Chapter 4 Alkanes Alkenes and Alkynes Nomenclature Conformational






![Give the name of the following compounds: 2 -Clorobicyclo[1. 1. 0]butane bicyclo[3. 3. 2]decane Give the name of the following compounds: 2 -Clorobicyclo[1. 1. 0]butane bicyclo[3. 3. 2]decane](https://slidetodoc.com/presentation_image/e9ed4e859d4db498e686f9d4dfdc9d50/image-33.jpg)
![Write the structural formula of the following: 7, 7 -Dichlorobicyclo[2. 2. 1]heptane bicyclo[3. 2. Write the structural formula of the following: 7, 7 -Dichlorobicyclo[2. 2. 1]heptane bicyclo[3. 2.](https://slidetodoc.com/presentation_image/e9ed4e859d4db498e686f9d4dfdc9d50/image-34.jpg)













- Slides: 106

Chapter 4 Alkanes, Alkenes and Alkynes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis

The branch of chemistry that deals with carbon compounds is called organic chemistry Common Elements in Organic Compounds

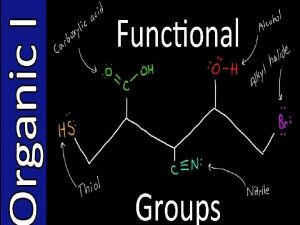
t Functional Groups èFunctional Group is a group of atoms that is largely responsible for the chemical behavior of the parent molecule. èFunctional group families are characterized by the presence of a certain arrangement of atoms called a functional group èA functional group is the site of most chemical reactivity of a molecule èThe functional group is responsible for many of the physical properties of a molecule

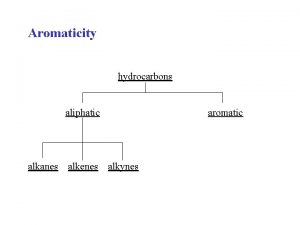
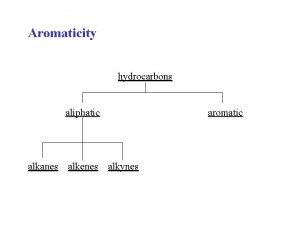
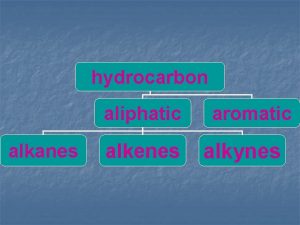
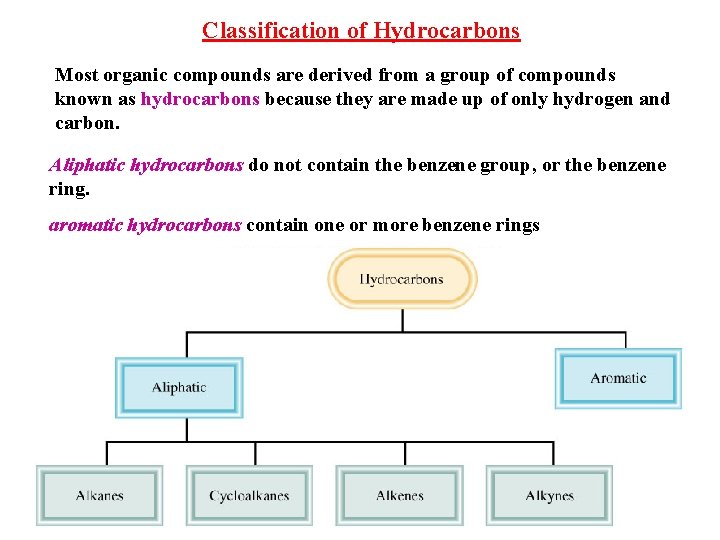
Classification of Hydrocarbons Most organic compounds are derived from a group of compounds known as hydrocarbons because they are made up of only hydrogen and carbon. Aliphatic hydrocarbons do not contain the benzene group, or the benzene ring. aromatic hydrocarbons contain one or more benzene rings

