The physical properties of alkane The physical properties

















- Slides: 36


The physical properties of alkane

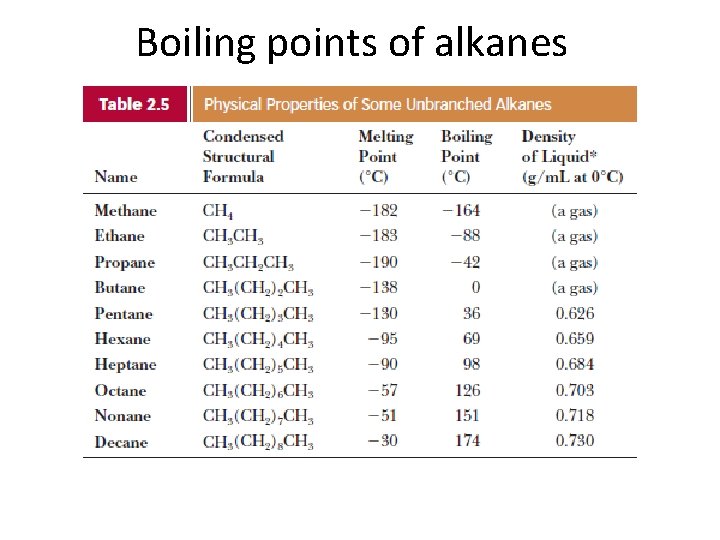
The physical properties of alkanes • Boiling points:

Boiling points of alkanes

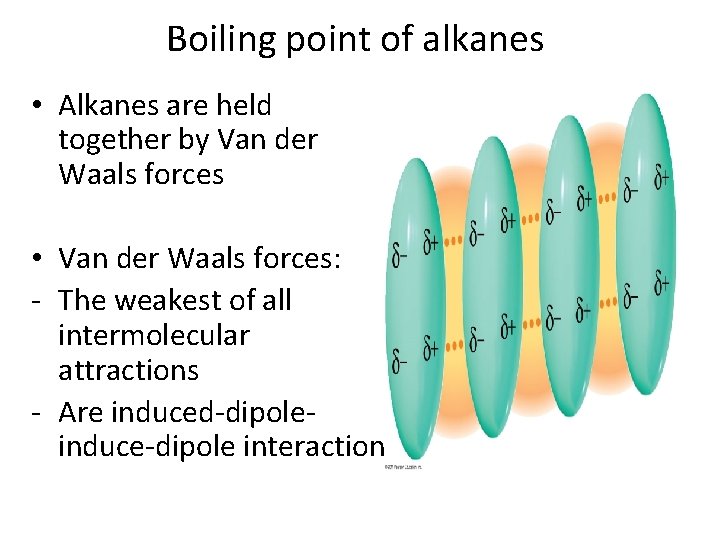
Boiling point of alkanes

Boiling point of alkanes • Alkanes are held together by Van der Waals forces • Van der Waals forces: - The weakest of all intermolecular attractions - Are induced-dipoleinduce-dipole interaction

Boiling point of alkanes • van der Waals forces depends on area of contacts between the molecules • Greater area of the contacts stronger van der Waals forces • Stronger van der Waals greater amount energy needed to break it higher boiling point

