EMULSIONs DEPARTMENT OF PHARMACEUTICS CHALAPATHI INSTITUTE OF PHARMACEUTICAL




































































- Slides: 92

EMULSIONs DEPARTMENT OF PHARMACEUTICS CHALAPATHI INSTITUTE OF PHARMACEUTICAL SCIENCES Lam, guntur

Outline � Definition � Classification � Identification of emulsion � Application of emulsion � Theory of emulsification � Formulation of emulsion � Emulsification techniques � Reference

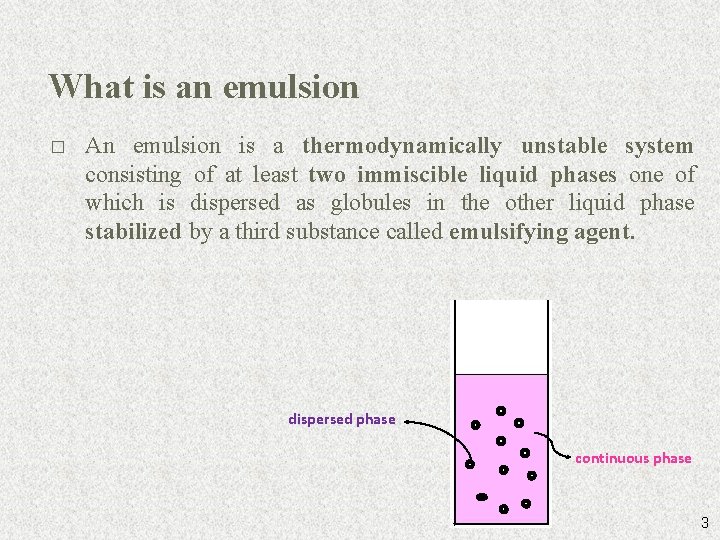
What is an emulsion � An emulsion is a thermodynamically unstable system consisting of at least two immiscible liquid phases one of which is dispersed as globules in the other liquid phase stabilized by a third substance called emulsifying agent. dispersed phase continuous phase 3

Pharmaceutical applications of emulsions: ØThey can mask the bitter taste and odor of drugs, e. g. castor oil, cod-liver oil etc. Ø They can be used to prolong the release of the drug thereby providing sustained release action. Ø Essential nutrients like carbohydrates, fats and vitamins can all be emulsified and can be administered to bed ridden patients as sterile intravenous emulsions. Ø Emulsions provide protection to drugs which are susceptible to oxidation or hydrolysis. Ø Intravenous emulsions of contrast media have been developed to assist in diagnosis. Ø Emulsions are used widely to formulate externally used products like lotions, creams, liniments etc. 4

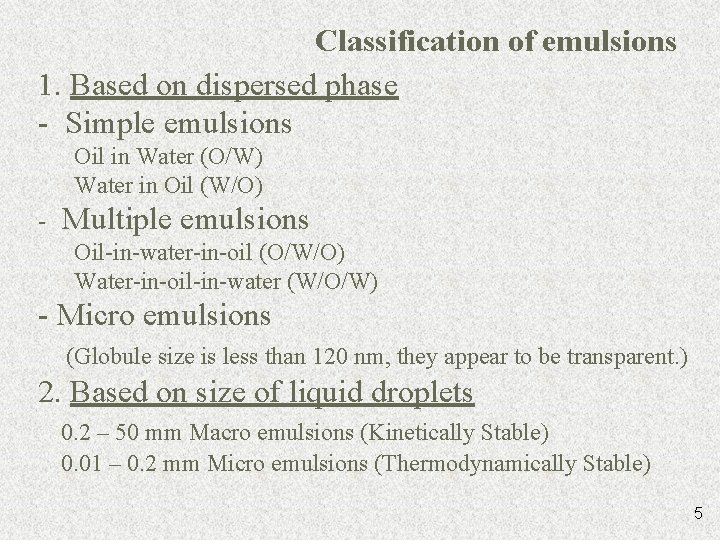
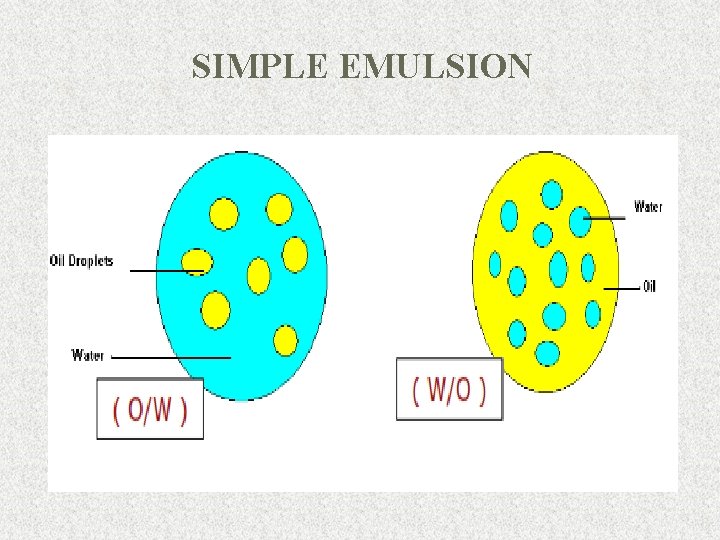
Classification of emulsions 1. Based on dispersed phase - Simple emulsions Oil in Water (O/W) Water in Oil (W/O) - Multiple emulsions Oil-in-water-in-oil (O/W/O) Water-in-oil-in-water (W/O/W) - Micro emulsions (Globule size is less than 120 nm, they appear to be transparent. ) 2. Based on size of liquid droplets 0. 2 – 50 mm Macro emulsions (Kinetically Stable) 0. 01 – 0. 2 mm Micro emulsions (Thermodynamically Stable) 5

SIMPLE EMULSION 6

DIFFERENCE BETWEEN O/W AND W/O EMULSIONS Oil in water emulsion (o/w) Water in oil emulsion (w/o) Water is the dispersion medium and oil is the dispersed phase Oil is the dispersion medium and water is the dispersed phase They are non greasy and easily removable from the skin surface They are greasy and not water washable They are used externally to provide cooling effect e. g. vanishing cream They are used externally to prevent evaporation of moisture from the surface of skin e. g. Cold cream Water soluble drugs are more quickly released from o/w emulsions Oil soluble drugs are more quickly released from w/o emulsions They are preferred formulations meant for internal use as bitter taste of oils can be masked. They are preferred formulations meant for external use like creams. O/W emulsions give a positive conductivity test as water is the external phase which is a good conductor of electricity. W/O emulsions go not give a positive conductivity test as oil is the external phase which is a poor conductor of electricity. 7