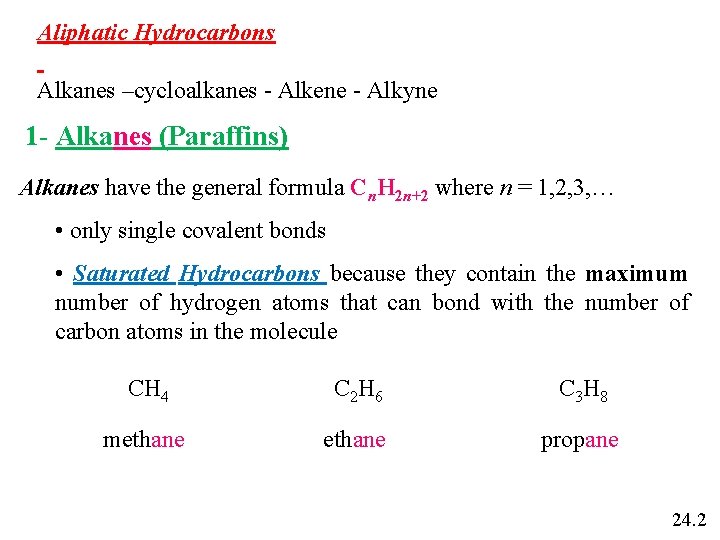
Aliphatic Hydrocarbons Alkanes –cycloalkanes - Alkene - Alkyne 1 - Alkanes (Paraffins) Alkanes have the general formula Cn. H 2 n+2 where n = 1, 2, 3, … • only single covalent bonds • Saturated Hydrocarbons because they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule CH 4 C 2 H 6 C 3 H 8 methane propane 24. 2

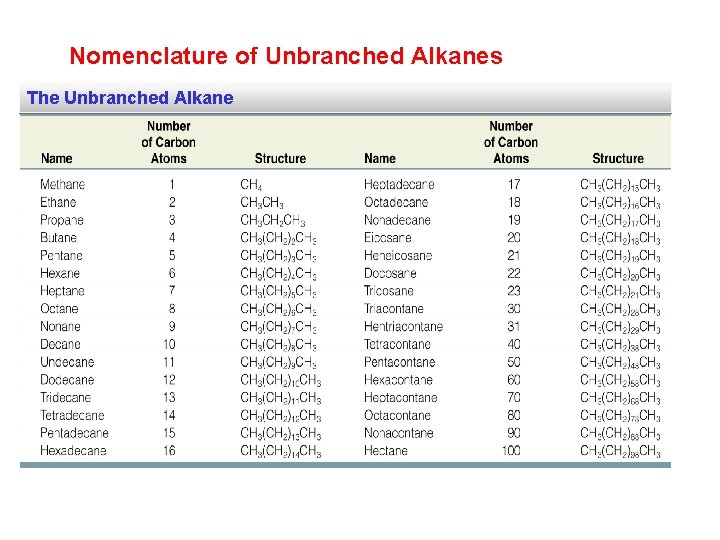
Nomenclature of Unbranched Alkanes The Unbranched Alkane

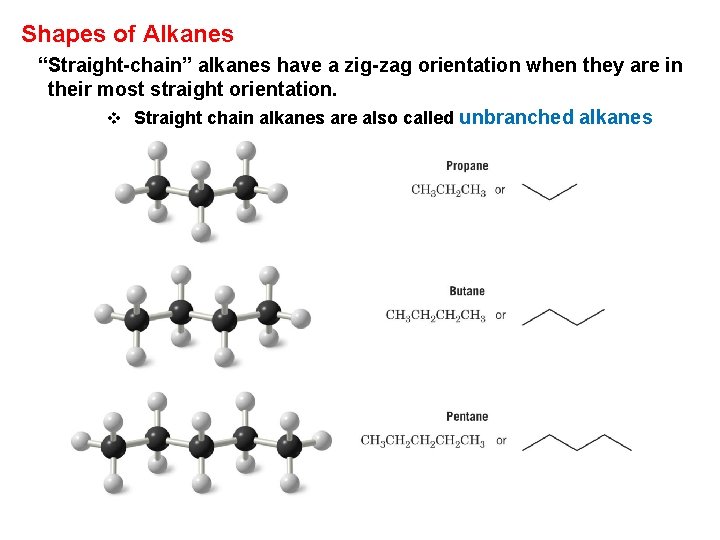
Shapes of Alkanes “Straight-chain” alkanes have a zig-zag orientation when they are in their most straight orientation. v Straight chain alkanes are also called unbranched alkanes

v Branched alkanes have at least one carbon which is attached to more than two other carbons

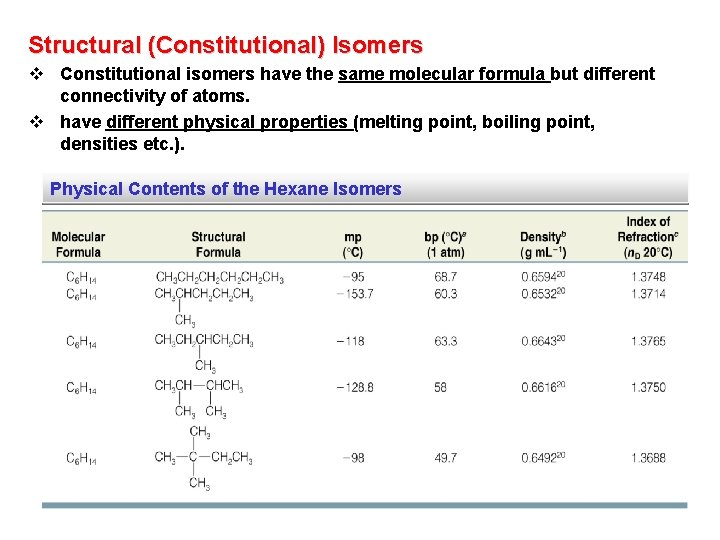
Structural (Constitutional) Isomers v Constitutional isomers have the same molecular formula but different connectivity of atoms. v have different physical properties (melting point, boiling point, densities etc. ). Physical Contents of the Hexane Isomers

