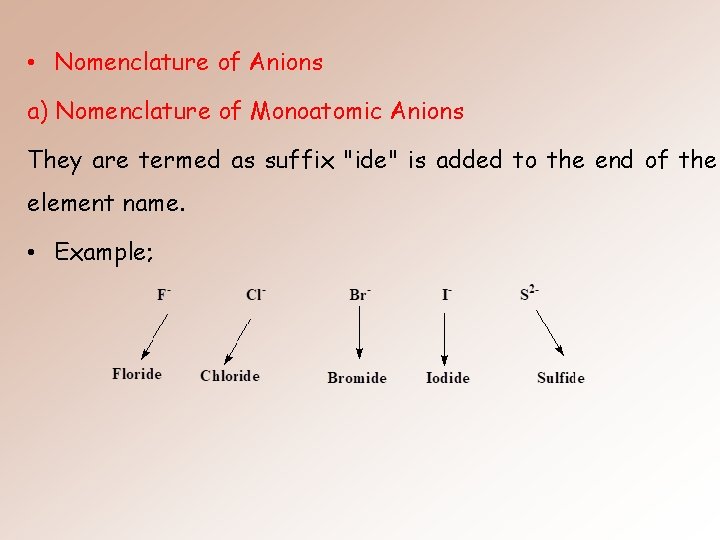
• Nomenclature of Anions a) Nomenclature of Monoatomic Anions They are termed as suffix "ide" is added to the end of the element name. • Example;

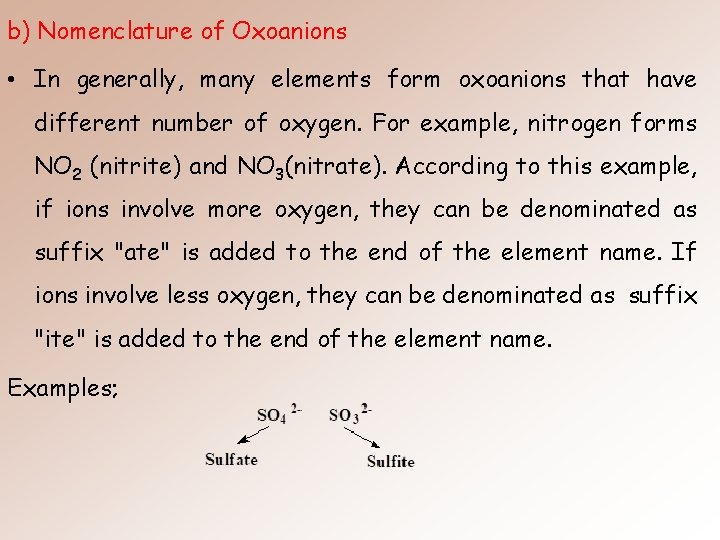
b) Nomenclature of Oxoanions • In generally, many elements form oxoanions that have different number of oxygen. For example, nitrogen forms NO 2 (nitrite) and NO 3(nitrate). According to this example, if ions involve more oxygen, they can be denominated as suffix "ate" is added to the end of the element name. If ions involve less oxygen, they can be denominated as suffix "ite" is added to the end of the element name. Examples;

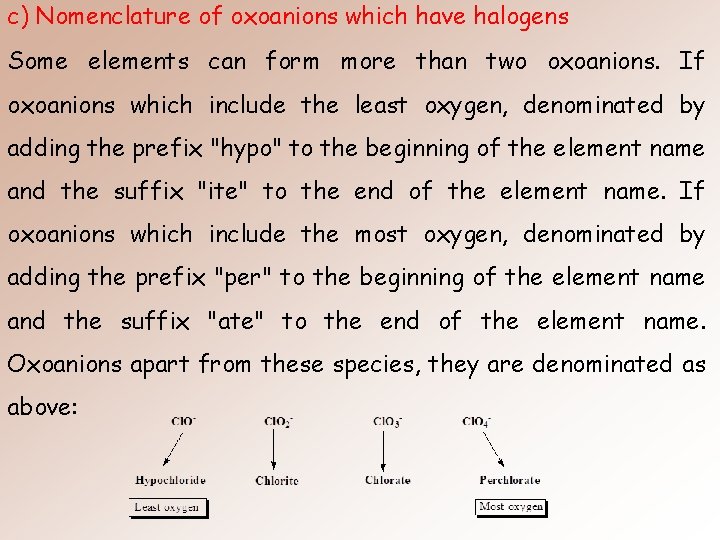
c) Nomenclature of oxoanions which have halogens Some elements can form more than two oxoanions. If oxoanions which include the least oxygen, denominated by adding the prefix "hypo" to the beginning of the element name and the suffix "ite" to the end of the element name. If oxoanions which include the most oxygen, denominated by adding the prefix "per" to the beginning of the element name and the suffix "ate" to the end of the element name. Oxoanions apart from these species, they are denominated as above:

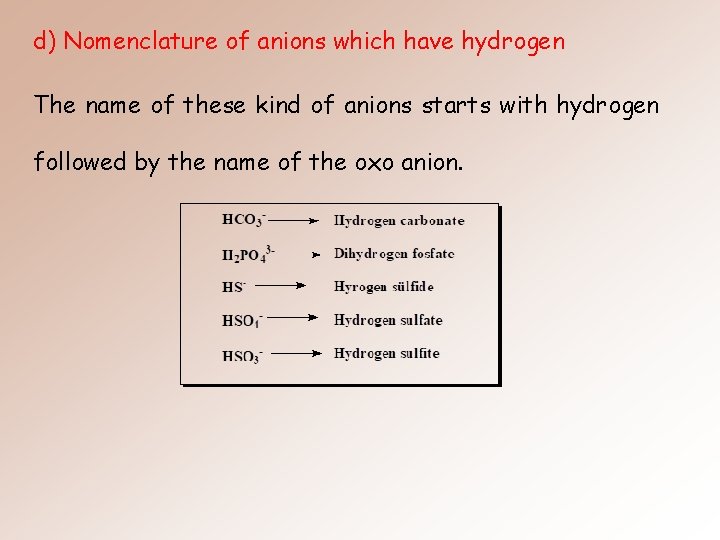
d) Nomenclature of anions which have hydrogen The name of these kind of anions starts with hydrogen followed by the name of the oxo anion.

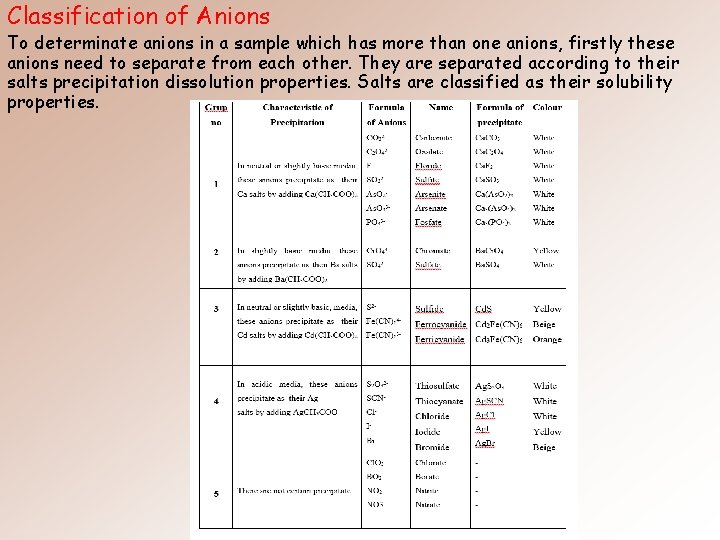
Classification of Anions To determinate anions in a sample which has more than one anions, firstly these anions need to separate from each other. They are separated according to their salts precipitation dissolution properties. Salts are classified as their solubility properties.

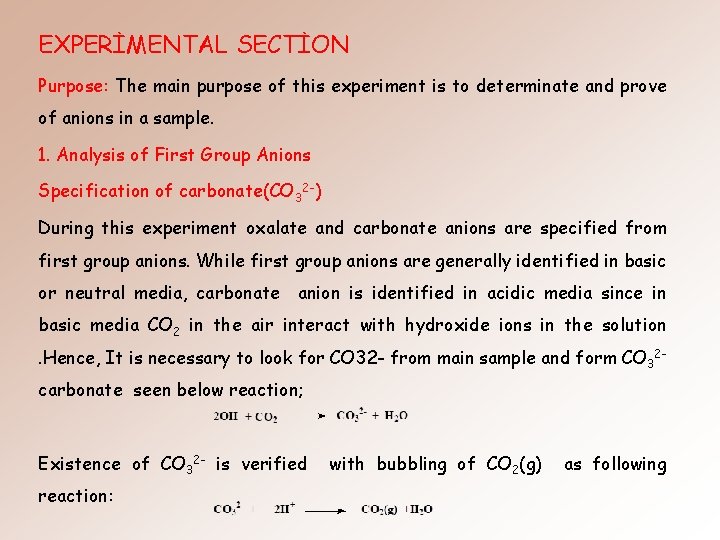
EXPERİMENTAL SECTİON Purpose: The main purpose of this experiment is to determinate and prove of anions in a sample. 1. Analysis of First Group Anions Specification of carbonate(CO 32 -) During this experiment oxalate and carbonate anions are specified from first group anions. While first group anions are generally identified in basic or neutral media, carbonate anion is identified in acidic media since in basic media CO 2 in the air interact with hydroxide ions in the solution. Hence, It is necessary to look for CO 32 - from main sample and form CO 32 carbonate seen below reaction; Existence of CO 32 - is verified reaction: with bubbling of CO 2(g) as following