Multiple emulsion � Also called emulsion within emulsion � They are developed with a view to delay the release of an active ingredient. They have three phases. � They may be oil-in-water-in-oil (o/w/o) or of water-in-oil-in-water (w/o/w). W/O/W SYSTEM water phase oil phase O/W/O SYSTEM 8

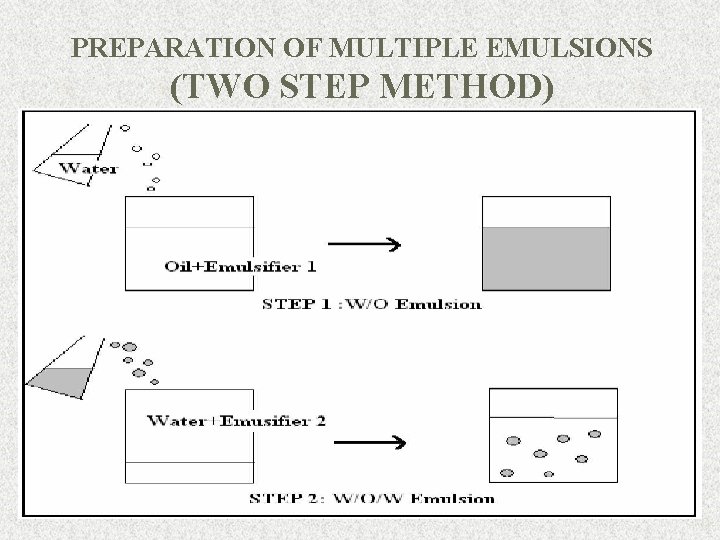
PREPARATION OF MULTIPLE EMULSIONS (TWO STEP METHOD) 9

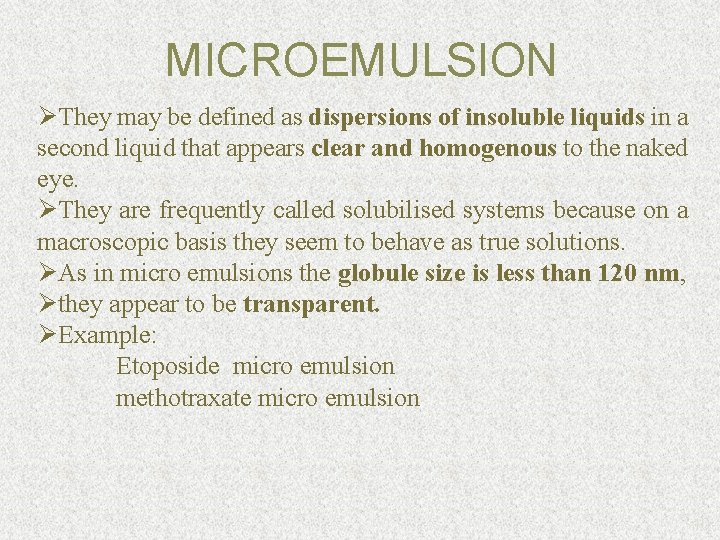
MICROEMULSION ØThey may be defined as dispersions of insoluble liquids in a second liquid that appears clear and homogenous to the naked eye. ØThey are frequently called solubilised systems because on a macroscopic basis they seem to behave as true solutions. ØAs in micro emulsions the globule size is less than 120 nm, Øthey appear to be transparent. ØExample: Etoposide micro emulsion methotraxate micro emulsion 10

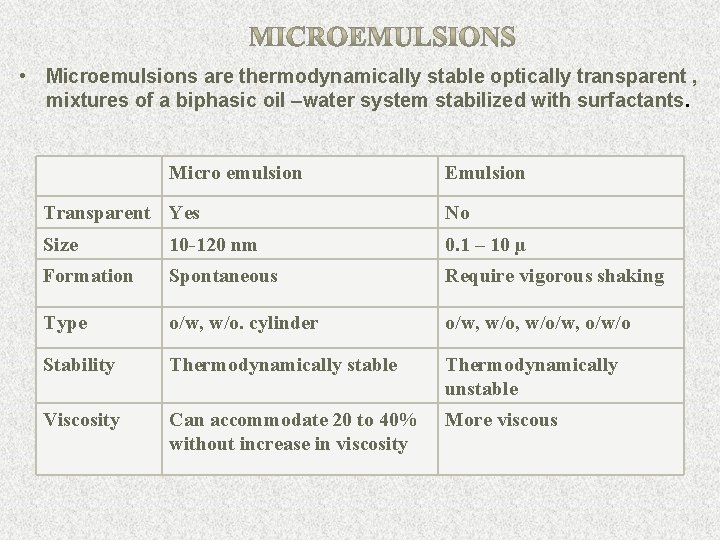
• Microemulsions are thermodynamically stable optically transparent , mixtures of a biphasic oil –water system stabilized with surfactants. Micro emulsion Emulsion Transparent Yes No Size 10 -120 nm 0. 1 – 10 µ Formation Spontaneous Require vigorous shaking Type o/w, w/o. cylinder o/w, w/o/w, o/w/o Stability Thermodynamically stable Thermodynamically unstable Viscosity Can accommodate 20 to 40% without increase in viscosity More viscous 11

Micro emulsion/macro emulsion MICRO � � � Thermodynamically stable Droplet size is 10 -100 nm (transparent) High surface area (200 m 2/g) MACRO � Kinetically stable 1 -10 microns (opaque) � Low surface area (15 m 2/g) � 12

PHARMACEUTICAL APPLICATIONS OF MICROEMULSIONS q Increase bioavailability of drugs poorly soluble in water. q Topical drug delivery systems ORAL PRODUCTS q It covers the unpleasant taste q Increases absorption rate O/W PARENTERAL USE EMULSION q i/v lipid nutrients q i/m – depot effect for water soluble antigenic material q Washable q Acceptable viscosity q Less greasy 13

Ø To mask the taste Ø O/W is convenient means of orally administration of waterinsoluble liquids Ø O/W emulsion facilitates the absorption of water-insoluble compounds comparing to their oily solution preparations (e. g. vitamins) Ø Oil-soluble drugs can be given parentrally in form of oil-in water emulsion. (e. g. Taxol) Ø and therapeutic uses. 14