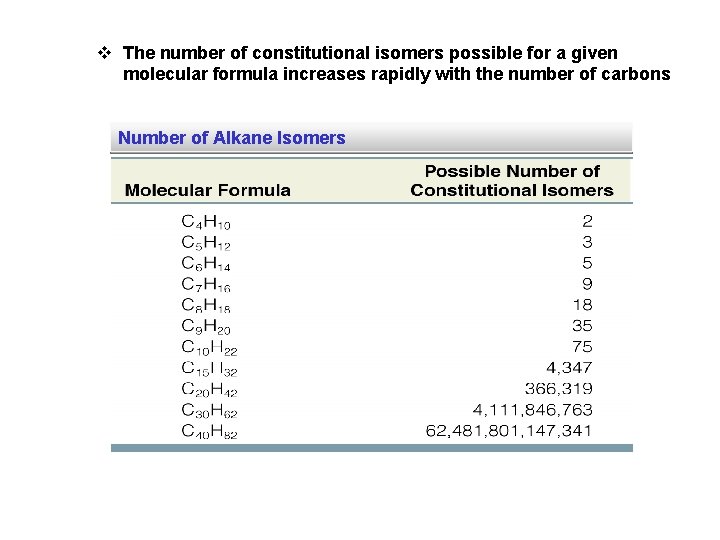
v The number of constitutional isomers possible for a given molecular formula increases rapidly with the number of carbons Number of Alkane Isomers

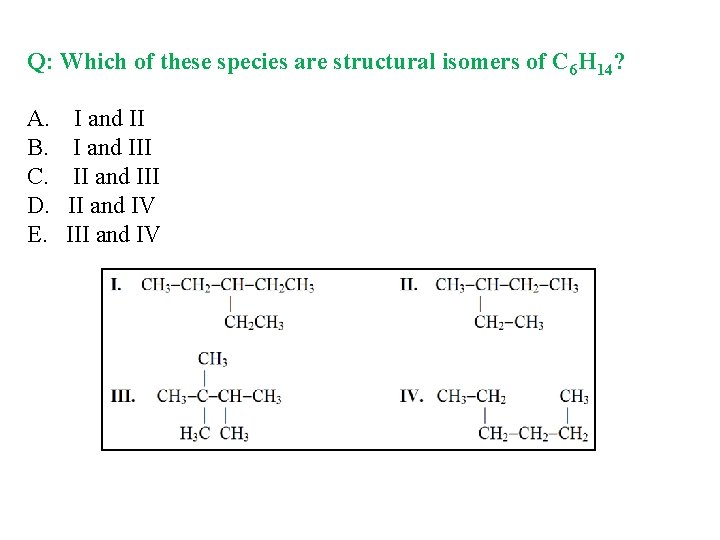
Q: Which of these species are structural isomers of C 6 H 14? A. I and II B. I and III C. II and III D. II and IV E. III and IV

