Chapter 11 Haloalkanes Alkenes and Alkynes Alkenes and

















- Slides: 23

Chapter 11 Haloalkanes, Alkenes, and Alkynes Alkenes and Alkynes Geometric Isomers of Alkenes Addition Reactions Robert J. O'Reilly 1

Saturated and Unsaturated Compounds l Saturated compounds (alkanes) have the maximum number of hydrogen atoms attached to each carbon atom l Unsaturated compounds have fewer hydrogen atoms attached to the carbon chain than alkanes l Unsaturated compounds contain double or triple bonds Robert J. O'Reilly 2

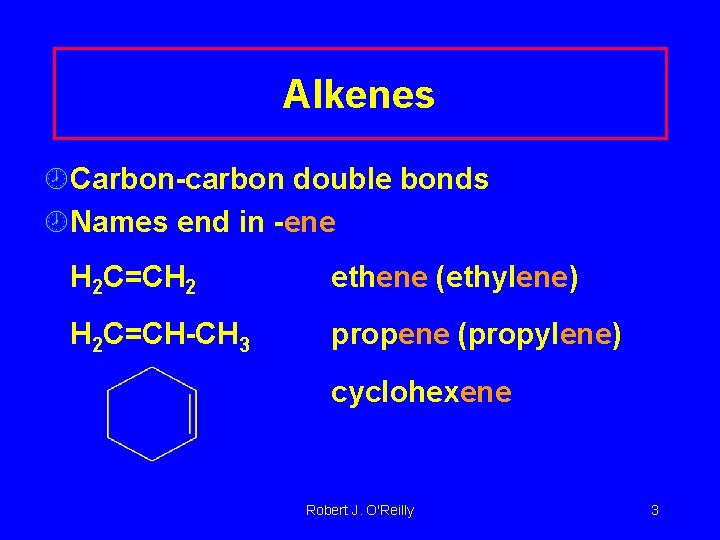
Alkenes ¾Carbon-carbon double bonds ¾Names end in -ene H 2 C=CH 2 ethene (ethylene) H 2 C=CH-CH 3 propene (propylene) cyclohexene Robert J. O'Reilly 3

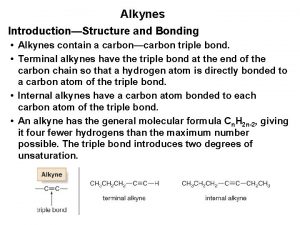
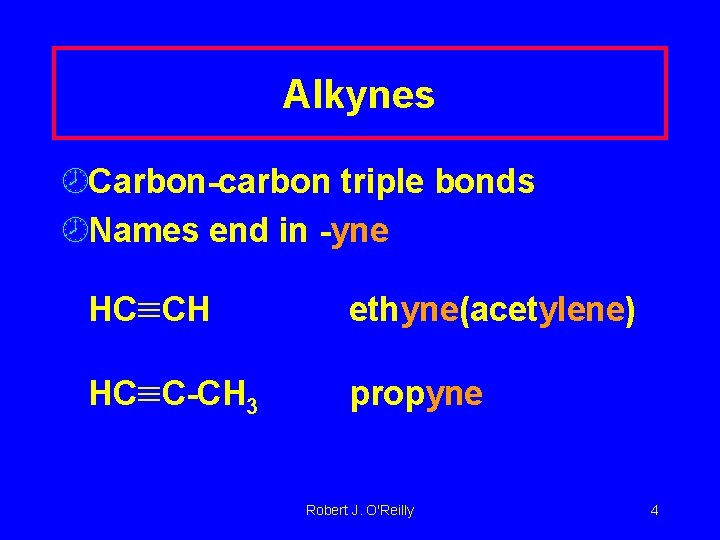
Alkynes ¾Carbon-carbon triple bonds ¾Names end in -yne HC CH ethyne(acetylene) HC C-CH 3 propyne Robert J. O'Reilly 4