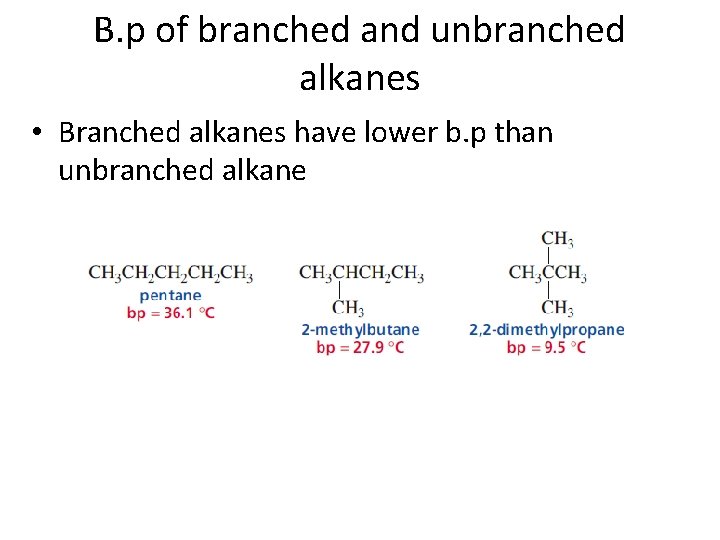
B. p of branched and unbranched alkanes • Branched alkanes have lower b. p than unbranched alkane

B. p of branched and unbranched alkanes • Branched alkane has lower area of contact compare to unbranched alkane • Branched alkane has more compact, spherical shape • Imagine: pentane is cigar and neopentane is tennis ball

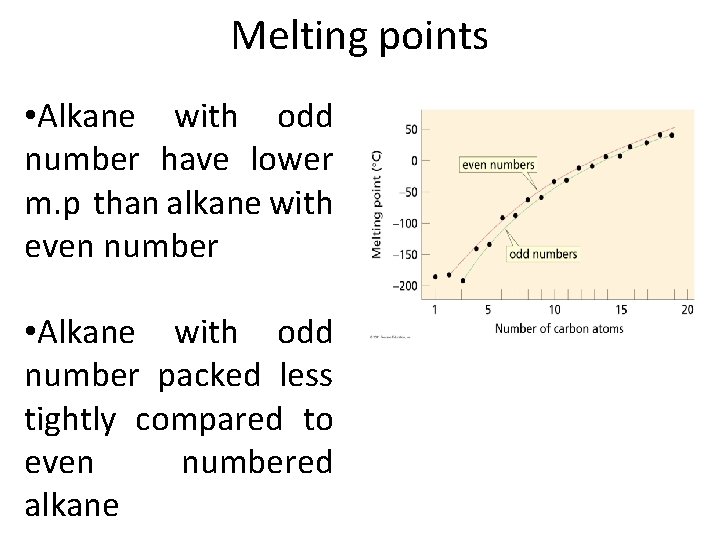
Melting points • Melting point (mp) of a compound is • Is influenced by packing (how well the individual molecules in a solid fit together in crystal lattice) • Greater packing Greater amount of energy needed to break the lattice and melt the compound

Melting points • Alkane with odd number have lower m. p than alkane with even number • Alkane with odd number packed less tightly compared to even numbered alkane

Solubility of alkane • Alkane are not soluble in water/ polar solvent • Alkane are soluble in organic solvent

Solubility of alkane • Solubility rule based on polarity of molecules: - Example: polar compound dissolve in polar solvent -Solvation: Interaction between the solvent and molecule/ ion dissolved in that solvent

Solubility of alkane

Solubility of alkane • Alkane is non-polar compound • No net charge – polar solvent not attracted to them

Solubility of alkane • When a molecular substance dissolves in water, you have to: • break the intermolecular forces within the substance. In the case of the alkanes, these are Van der Waals forces. • break the intermolecular forces in the water so that the substance can fit between the water molecules. In water the main intermolecular attractions are hydrogen bonds.

Solubility of alkane • Breaking any intermolecular forces cause energy • Making a new intermolecular forces release energy • Possible interaction between alkane and water is only van der Waals forces

Solubility of alkane • Amount of energy release if alkane make a new van der Waals bond between alkane and water is small • The small amount of energy released will not be enough to break the hydrogen bonds of water • Therefore alkane are not dissolved in water

Solubility of alkane • Breaking any intermolecular forces cause energy • Making a new intermolecular forces release energy • Possible interaction between alkane and water is only van der Waals forces

Exercise • Arrange the alkane in each set in order of decreasing boiling point (a) Butane, decane, hexane (b) 2 -methylheptane, octane, 2, 2, 4 trimethylpentane

Exercise • Explain why has a lower boiling point than CH 3 CH 2 CH 3.