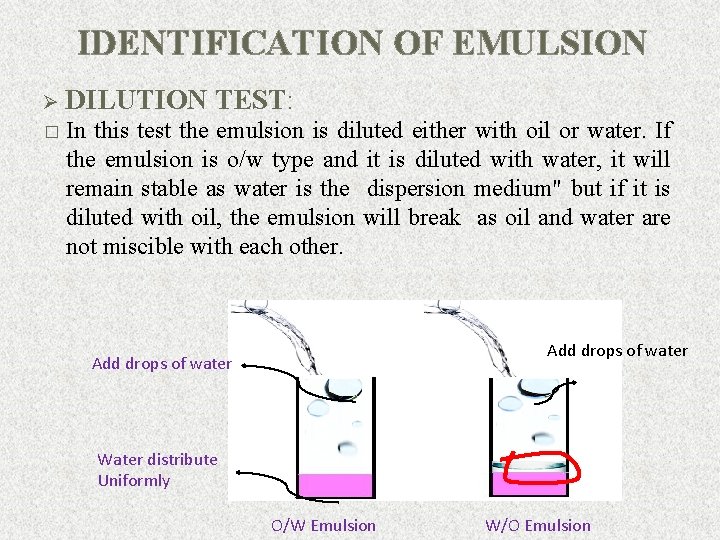
IDENTIFICATION OF EMULSION Ø DILUTION TEST: � In this test the emulsion is diluted either with oil or water. If the emulsion is o/w type and it is diluted with water, it will remain stable as water is the dispersion medium" but if it is diluted with oil, the emulsion will break as oil and water are not miscible with each other. Add drops of water Water distribute Uniformly O/W Emulsion W/O Emulsion 15

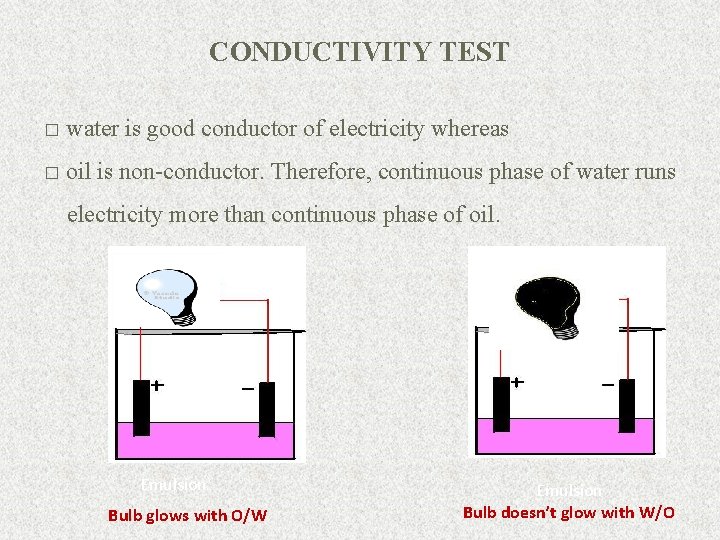
CONDUCTIVITY TEST � water is good conductor of electricity whereas � oil is non-conductor. Therefore, continuous phase of water runs electricity more than continuous phase of oil. Emulsion Bulb glows with O/W Emulsion Bulb doesn’t glow with W/O 16

Dye Solubility test: � DYE TEST Water soluble dye (methylene blue) will be taken up by the aqueous phase where as oil soluble dye will be taken by oily phase. � When microscopically it is observed that water soluble dye is taken up by the continuous phase , it is o/w emulsion. � If the dye is not taken up by the continuous phase , test is repeated with oil soluble dye. . Coloring of continuous phase confirms w/o emulsion. This test can fail if ionic emulsions are present. 17

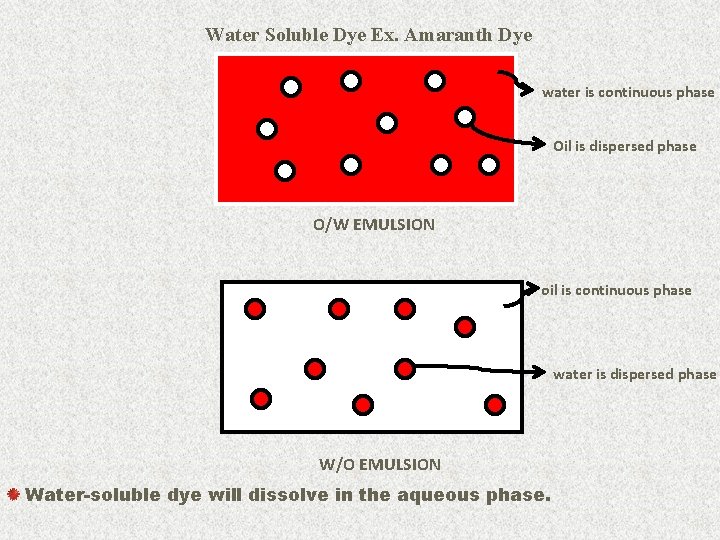
Water Soluble Dye Ex. Amaranth Dye water is continuous phase Oil is dispersed phase O/W EMULSION oil is continuous phase water is dispersed phase W/O EMULSION Water-soluble dye will dissolve in the aqueous phase. 18

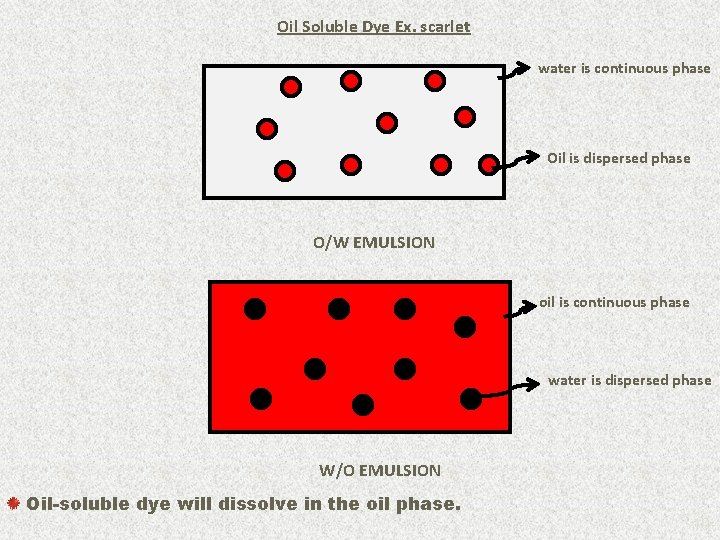
Oil Soluble Dye Ex. scarlet water is continuous phase Oil is dispersed phase O/W EMULSION oil is continuous phase water is dispersed phase W/O EMULSION Oil-soluble dye will dissolve in the oil phase. 19