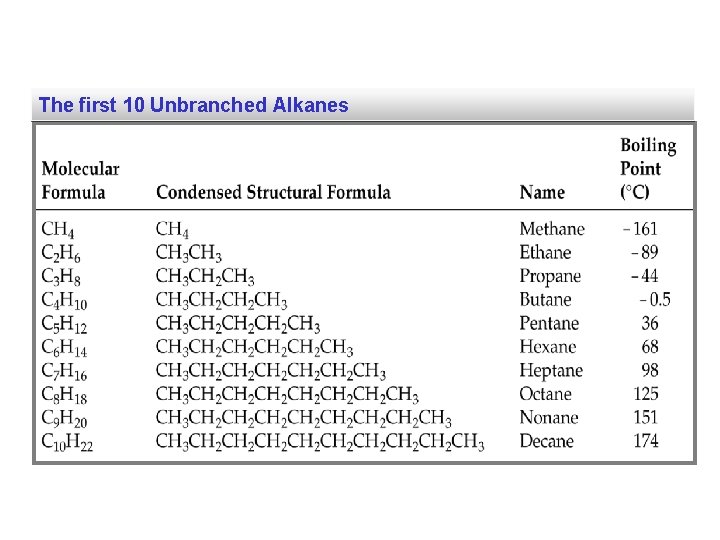
The first 10 Unbranched Alkanes

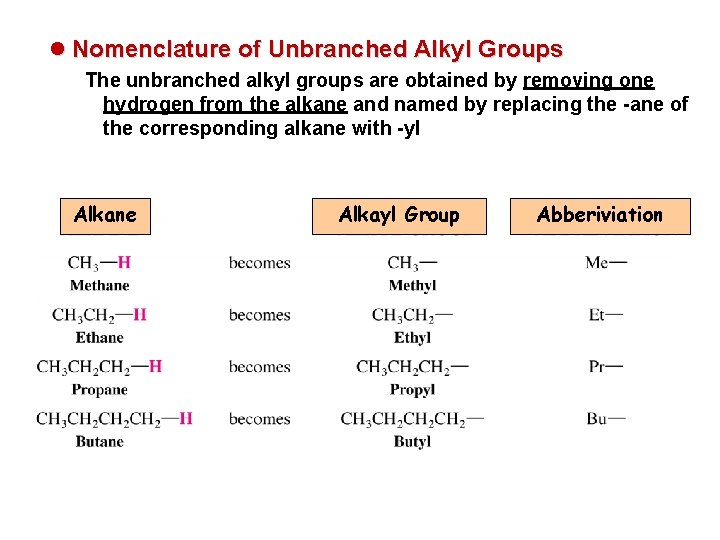
l Nomenclature of Unbranched Alkyl Groups The unbranched alkyl groups are obtained by removing one hydrogen from the alkane and named by replacing the -ane of the corresponding alkane with -yl Alkane Alkayl Group Abberiviation

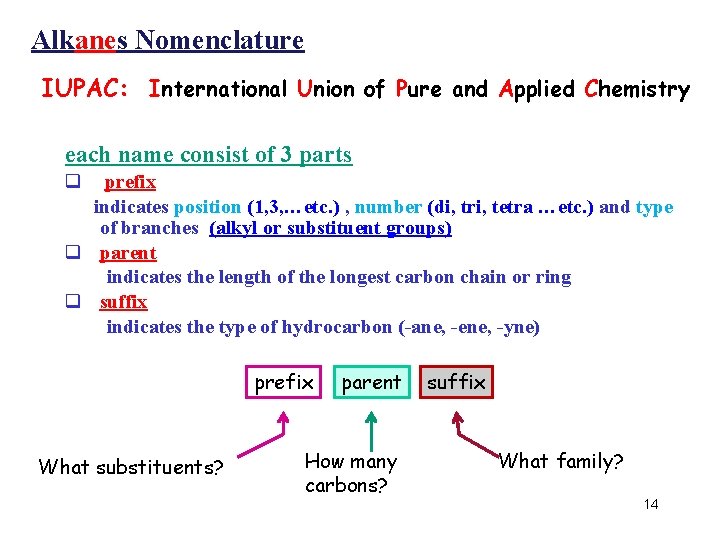
Alkanes Nomenclature IUPAC: International Union of Pure and Applied Chemistry • each name consist of 3 parts q prefix indicates position (1, 3, …etc. ) , number (di, tri, tetra …etc. ) and type of branches (alkyl or substituent groups) q parent indicates the length of the longest carbon chain or ring q suffix indicates the type of hydrocarbon (-ane, -ene, -yne) prefix What substituents? parent How many carbons? suffix What family? 14

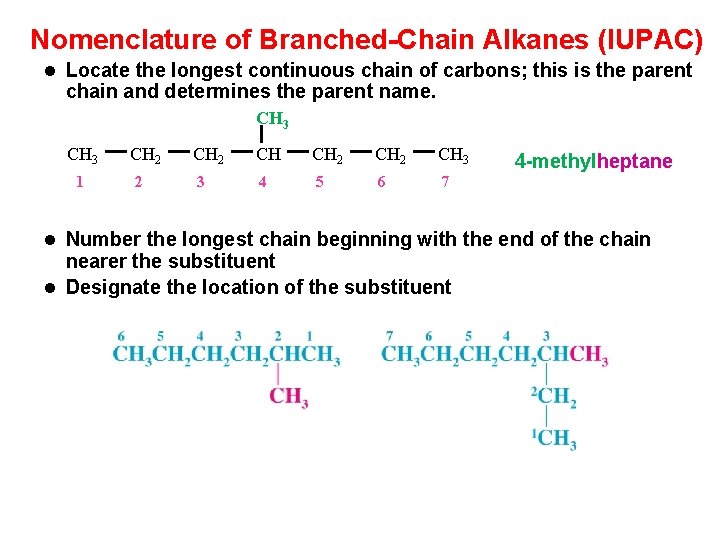
Nomenclature of Branched-Chain Alkanes (IUPAC) l Locate the longest continuous chain of carbons; this is the parent chain and determines the parent name. CH 3 1 CH 2 CH 3 2 3 4 5 6 7 4 -methylheptane l Number the longest chain beginning with the end of the chain nearer the substituent l Designate the location of the substituent