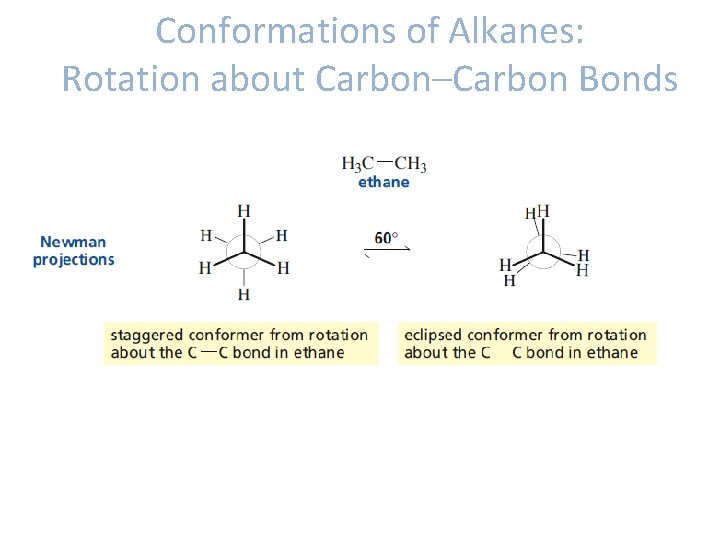
Conformations of Alkanes: Rotation about Carbon–Carbon Bonds • Conformations: different spatial arrangements of the atoms that result from rotation about a single bond. • Conformational isomers/conformers- molecules with different conformations.

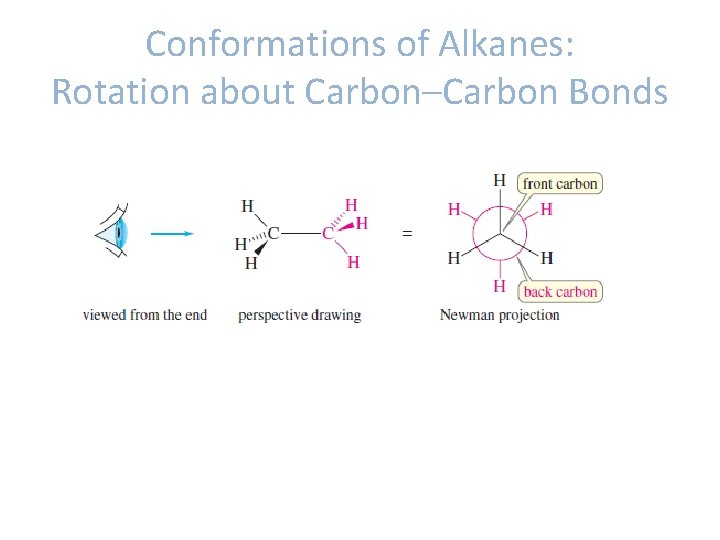
Conformations of Alkanes: Rotation about Carbon–Carbon Bonds • Ethane -The conformers produced by rotation about carbon-carbon bond of ethane: staggered conformer eclipsed conformer.