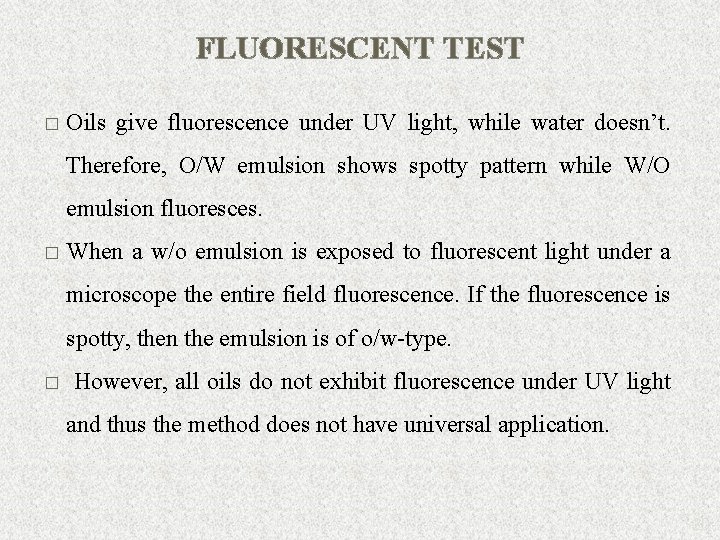
FLUORESCENT TEST � Oils give fluorescence under UV light, while water doesn’t. Therefore, O/W emulsion shows spotty pattern while W/O emulsion fluoresces. � When a w/o emulsion is exposed to fluorescent light under a microscope the entire field fluorescence. If the fluorescence is spotty, then the emulsion is of o/w-type. � However, all oils do not exhibit fluorescence under UV light and thus the method does not have universal application. 20

COCL 2/ FILTER PAPER TEST v Filter paper impregnated with Co. Cl 2 and dried appear to be blue but when dipped in o/w emulsion changes to pink. v This test may fail if emulsion unstable or breaks in presence of electrolyte 21

CREAMING TEST • The direction of creaming identifies the emulsion type, if the densities of aqueous and oil phases are known. • Water-in-oil emulsions normally cream downward as oil is usually less dense than water. • Oil-in-water emulsions normally cream upwards. 22

APPLICATIONS OF EMULSIONS • For prolonged action • Taste masking • Improved stability • Parenteral preparation • Enzyme entrapment • Increased Oral bioavailability 23

INSTABILITY OF EMULSION • Emulsification is not a spontaneous process and hence emulsions have minimal stability. • Reasons for instability can be understood from the nature of immiscible phases and their interfacial properties. When two immiscible liquids are agitated together polar (aqueous) and non polar (oil) liquids are mixed together one of the liquids forms small droplets and gets dispersed in the other liquid forms an emulsion. 24

• When left aside, droplets fuse themselves and finally separate as two layers. This in an indication of instability of an emulsion. • Except in the case of very dilute oil-in-water emulsions (oil hydrosols), which are somewhat stable, the liquids separate rapidly into two clearly defined layers. • The state of instability may be described by the fact that the cohesive force between the molecules of each separate liquid is greater than the adhesive force between the two liquids. • Any attempt to increase the adhesive forces between these phases can produce a stable emulsion. • A system is said to be thermodynamically stable, if it possesses low surface free energy. • The higher the interfacial area, the greater is the interfacial free energy, and hence lower the stability. 25

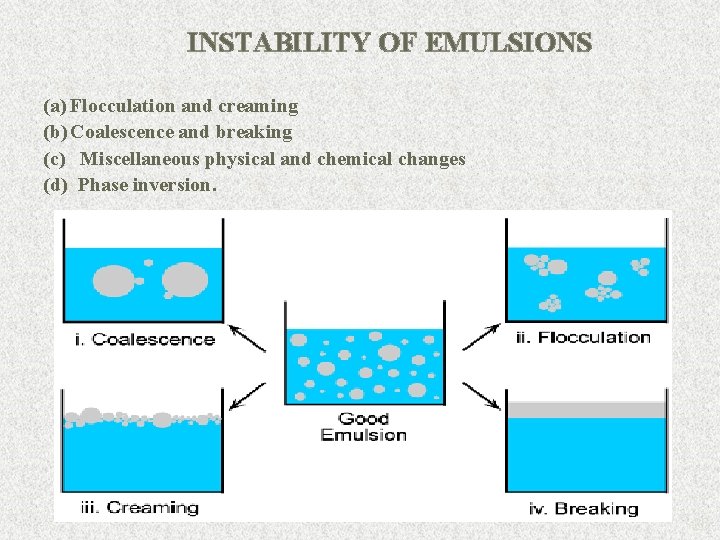
INSTABILITY OF EMULSIONS (a) Flocculation and creaming (b) Coalescence and breaking (c) Miscellaneous physical and chemical changes (d) Phase inversion. 26

Flocculation • Neighboring globules come closer to each other and form colonies in the continuous phase. This aggregation of globules is not clearly visible. • This is the initial stage that leads to instability. • Flocculation of the dispersed phase may take place before, during or after creaming. 27

• The extent of flocculation of globules depends on (a) globule size distribution. (b) charge on the globule surface. (c) viscosity of the external medium. (a) Globule size distribution • Uniform sized globules prevent flocculation. • This can be achieved by proper size reduction process. (b) Charge on the globule surface • A charge on the globules exert repulsive forces with the neighboring globules. • This can be achieved by using ionic emulsifying agent, electrolytes etc. 28

(c) Viscosity of the external medium. • If the viscosity of the external medium is increased, the globules become relatively immobile and flocculation can be prevented. • This can be obtained by adding viscosity improving agents (thickening agents) such as hydrocolloids or waxes. • Floccules slowly move either upward or downward leading to creaming. • Flocculation is due to the interaction of attractive and repulsive forces, whereas creaming is due to density differences in the two phases. 29

Creaming • Creaming is the concentration of globules at the top or bottom of the emulsion. • Droplets larger than 1 mm may settle preferentially to the top or the bottom under gravitational forces. • Creaming may also be observed on account of the difference of individual globules (movement rather than flocs). • It can be observed by a difference in color shade of the layers. 30

• It is a reversible process, i. e. , cream can be redispersed easily by agitation, this is possible because the oil globules are still surrounded by the protective sheath of the emulsifier. • Creaming results in a lack of uniformity of drug distribution. This leads to variable dosage. Therefore, the emulsion should be shaken thoroughly before use. • Creaming is of two types, upward creaming and downward creaming 31

• Upward creaming, is due to the dispersed phase is less dense than the continuous phase. This is normally observed in o/w emulsions. The velocity of sedimentation becomes negative. • Downward creaming occurs if the dispersed phase is heavier than the continuous phase. Due to gravitational pull, the globules settle down. This is normally observed in w/o emulsions. • Since creaming involves the movement of globules in an emulsion, Stokes’ law can be applied. ν = d 2 (ρs – ρ0) 18 η 0 ν = terminal velocity in cm/sec, d is the diameter of the particle in cm, ρs and ρ0 are the densities of the dispersed phase and dispersion medium respectively, g is the acceleration due to gravity and η 0 is the viscosity of the dispersion medium in poise. 32