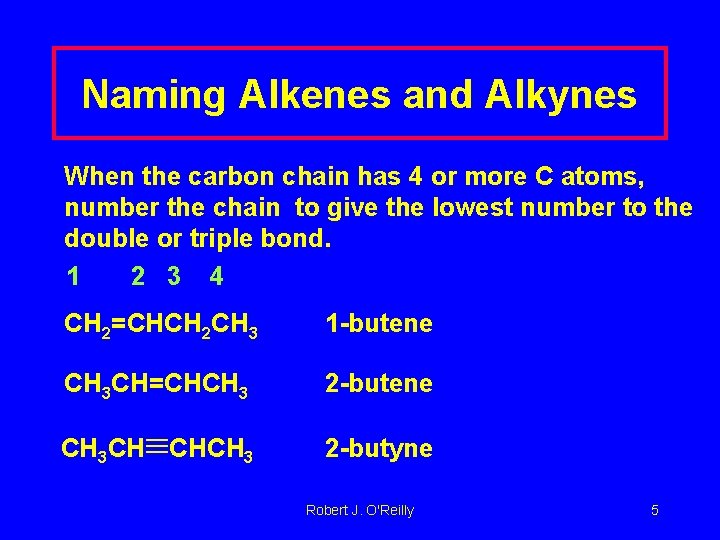
Naming Alkenes and Alkynes When the carbon chain has 4 or more C atoms, number the chain to give the lowest number to the double or triple bond. 1 2 3 4 CH 2=CHCH 2 CH 3 1 -butene CH 3 CH=CHCH 3 2 -butene 2 -butyne CH 3 CH CHCH 3 Robert J. O'Reilly 5

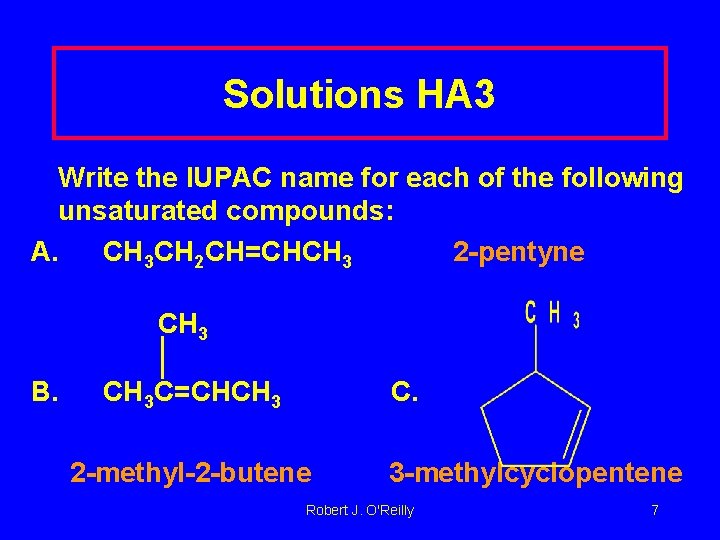
Learning Check HA 3 Write the IUPAC name for each of the following unsaturated compounds: A. CH 3 CH 2 C CCH 3 B. CH 3 C=CHCH 3 C. Robert J. O'Reilly 6

Solutions HA 3 Write the IUPAC name for each of the following unsaturated compounds: A. CH 3 CH 2 CH=CHCH 3 2 -pentyne CH 3 B. CH 3 C=CHCH 3 C. 2 -methyl-2 -butene 3 -methylcyclopentene Robert J. O'Reilly 7

Cis and Trans Isomers ¾Double bond is fixed ¾Cis/trans Isomers are possible CH 3 CH = CH cis trans Robert J. O'Reilly CH 3 8

Hydrogenation l Adds a hydrogen atom to each carbon atom of a double bond H H H–C=C–H + H 2 H H Ni H–C–C–H H H ethene Robert J. O'Reilly ethane 9

Products of Hydrogenation Adding H 2 to vegetable oils produces compounds with higher melting points l Margarines l Soft margarines l Shortenings (solid) Robert J. O'Reilly 10

Learning Check HA 4 What is the product of adding H 2 (Ni catalyst) to 1 -butene? Robert J. O'Reilly 11