Conformations of Alkanes: Rotation about Carbon–Carbon Bonds

Conformations of Alkanes: Rotation about Carbon–Carbon Bonds

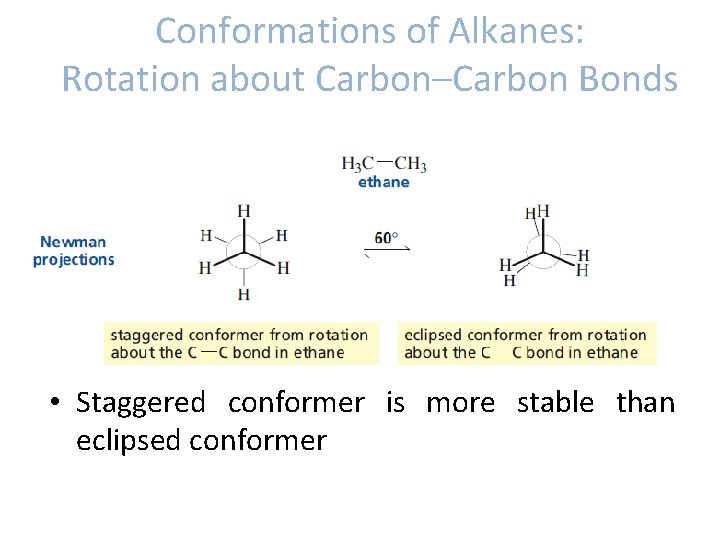
Conformations of Alkanes: Rotation about Carbon–Carbon Bonds • Staggered conformer is more stable than eclipsed conformer

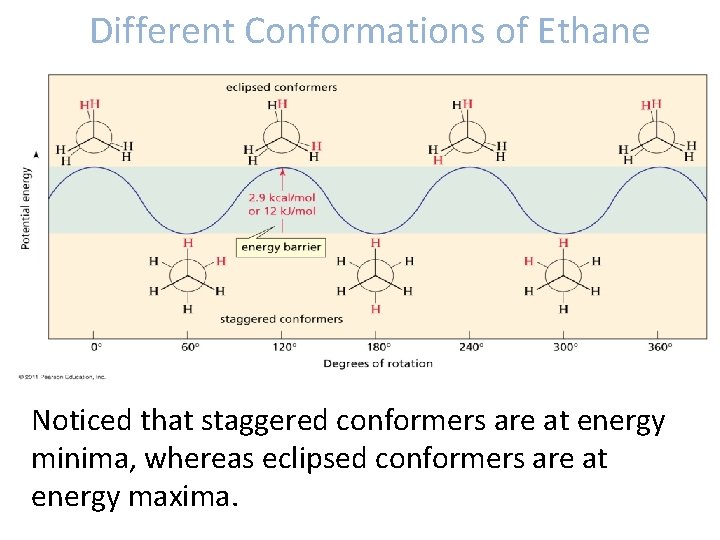
Different Conformations of Ethane Noticed that staggered conformers are at energy minima, whereas eclipsed conformers are at energy maxima.

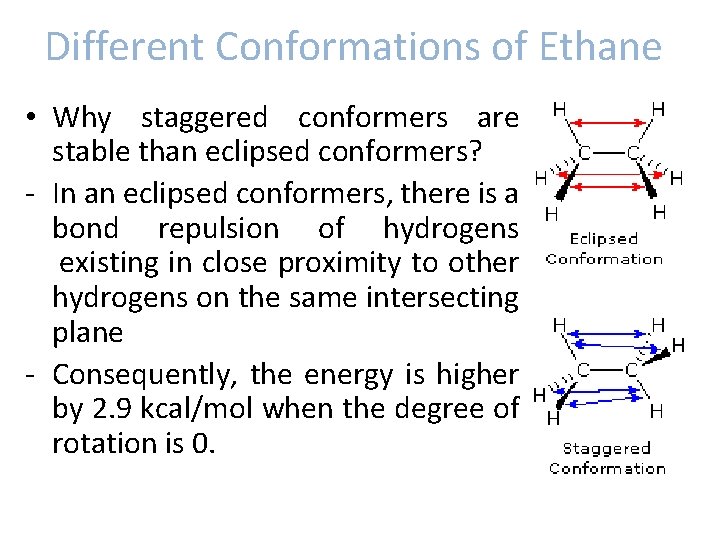
Different Conformations of Ethane • Why staggered conformers are stable than eclipsed conformers? - In an eclipsed conformers, there is a bond repulsion of hydrogens existing in close proximity to other hydrogens on the same intersecting plane - Consequently, the energy is higher by 2. 9 kcal/mol when the degree of rotation is 0.