• Creaming is influenced by, – Globule size – Viscosity of the dispersion medium – Difference in the densities of dispersed phase and dispersion medium. Creaming can be reduced or prevented by: 1. Reducing the particle size by homogenization. Doubling the diameter of oil globules increases the creaming rate by a factor of four. 2. Increasing the viscosity of the external phase by adding the thickening agents such as methyl cellulose tragacanth or sodium alginate. 3. Reducing the difference in the densities between the dispersed phase and dispersion medium. 4. Adjusting the continuous phase and dispersed phase densities to the same value should eliminate the tendency to cream. 5. To make densities equal, oil soluble substances such as bromoform, β-bromo naphthalene are added to the oil phase (rarely used technique). 33

COALESCENCE • If the sizes of globules are not uniform, globules of smaller size occupy the spaces between the larger globules. A few globules tend to fuse with each other and form bigger globules. • This type of closed packing induces greater cohesion which leads to coalescence. • In this process, the emulsifier film around the globules is DESTROYED to a certain extent. This step can be recognized by increased globule size and reduced number of globules. 34

Coalescence is observed due to: Ø Insufficient amount of the emulsifying agent. Ø Altered partitioning of the emulsifying agent. Ø Incompatibilities between emulsifying agents. • Phase volume ratio of an emulsion has a secondary influence on the stability of the product and represents the relative volume of water to oil in emulsion. • At higher ratio (>74% of oil to water), globules are closely packed, wherein small globules occupy the void spaces between bigger globules. • Thus globules get compressed and become irregular in shape, which leads to fusion of adjacent globules. • Ostwald and others have shown that if one attempts to incorporate more than about 74% of oil in an o/w emulsion, the oil globules often coalesce and the emulsion breaks. • This value known as the critical point, is defined as the concentration of the dispersed phase above which the emulsifying agent cannot produce a stable emulsion of the desired type. 35

BREAKING • Separation of the internal phase from the external phase is called breaking of the emulsion. • This is indicated by complete separation of oil and aqueous phases, is an irreversible process, i. e. , simple mixing fails. It is to resuspend the globules into an uniform emulsion. • In breaking, the protective sheath around the globules is completely destroyed and oil tends to coalesce. 36

PHASE INVERSION • This involves the change of emulsion type from o/w to w/o or vice versa. • When we intend to prepare one type of emulsion say o/w, and if the final emulsion turns out to be w/o, it can be termed as a sign of instability. 37

FORMULATION OF EMULSION � Aqueous phase: purified water � Organic phase: arachis oil, cod liver oil, sesame oil, castor oil � Emulsifier: acacia, SLS, tween, bentonite � Antioxidant: sodium bisulphite, sodium nitrite � Preservatives: methyl and propyl paraben 38

Emulsifying agents � Emulsifier or surface active agent (SAA) is molecule which has two parts, one is hydrophilic and the other is hydrophobic. Upon the addition of SAA, it tends to form monolayer film at the oil/water interface. Mechanism of action of emulsifying agents: � When two immiscible liquids are agitated together so that one of the liquids is dispersed as small droplets in the other. � To prevent coalescence between globules, it is necessary to use emulsifying agent. 39

CLASSIFICATION OF EMULSIFYING AGENTS Natural emulsifying agents from vegetable sources Natural emulsifying agents from animal sources Synthetic emulsifying agents Finally divided emulsifying agents Auxiliary divided emulsifying agents. Saponins Alcohols 40

Properties of an ideal emulsifying agent � Reduce the interfacial tension between the two immiscible liquids. � Physically and chemically stable, inert and compatible with the other ingredients of the formulation. � Completely non irritant and non toxic in the concentrations used. � Organoleptically inert i. e. should not impart any colour, odour or taste to the preparation. � Form a coherent film around the globules of the dispersed phase and should prevent the coalescence of the droplets of the dispersed phase. � Produce and maintain the required viscosity of the preparation. 41

A) NATURAL EMULSIFYING AGENTS FROM VEGETABLE SOURCES � Obtained from vegetable source � Anionic in nature � Produce O/W emulsions � Capable of emulsifying a large number of substances Example: Ø Acacia Ø Tragacanth Ø Agar Ø Chondrus Ø Pectin Ø starch 42

ACACIA � Emulsions formed are O/W. � Stable over wide range of PH. � Soluble in water � Low viscosity that’s why creaming takes place � Should be preserved properly 43

TRAGACANTH � It alone is rarely used as an emulsifying agent because it doesn't reduce interfacial tension. � Produces very coarse and thick emulsion with increase viscosity. � Stable emulsion is produce when use along with acacia. 44

AGAR � Good emulsifying agents because they forms a very coarse and viscous emulsion � Used as thickening agent along with acacia. 45

CHONDRUS � Like agar it is not used as primary emulsifier. � It is used as a thickening agent. � Used along with acacia for the emulsification of cod-liver oil. 46

PECTIN � It is a purified complex carbohydrate obtain from rind of citrus fruit. � It act as a emulsion stabilizer in acacia emulsions. � Mucilage of pectin is prepared before adding it to emulsion. � To prevent lump formation pectin is triturated with small amount of alcohol or glycerol. 47

B) NATURAL EMULSIFYING AGENTS FROM ANIMAL SOURCES � Obtained from animals body. Examples : � Gelatin, � Egg yolk, � Wool fat. 48

GELATIN � Mainly used for the emulsification of liquid paraffin. � Protein in nature � It possesses isoelectronic point. � Prone to bacterial growth � Suitable preservative should be added. 49

EGG YOLK � It itself is an emulsion because of the presence of lecithin and cholesterol � Rarely used in industrial preparations because it is spoiled during transportation. � Used in extemporaneous preparations. 50

WOOL FAT � Also known as Anhydrous Lanolin � It is generally used in emulsion meant for external application. � It produces water in oil emulsions � It can absorb 50% of water 51

C) SEMI SYNTHETIC POLYSACCHARIDES � Methyl cellulose � Sodium carboxy methyl cellulose 52

METHYL CELLULOSE � Synthetic derivative of cellulose � Used as suspending, thickening and emulsifying agent. � Commonly used for emulsification of mineral and vegetable oil. � Soluble in hot water therefore mucilage can be prepared easily. � Emulsions prepared are very stable. 53

SODIUM CARBOXY METHYL CELLULOSE � Not a true emulsifier � Used as an emulsion stabilizer. � Soluble in cold as well as hot water. 54

D) SYNTHETIC EMULSIFYING AGENTS � Group includes surface active agents used as emulsifying agents. � Classified by the ionic charges they posses. � Anionic, Cationic, Non-ionic 55