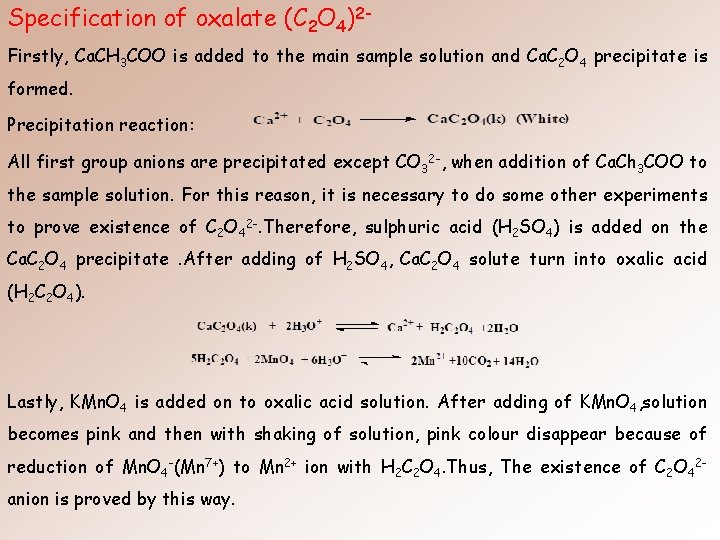
Specification of oxalate (C 2 O 4)2 Firstly, Ca. CH 3 COO is added to the main sample solution and Ca. C 2 O 4 precipitate is formed. Precipitation reaction: All first group anions are precipitated except CO 32 -, when addition of Ca. Ch 3 COO to the sample solution. For this reason, it is necessary to do some other experiments to prove existence of C 2 O 42 -. Therefore, sulphuric acid (H 2 SO 4) is added on the Ca. C 2 O 4 precipitate. After adding of H 2 SO 4, Ca. C 2 O 4 solute turn into oxalic acid (H 2 C 2 O 4). Lastly, KMn. O 4 is added on to oxalic acid solution. After adding of KMn. O 4, solution becomes pink and then with shaking of solution, pink colour disappear because of reduction of Mn. O 4 -(Mn 7+) to Mn 2+ ion with H 2 C 2 O 4. Thus, The existence of C 2 O 42 anion is proved by this way.

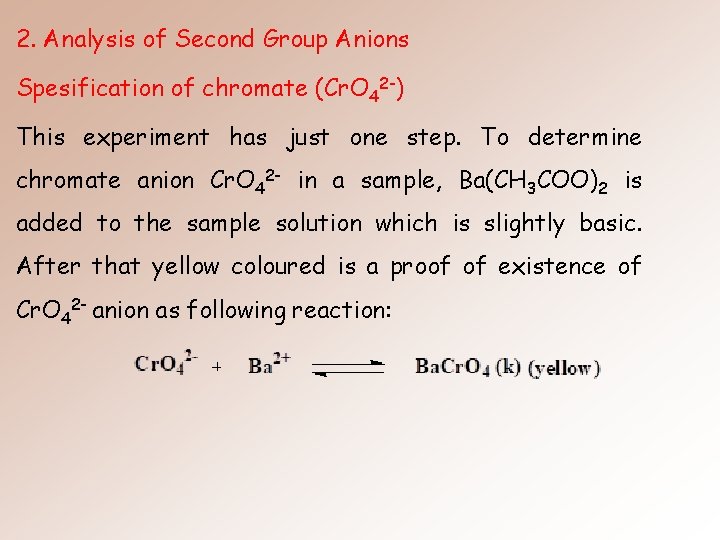
2. Analysis of Second Group Anions Spesification of chromate (Cr. O 42 -) This experiment has just one step. To determine chromate anion Cr. O 42 - in a sample, Ba(CH 3 COO)2 is added to the sample solution which is slightly basic. After that yellow coloured is a proof of existence of Cr. O 42 - anion as following reaction:

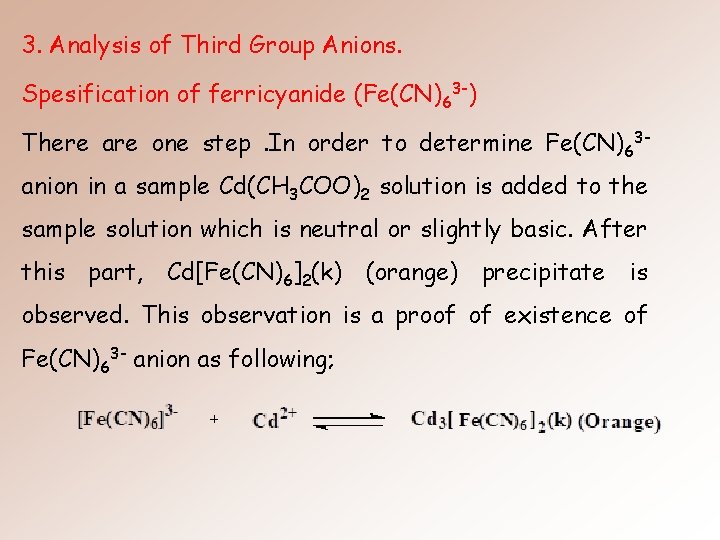
3. Analysis of Third Group Anions. Spesification of ferricyanide (Fe(CN)63 -) There are one step. In order to determine Fe(CN)63 anion in a sample Cd(CH 3 COO)2 solution is added to the sample solution which is neutral or slightly basic. After this part, Cd[Fe(CN)6]2(k) (orange) precipitate is observed. This observation is a proof of existence of Fe(CN)63 - anion as following;

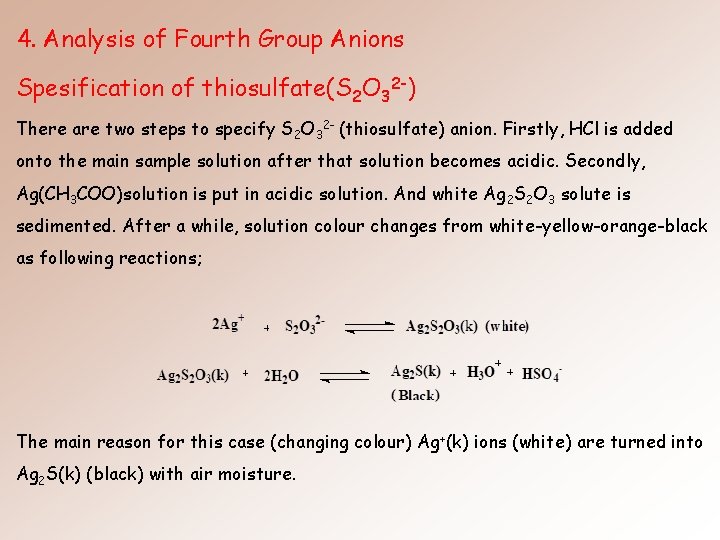
4. Analysis of Fourth Group Anions Spesification of thiosulfate(S 2 O 32 -) There are two steps to specify S 2 O 32 - (thiosulfate) anion. Firstly, HCl is added onto the main sample solution after that solution becomes acidic. Secondly, Ag(CH 3 COO)solution is put in acidic solution. And white Ag 2 S 2 O 3 solute is sedimented. After a while, solution colour changes from white-yellow-orange-black as following reactions; The main reason for this case (changing colour) Ag+(k) ions (white) are turned into Ag 2 S(k) (black) with air moisture.
 Mikael ferm
Mikael ferm How are anions divided into groups
How are anions divided into groups Flow of anions and cations in an electrochemical cell
Flow of anions and cations in an electrochemical cell Hf acid or base
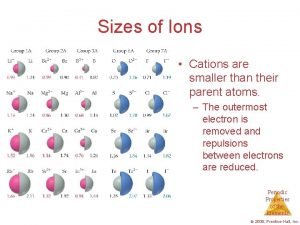
Hf acid or base Sizes of ions
Sizes of ions Classification of anions
Classification of anions Unmeasured anions
Unmeasured anions Three ionic compounds
Three ionic compounds Which is an example of a polyatomic ion?h2co3-mg+ne+ -
Which is an example of a polyatomic ion?h2co3-mg+ne+ - 5 anions
5 anions Major physiological anions
Major physiological anions Cations and anions table
Cations and anions table Examples ionic compounds
Examples ionic compounds Polyatomic ions game
Polyatomic ions game