proteins Proteins are divided into two types fibrous
proteins • Proteins are divided into two types: – fibrous proteins are • insoluble in water • used mainly for structural purposes – globular proteins are • More or less soluble in water • Used mainly for nonstructural purposes

Proteins properties • Proteins behave as zwitterions. • Proteins also have an isoelectric point, p. I. – At its isoelectric point, the protein has no net charge. – At any p. H above (more basic than) its p. I, it has a net negative charge. – At any p. H below (more acidic than) its p. I, it has a net positive charge. – Hemoglobin, for example, has an almost equal number of acidic and basic side chains; its p. I is 6. 8. – Serum albumin has more acidic side chains; its p. I is 4. 9. • Proteins are least soluble in water at their isoelectric points and can be precipitated from solution at this p. H.

Levels of structure • Primary structure: the sequence of amino acids in a polypeptide chain; read from the N-terminal amino acid to the C-terminal amino acid. • Secondary structure: conformations of amino acids in localized regions of a polypeptide chain; examples are α-helix, β-pleated sheet, and random coil. • Tertiary structure: the complete three-dimensional arrangement of atoms of a polypeptide chain. • Quaternary structure: the spatial relationship and interactions between subunits in a protein that has more than one polypeptide chain.

Primary structure • Primary structure: the sequence of amino acids in a polypeptide chain. • The number peptides possible from the 20 protein-derived amino acids is enormous.

Primary structure • How important is the exact amino acid sequence? – Human insulin consists of two polypeptide chains having a total of 51 amino acids; the two chains are connected by three interchain disulfide bonds. – In the table are differences between four types of insulin.
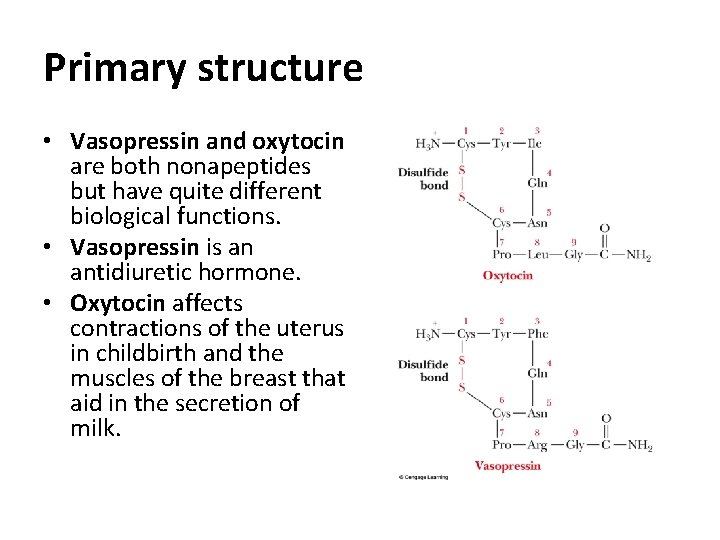
Primary structure • Vasopressin and oxytocin are both nonapeptides but have quite different biological functions. • Vasopressin is an antidiuretic hormone. • Oxytocin affects contractions of the uterus in childbirth and the muscles of the breast that aid in the secretion of milk.
Secondary structure • Secondary structure: conformations of amino acids in localized regions of a polypeptide chain. – The most common types of secondary structure are : • α-helix • β-pleated sheet.

α-Helix structure • α-Helix structure: a type of secondary structure in which a section of polypeptide chain coils into a spiral, most commonly a right-handed spiral.
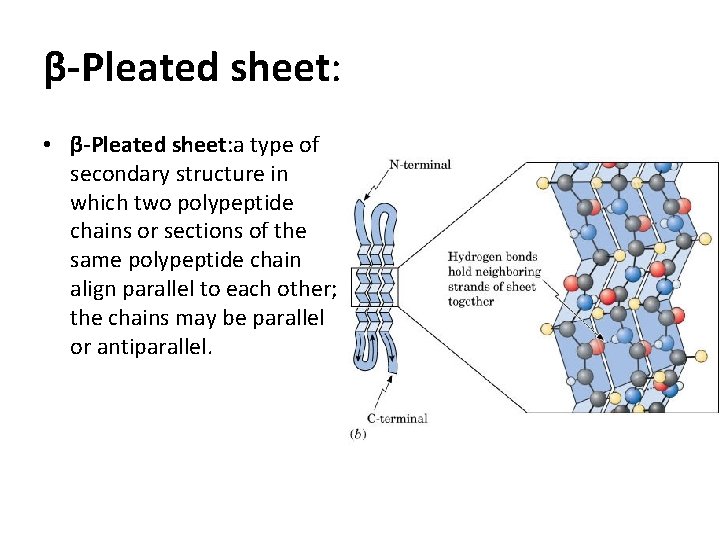
β-Pleated sheet: • β-Pleated sheet: a type of secondary structure in which two polypeptide chains or sections of the same polypeptide chain align parallel to each other; the chains may be parallel or antiparallel.