E)INORGANIC EMULSIFYING AGENTS � Inorganic substances like milk of magnesia, magnesium oxide are used in pharmaceutical emulsions. � Produce O/W emulsion 56

F) ALCOHOLS �Examples are cholesterol, carbowaxes, lecithin 57

LACITHIN � Phospholipids in nature. � Obtained from nerve cells and Soya beans. � Show surface activity. � Highly stable and used for making W/O emulsions. � Chemicals used to overcome bad smell. 58

CHOLESTEROL � Used to prepare W/O emulsions � Ability to absorb water is low. 59

G) SAPONINS � Rarely used as emulsifying agents. � Quillaia tincture and liquid extracts may be used. 60

FINELY DIVIDED EMULSIFYING AGENTS � Having suitable hydrophilic and lipophilic properties. � If wetted with oil W/O emulsions are formed. � If wetted with water O/W emulsions are formed. � Clay, Pt, Ag in finally divided form is used as emulsifying agent. 61

AUXILLARY EMULSIFYING AGENTS � Normally incapable of forming stable emulsion but main function is thickening agent. � Agar and stearic acid. 62

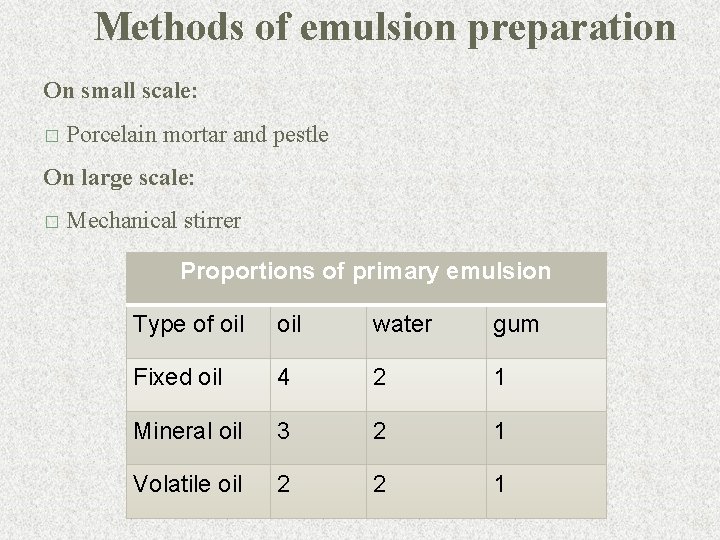
Methods of emulsion preparation On small scale: � Porcelain mortar and pestle On large scale: � Mechanical stirrer Proportions of primary emulsion Type of oil water gum Fixed oil 4 2 1 Mineral oil 3 2 1 Volatile oil 2 2 1 63

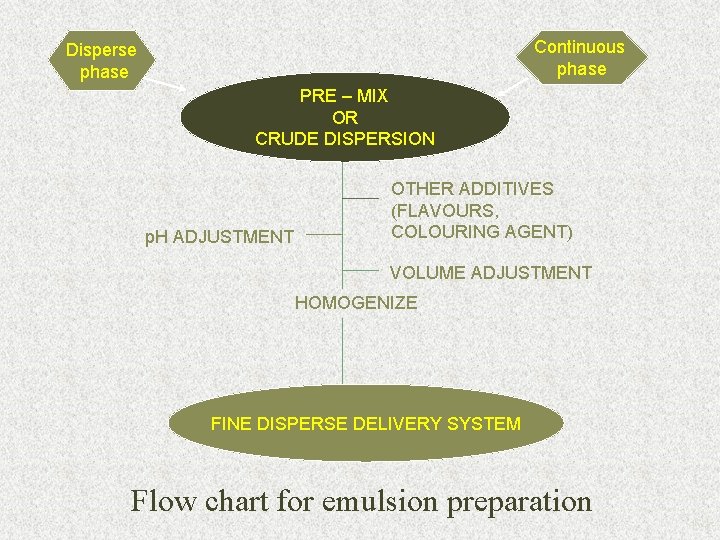
Continuous phase Disperse phase PRE – MIX OR CRUDE DISPERSION p. H ADJUSTMENT OTHER ADDITIVES (FLAVOURS, COLOURING AGENT) VOLUME ADJUSTMENT HOMOGENIZE FINE DISPERSE DELIVERY SYSTEM Flow chart for emulsion preparation 64

Methods of emulsion preparation: Continental or dry gum method: Emulsifier is triturated with the oil in perfectly dry porcelain mortar water is added at once triturate immediately, rapidly and continuously (until get a clicking sound and thick white cream is formed, this is primary emulsion) the remaining quantity of water is slowly added to form the final emulsion 65

Example for dry gum method Ingredients: Cod liver oil 50 ml Acacia 12. 5 gm Syrup 10 ml Flavor oil 0. 4 ml Purified oil up to 100 ml Method: • Accurately weigh or measure each ingredient. Place cod liver oil in dry mortar. Add acacia and give it a very quick mix. Add 25 ml of water and immediately triturate to form thick white , homogenous primary emulsion. Add flavor and mix. Add syrup and mix. Add sufficient water to total 100 ml. 66

English or Wet Gum Method triturate gum with water in a mortar to form a mucilage oil is added slowly in portions the mixture is triturated after adding all of the oil, thoroughly mixed for several minute to form the primary emulsion Once the primary emulsion has been formed remaining quantity of water is added to make the final emulsion. 67

Bottle or Forbes Bottle Method - It is extemporaneous preparation for volatile oils or oil with low viscosity. gum + oil (dry bottle) Shake water (volume equal to oil) is added in portions with vigorous shaking to form primary emulsion remaining quantity of water is added to make the final emulsion 68

Auxiliary method � An emulsion prepared by other methods can also be improved by passing it through a hand homogenizer, which forces the emulsion through a very small orifice, reducing the dispersed droplet size to about 5 microns or less. 69

In situ soap method Calcium Soaps : � w/o emulsions contain oils such as oleic acid , in combination with lime water ( calcium hydroxide solution, USP). � Prepared by mixing equal volumes of oil and lime water. 70

In situ soap method – Example Nascent soap � Oil Phase : Olive oil / oleic acid ; olive oil may be replaces by other oils but oleic acid must be added. � Lime water : Ca(OH)2 should be freshly prepared. � The emulsion formed is w/o � Method of preparation : Bottle method � Mortar method : When the formulation contains solid insoluble such as zinc oxide and calamine. 71

Preparation of emulsions- large scale Mechanical equipment for emulsification (Agitation) � Mechanical stirrers -Propeller type mixers -Turbine mixers � Homogenizers � Colloid mills � Ultrasonifiers 72