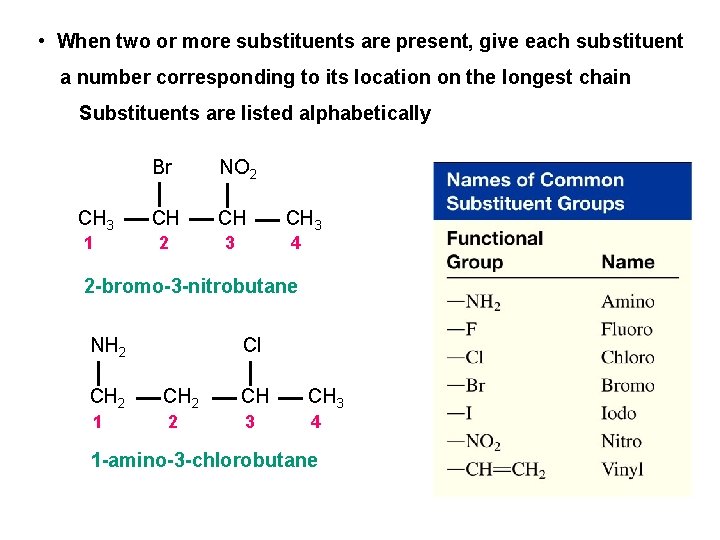
• When two or more substituents are present, give each substituent a number corresponding to its location on the longest chain Substituents are listed alphabetically CH 3 1 Br NO 2 CH CH 2 3 CH 3 4 2 -bromo-3 -nitrobutane NH 2 Cl CH 2 CH CH 3 1 2 3 4 1 -amino-3 -chlorobutane

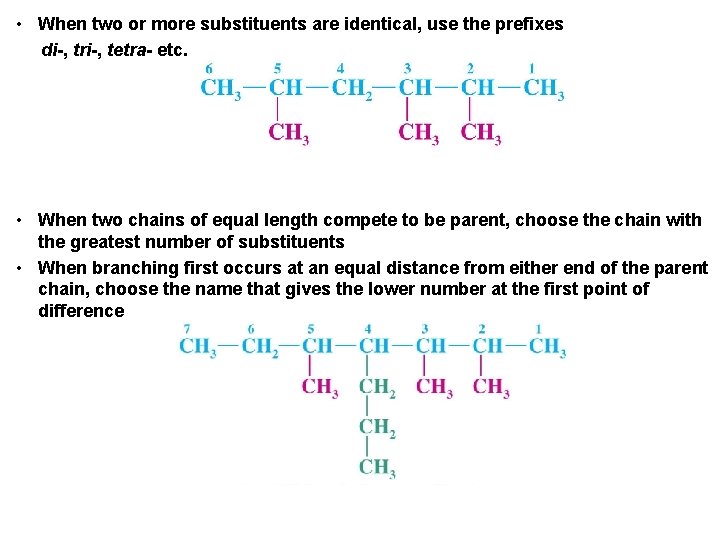
• When two or more substituents are identical, use the prefixes di-, tri-, tetra- etc. • When two chains of equal length compete to be parent, choose the chain with the greatest number of substituents • When branching first occurs at an equal distance from either end of the parent chain, choose the name that gives the lower number at the first point of difference

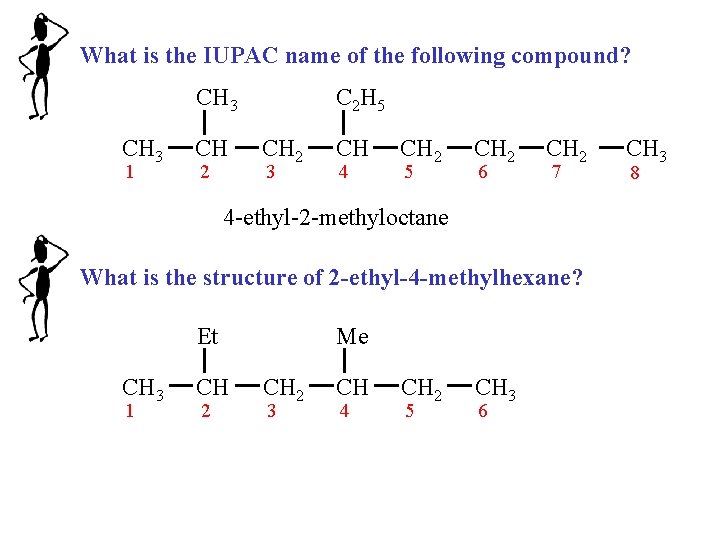
What is the IUPAC name of the following compound? CH 3 1 CH 2 C 2 H 5 CH 2 3 CH 4 CH 2 5 CH 2 6 CH 2 7 4 -ethyl-2 -methyloctane What is the structure of 2 -ethyl-4 -methylhexane? Et CH 3 1 CH 2 Me CH 2 3 CH 4 CH 2 5 CH 3 6 CH 3 8