Solution HA 4 What is the product of adding H 2 (Ni catalyst) to 1 -butene? Ni CH 2=CHCH 2 CH 3 + H 2 CH 3 CH 2 CH 3 Robert J. O'Reilly 12

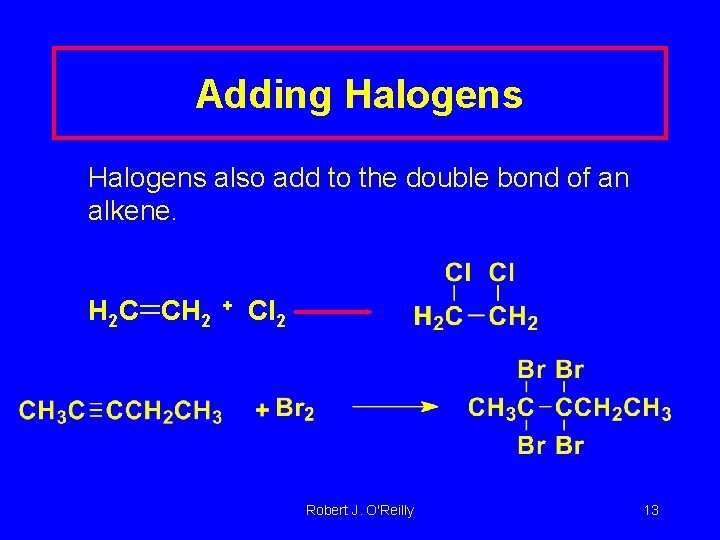
Adding Halogens also add to the double bond of an alkene. H 2 C CH 2 + Cl 2 Robert J. O'Reilly 13

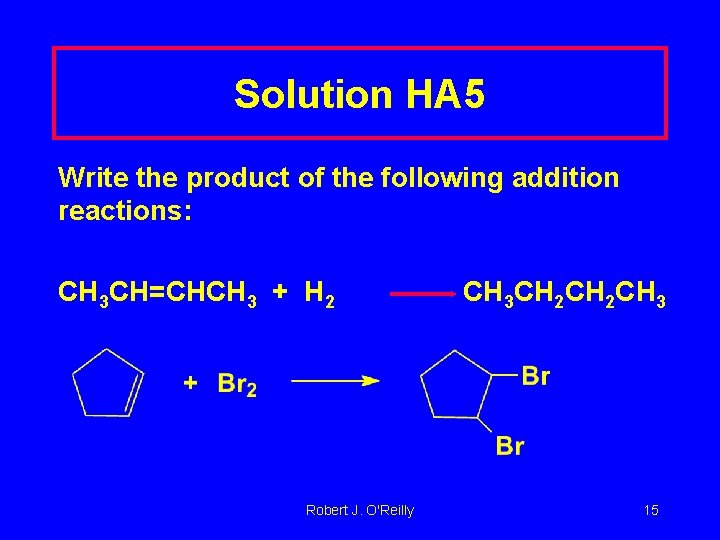
Learning Check HA 5 Write the product of the following addition reactions: CH 3 CH=CHCH 3 + H 2 Robert J. O'Reilly 14

Solution HA 5 Write the product of the following addition reactions: CH 3 CH=CHCH 3 + H 2 Robert J. O'Reilly CH 3 CH 2 CH 3 15

Unsaturated Fatty Acids l Fatty acids in vegetable oils are omega-6 acids (the first double bond occurs at carbon 6 counting from the methyl group) l A common omega-6 acid is linoleic acid CH 3 CH 2 CH 2 CH=CHCH 2 CH=CH(CH 2)7 COOH 6 linoleic acid, a fatty acid Robert J. O'Reilly 16

Trans Fats 1. In vegetable oils, the unsaturated fats usually contain cis double bonds. 2. During hydrogenation, some cis double bonds are converted to trans double bonds (more stable) causing a change in the fatty acid structure 3. If a label states “partially” or “fully hydrogenated”, the fats contain trans fatty acids. Robert J. O'Reilly 17