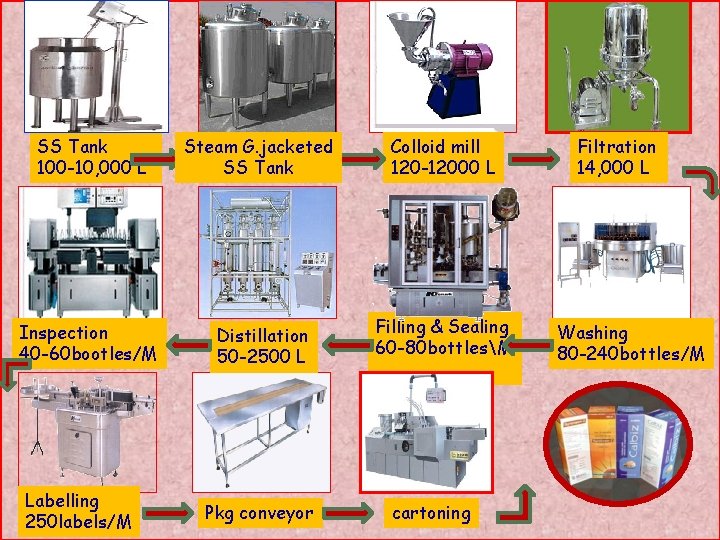
Flow of material: SS Tank 100 -10, 000 L Steam G. jacketed SS Tank Inspection 40 -60 bootles/M Distillation 50 -2500 L Labelling 250 labels/M Colloid mill 120 -12000 L Filling & Sealing 60 -80 bottlesM Paper: -910102 Pkg conveyor cartoning Jignasha R. Bhuria(2009 -2010) Filtration 14, 000 L Washing 80 -240 bottles/M 73 73

Mixing & Storage Tanks üManufactured from SS & is Argon welded. üThe tank will be mounted on 4 legs with castors for movements. üAvailable from 50 -10, 000 liters. 74

SS Tank with Stirrer ü With SS steam jacketed & insulation with SS cladding. ü Different type of stirrer (paddle/ anchor/propeller) available. ü Electric heating also possible for small scale. ü Capacity: -100 ltres to 10, 000 ltrs 75

Jacketed kettle / SS Tank (steam, gas or electrically heated) 76

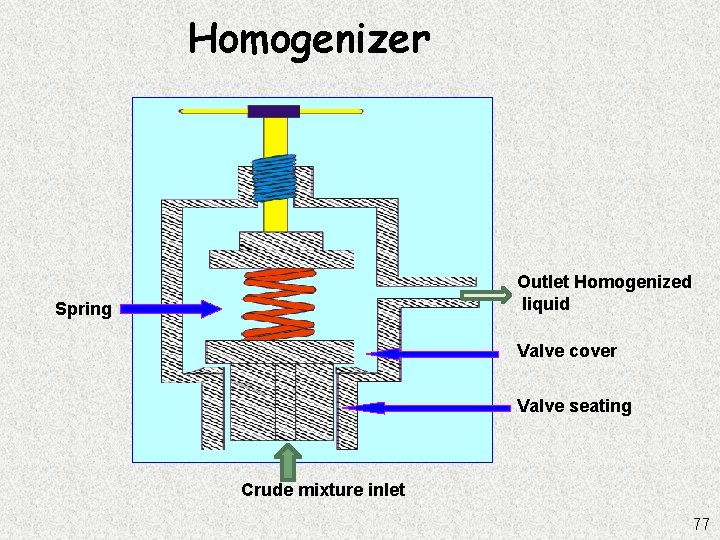
Homogenizer Outlet Homogenized liquid Spring Valve cover Valve seating Crude mixture inlet 77

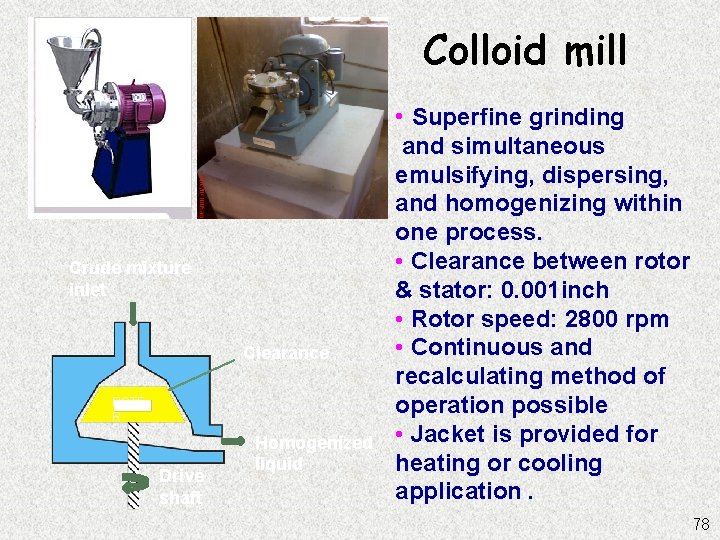
Colloid mill • Superfine grinding Crude mixture inlet Clearance ROTE R Drive shaft Homogenized liquid and simultaneous emulsifying, dispersing, and homogenizing within one process. • Clearance between rotor & stator: 0. 001 inch • Rotor speed: 2800 rpm • Continuous and recalculating method of operation possible • Jacket is provided for heating or cooling application. 78

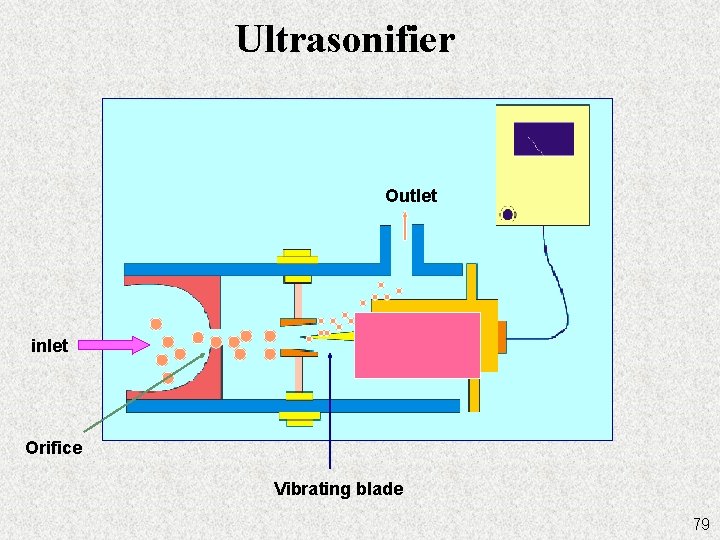
Ultrasonifier Outlet inlet Orifice Vibrating blade 79