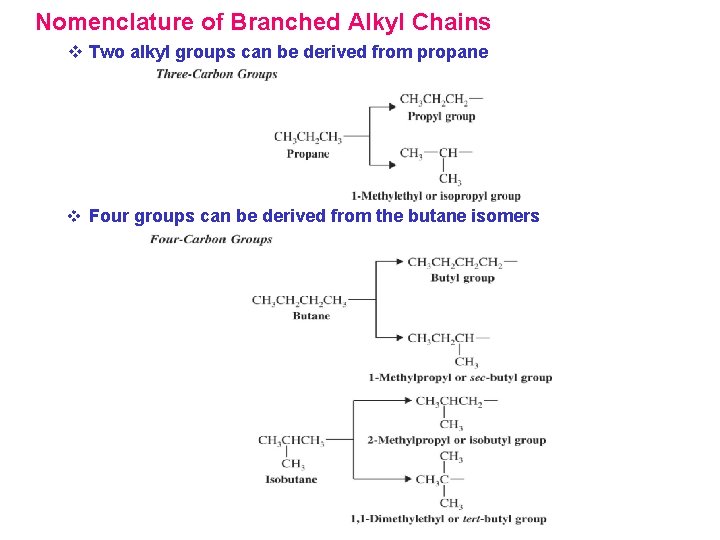
Nomenclature of Branched Alkyl Chains v Two alkyl groups can be derived from propane v Four groups can be derived from the butane isomers

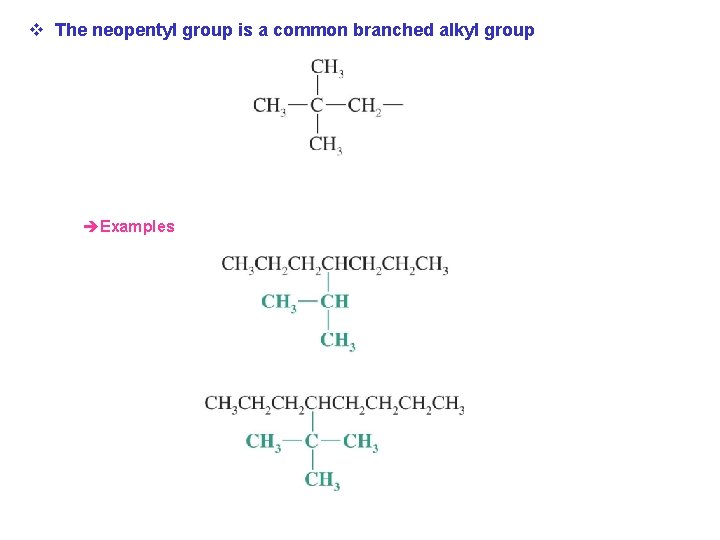
v The neopentyl group is a common branched alkyl group èExamples

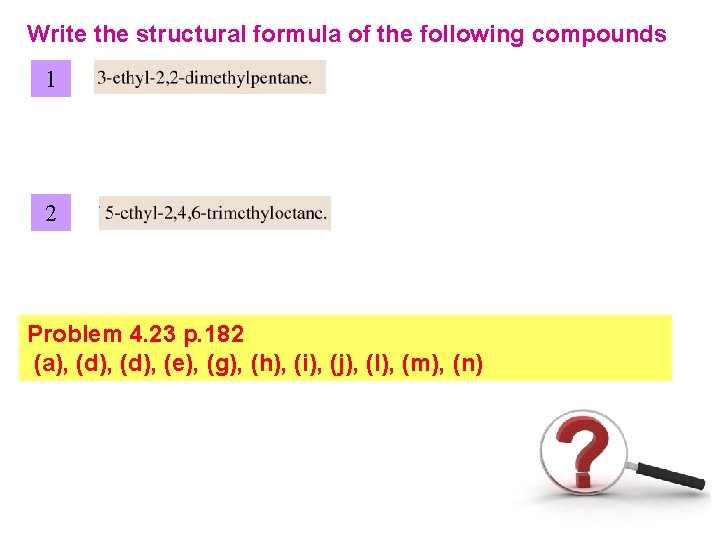
Write the structural formula of the following compounds 1 2 Problem 4. 23 p. 182 (a), (d), (e), (g), (h), (i), (j), (l), (m), (n)