Trans Fats u. In the US, it is estimated that 2 -4% of our total Calories is in the form of trans fatty acid. utrans fatty acids behave like saturated fatty acids in the body. u. Several studies reported that trans fatty acids raise LDL-cholesterol. Some studies also report that trans fatty acid lower HDL-cholesterol u. The trans fatty acids controversy will continue to be debated. Robert J. O'Reilly 18

Fats and Atheroschlerosis l Inuit people of Alaska have a high fat diet and high blood cholesterol levels, but a very low occurrence of atherosclerosis and heart attacks. l Fat in the Intuit diet was primarily from fish such as salmon, tuna and herring rather than from land animals (as in the American diet). Robert J. O'Reilly 19

Omega-3 Fatty Acids 1. Fatty acids in the fish oils are mostly the omega-3 type (first double bond occurs at the third carbon counting from the methyl group). 2. linolenic acid 18 carbon atoms CH 3 CH 2 CH=CHCH 2 CH=CH(CH 2)7 COOH 1. eicosapentaenoic acid (EPA) 20 carbon atoms CH 3 CH 2(CH=CHCH 2)5(CH 2)2 COOH Robert J. O'Reilly 20

Atherosclerosis l Plaques of cholesterol adhere to the walls of the blood vessels l Blood pressure rises as blood squeezes through smaller blood vessels l Blood clots may form l Omega-3 fatty acids decrease the “sticking” of blood platelets (fewer blood clots) l Omega-3 fatty acids can increase bleeding time Robert J. O'Reilly 21

Learning Check HA 6 (1) Ture or (2) False A. ____ There are more unsaturated fats in vegetable oils. B. ____ Vegetable oils have more omega-3 oils than found in fish. C. ____ Hydrogenation of oils converts some cis -double bonds to trans- double bonds. D. ____ Animal fats have more saturated fats. Robert J. O'Reilly 22

Solution HA 6 (1) True or (2) False A. _T__ There are more unsaturated fats in vegetable oils. B. _F__ Vegetable oils have more omega-3 oils than found in fish. C. _T__ Hydrogenation of oils converts some cis -double bonds to trans- double bonds. D. _T__ Animal fats have more saturated fats. Robert J. O'Reilly 23
 Alkanes alkenes alkynes
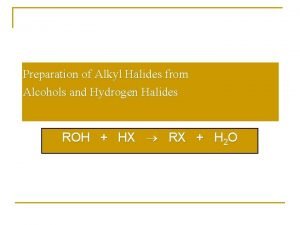
Alkanes alkenes alkynes Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Haloalkanes general formula
Haloalkanes general formula Functional group table
Functional group table C-x bond polarity
C-x bond polarity Leaving group order
Leaving group order Halogenation of alkynes
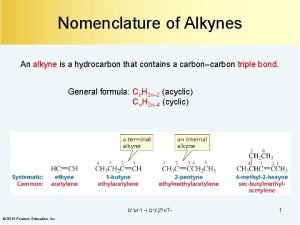
Halogenation of alkynes Naming alkynes
Naming alkynes Ozonolysis of alkenes mechanism
Ozonolysis of alkenes mechanism Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Alkenes general formula
Alkenes general formula Hybridization of alkynes
Hybridization of alkynes Alkynes structural formula
Alkynes structural formula Preparation of alkynes
Preparation of alkynes Alkynes
Alkynes Dihydroxylation
Dihydroxylation Hydroboration of alkynes
Hydroboration of alkynes First 10 members of alkynes
First 10 members of alkynes Benzalacetone
Benzalacetone Combustion reaction of alkanes
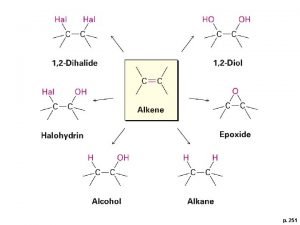
Combustion reaction of alkanes Alkenes reactions and synthesis
Alkenes reactions and synthesis Addition polymerisation animation
Addition polymerisation animation