Bottle washing machine 80

Bottle filling machine 81

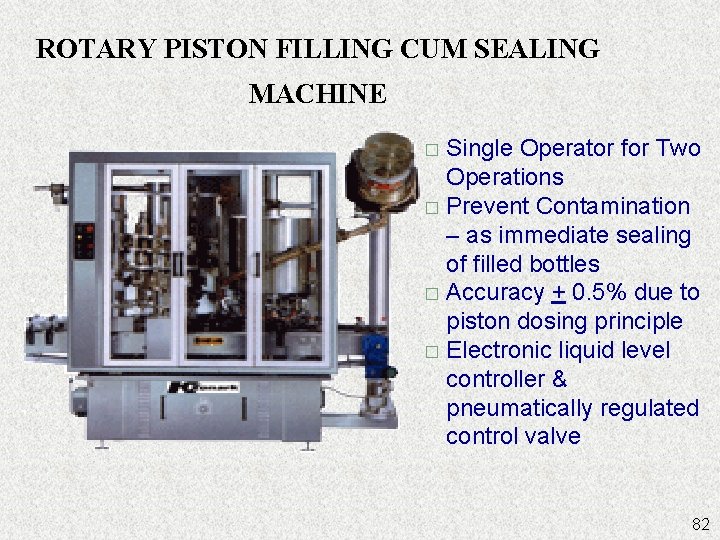
ROTARY PISTON FILLING CUM SEALING MACHINE Single Operator for Two Operations � Prevent Contamination – as immediate sealing of filled bottles � Accuracy + 0. 5% due to piston dosing principle � Electronic liquid level controller & pneumatically regulated control valve � 82

Bottle Inspection Machine 83

Automatic High Speed Labeling Machine Application - Patch Body Shape of Container: Flat/Elliptical/Oval/Square Size of label: 15 -130 mm Output: 50 -120 containers / minute 84

Packing conveyor belt 85

Bottle trimming & cartooning machine 86

Packing, Labelling And Storage Depending on the use, emulsions should be packed in suitable containers. � Emulsions meant for oral use are usually packed in well filled bottles having an air tight closure. � Light sensitive products are packed in amber coloured bottles. � For viscous emulsions, wide mouth bottles should be used. � The label on the emulsion should mention that these products have to be shaken thoroughly before use. � External use products should clearly mention on their label that they are meant for external use only. � Emulsions should be stored in a cool place but refrigeration should be avoided as this low temperature can adversely effect the stability of preparation. � 87

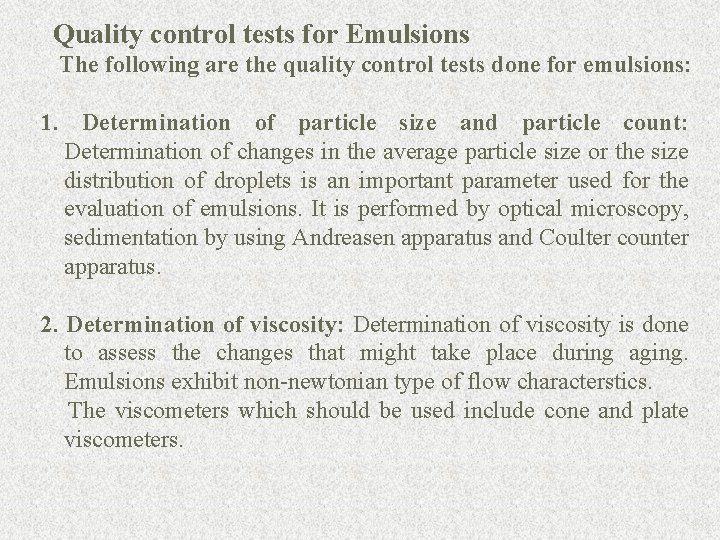
Quality control tests for Emulsions The following are the quality control tests done for emulsions: 1. Determination of particle size and particle count: Determination of changes in the average particle size or the size distribution of droplets is an important parameter used for the evaluation of emulsions. It is performed by optical microscopy, sedimentation by using Andreasen apparatus and Coulter counter apparatus. 2. Determination of viscosity: Determination of viscosity is done to assess the changes that might take place during aging. Emulsions exhibit non-newtonian type of flow characterstics. The viscometers which should be used include cone and plate viscometers. 88

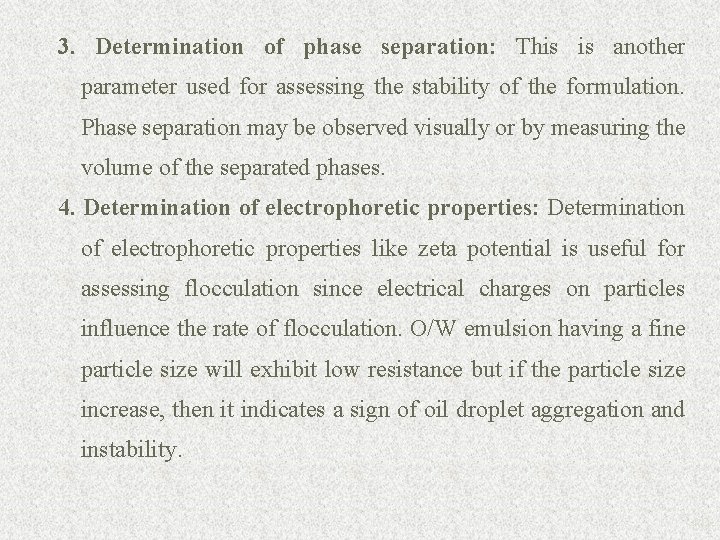
3. Determination of phase separation: This is another parameter used for assessing the stability of the formulation. Phase separation may be observed visually or by measuring the volume of the separated phases. 4. Determination of electrophoretic properties: Determination of electrophoretic properties like zeta potential is useful for assessing flocculation since electrical charges on particles influence the rate of flocculation. O/W emulsion having a fine particle size will exhibit low resistance but if the particle size increase, then it indicates a sign of oil droplet aggregation and instability. 89

PRODUCT VALIDATION v § § § § § Major test parameters used for final product testing Appearance p. H Viscosity Specific gravity Microbial count Leakage test for filled bottle Check the cap sealing Fill volume determination Particulate matter testing Stress test 90

REFERENCE • Theory & Practice of Industrial pharmacy by Leon Lachman. • CVS Subrahmanyam , Textbook of Physical Pharmaceutics, edition 2007, Vallabh Prakashan, PP-395 -426 • Aulton, Michael E. , ed (2007). Aulton's Pharmaceutics: The Design and Manufacture of Medicines (3 rd ed. ). Churchill Livingstone. • Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems, 9 th edition, pg no. 376 • Validation of Disperse System: Pharmaceutical Dosage Form: Disperse Systems: Volume 3 (Part B). 91

92