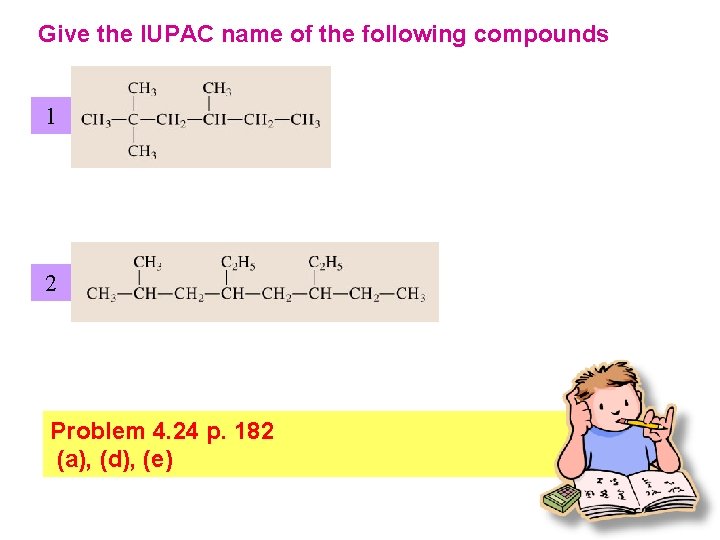
Give the IUPAC name of the following compounds 1 2 Problem 4. 24 p. 182 (a), (d), (e)

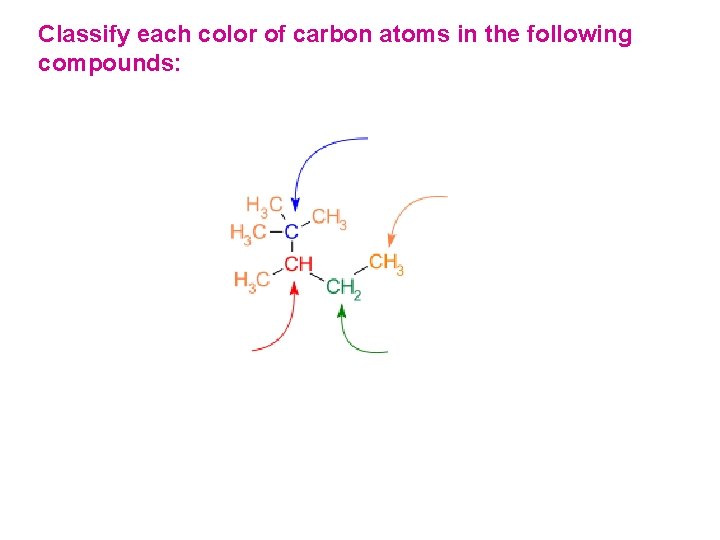
Classification of Hydrogen Atoms Hydrogen atoms take their classification from the carbon they are attached to. 1° Hydrogen atoms Primary Hydrogen atoms 3° Hydrogen atoms Tertiary Hydrogen atoms 2° Hydrogen atoms Secondary Hydrogen atoms

Classify each color of carbon atoms in the following compounds:

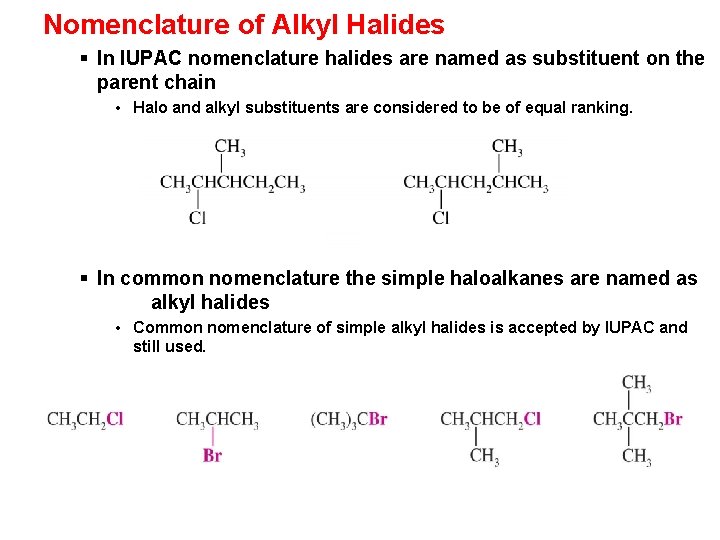
Nomenclature of Alkyl Halides § In IUPAC nomenclature halides are named as substituent on the parent chain • Halo and alkyl substituents are considered to be of equal ranking. § In common nomenclature the simple haloalkanes are named as alkyl halides • Common nomenclature of simple alkyl halides is accepted by IUPAC and still used.