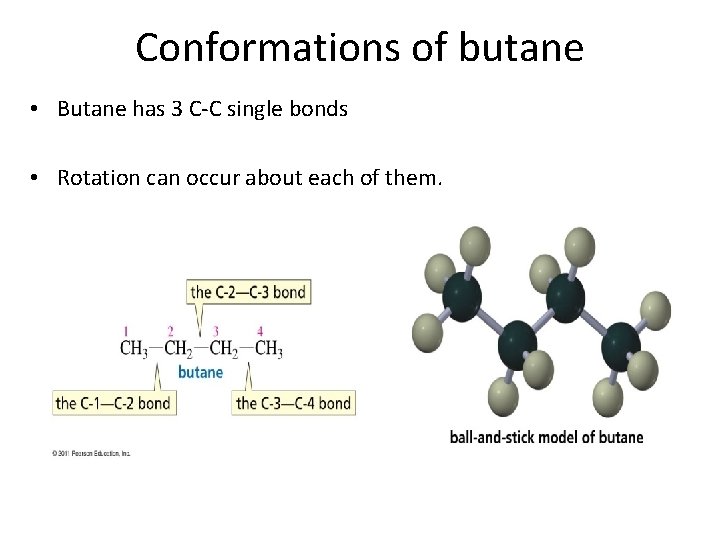
Conformations of butane • Butane has 3 C-C single bonds • Rotation can occur about each of them.

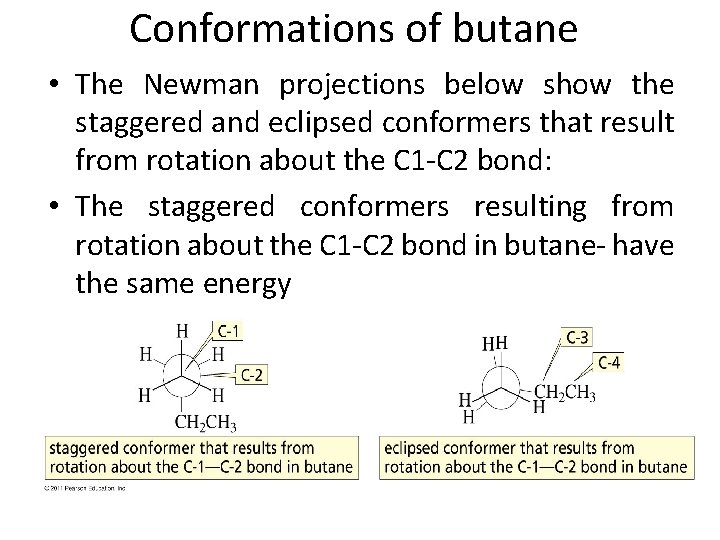
Conformations of butane • The Newman projections below show the staggered and eclipsed conformers that result from rotation about the C 1 -C 2 bond: • The staggered conformers resulting from rotation about the C 1 -C 2 bond in butane- have the same energy

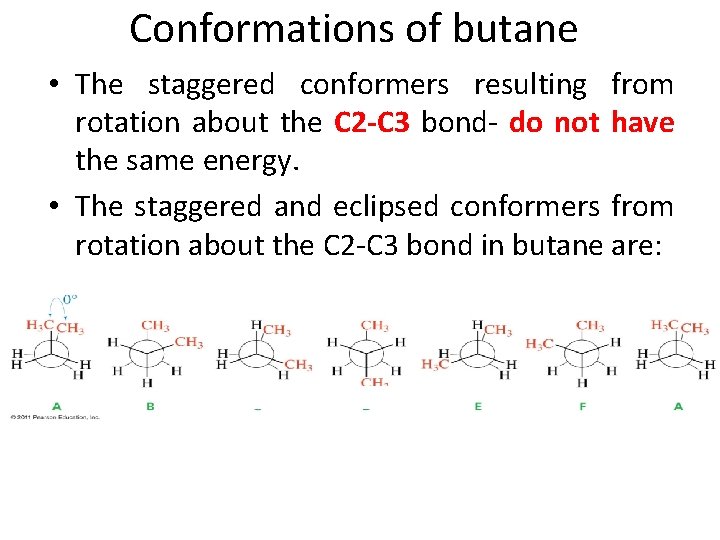
Conformations of butane • The staggered conformers resulting from rotation about the C 2 -C 3 bond- do not have the same energy. • The staggered and eclipsed conformers from rotation about the C 2 -C 3 bond in butane are:

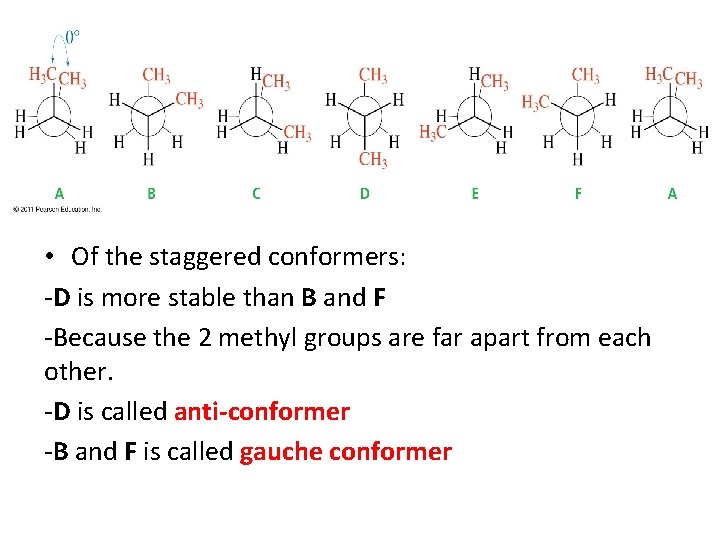
• Of the staggered conformers: -D is more stable than B and F -Because the 2 methyl groups are far apart from each other. -D is called anti-conformer -B and F is called gauche conformer


Conformations of butane • Anti and gauche conformers do not have the same energy Steric strain • Steric strain: strain experienced by a molecule when atoms or groups are close enough to one another for their electron clouds to repel each other. Thus possesses additional energy. • Steric strain in molecules increase as the size of the group increase

Conformations of butane • Why A has the highest potential energy compare to other conformers?

Exercise • Draw all the staggered and eclipsed conformers that result from the rotation about the C-2 and C-3 bond of pentane • Draw a potential energy diagram for a rotation of C-2 and C-3 bond of pentane through 360, starting with the least conformers
 Butyl isomers
Butyl isomers Alkane common name
Alkane common name Alkane functional group
Alkane functional group Alkane organic chemistry
Alkane organic chemistry Tollens reagent
Tollens reagent General molecular formula of alkene
General molecular formula of alkene Methane ethane propane butane pentane
Methane ethane propane butane pentane R-x functional group
R-x functional group Stability of chair and boat conformation
Stability of chair and boat conformation Homologe reihe der alkane
Homologe reihe der alkane Nomenclature
Nomenclature Alkane viscosity
Alkane viscosity Benzolring
Benzolring Alkane complete combustion
Alkane complete combustion Vereinfachte strukturformel alkane
Vereinfachte strukturformel alkane 2 3 dimethylpentane
2 3 dimethylpentane Is alkane an organic compound
Is alkane an organic compound Name
Name The polar group
The polar group Alkyne to alkene
Alkyne to alkene Hydrocarbons class 11
Hydrocarbons class 11 Meth eth prop
Meth eth prop Chemistry cracking
Chemistry cracking Methan ethan propan
Methan ethan propan Chemical property definition
Chemical property definition Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