Collagen triple helix – Consists of three polypeptide chains wrapped around each other in a ropelike twist to form a triple helix called tropocollagen. – 30% of amino acids in each chain are Pro and Lhydroxyproline (Hyp); Lhydroxylysine (Hyl) also occurs. – Every third position is Gly and repeating sequences are X-Pro. Gly and X-Hyp-Gly. – The three strands are held together by hydrogen bonding involving hydroxyproline and hydroxylysine.
Tertiary structure • Tertiary structure: the overall conformation of an entire polypeptide chain. • Tertiary structure is stabilized in five ways: – Covalent bonds, as for example, the formation of disulfide bonds between cysteine side chains. – Hydrogen bonding between polar groups of side chains, as for example between the -OH groups of serine and threonine. – Salt bridges, as for example, the attraction of the -NH 3+ group of lysine and the -COO- group of aspartic acid. – Hydrophobic interactions, as for example, between the nonpolar side chains of phenylalanine and isoleucine. – Metal ion coordination, as for example, between two glutamic side chain

Tertiary structure

Quaternary structure • Quaternary structure: the arrangement of polypeptide chains into a noncovalently bonded aggregation. – The individual chains are held in together by hydrogen bonds, salt bridges, and hydrophobic interactions. • Hemoglobin – Adult hemoglobin: two alpha chains of 141 amino acids each, and two beta chains of 146 amino acids each. – Each chain surrounds an iron-containing heme unit. – Fetal hemoglobin: two alpha chains and two gamma chains; fetal hemoglobin has a greater affinity for oxygen than does adult hemoglobin.
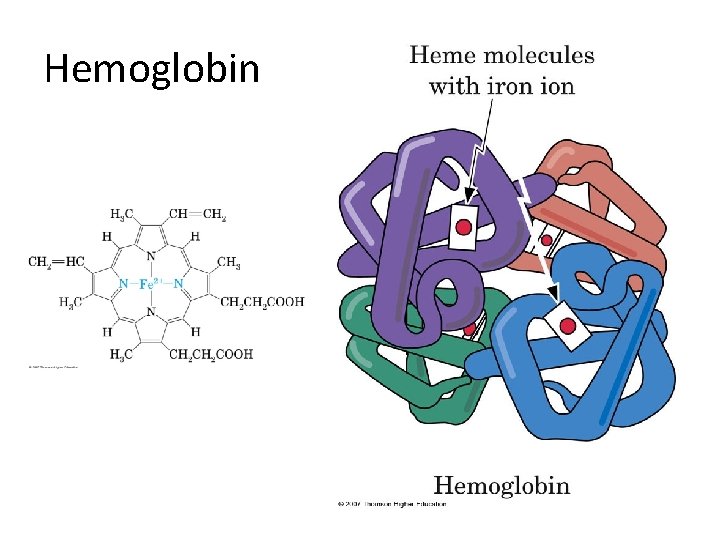
Hemoglobin
Denatured proteins • Denaturation: the process of destroying the native conformation of a protein by chemical or physical means. – Some denaturations are reversible, while others permanently damage the protein. • Denaturing agents include: – Heat: heat can disrupt hydrogen bonding; in globular proteins, it can cause unfolding of polypeptide chains with the result that coagulation and precipitation may take place.

Denatured proteins (Cont’d) – 6 M aqueous urea: disrupts hydrogen bonding. – Surface-active agents: detergents such as sodium dodecylbenzenesulfate (SDS) disrupt hydrogen bonding. – Reducing agents: 2 -mercaptoethanol (HOCH 2 SH) cleaves disulfide bonds by reducing -S-S- groups to -SH groups. – Heavy metal ions: transition metal ions such as Pb 2+, Hg 2+, and Cd 2+ form water-insoluble salts with -SH groups; Hg 2+ for example forms -S-Hg-S-. – Alcohols: 70% ethanol, for example, which denatures proteins, is used to sterilize skin before injections.
- Slides: 16