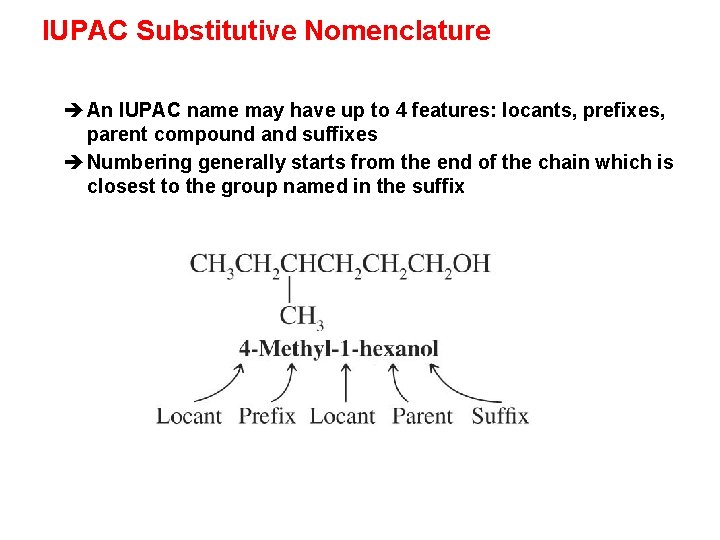
IUPAC Substitutive Nomenclature è An IUPAC name may have up to 4 features: locants, prefixes, parent compound and suffixes è Numbering generally starts from the end of the chain which is closest to the group named in the suffix

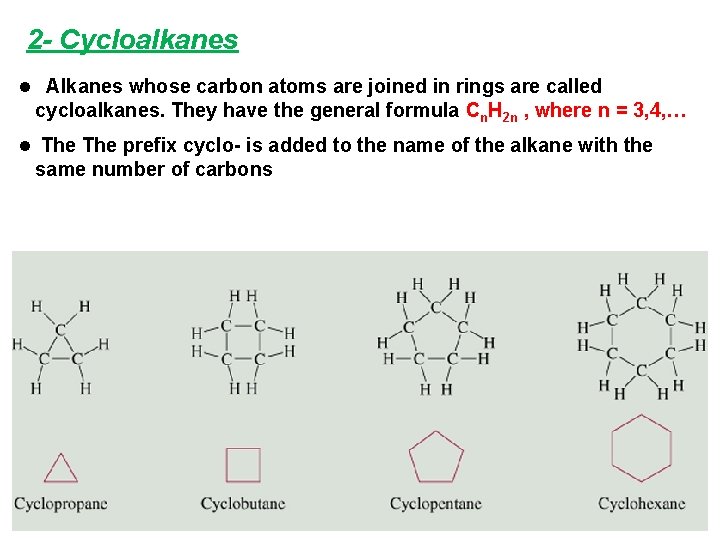
2 - Cycloalkanes l Alkanes whose carbon atoms are joined in rings are called cycloalkanes. They have the general formula Cn. H 2 n , where n = 3, 4, … l The prefix cyclo- is added to the name of the alkane with the same number of carbons

Nomenclature of Cycloalkanes A. When one substituent is present it is assumed to be at position one and is not numbered. B. When two alkyl substituents are present the one with alphabetical priority is given position 1 ×

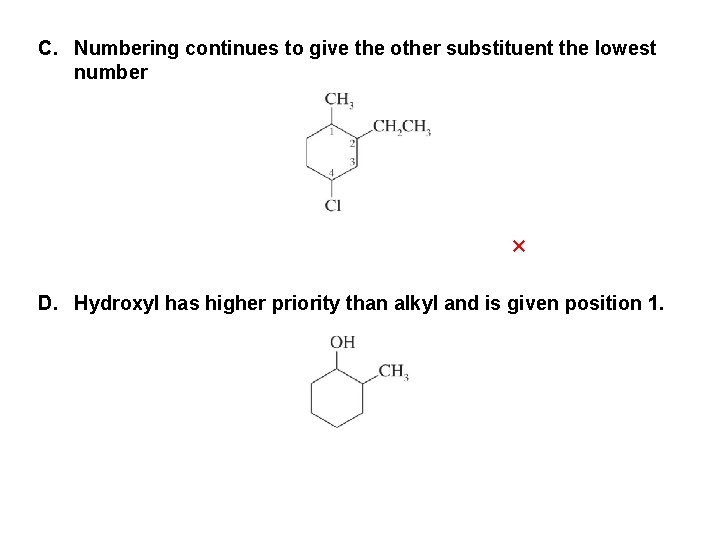
C. Numbering continues to give the other substituent the lowest number × D. Hydroxyl has higher priority than alkyl and is given position 1.

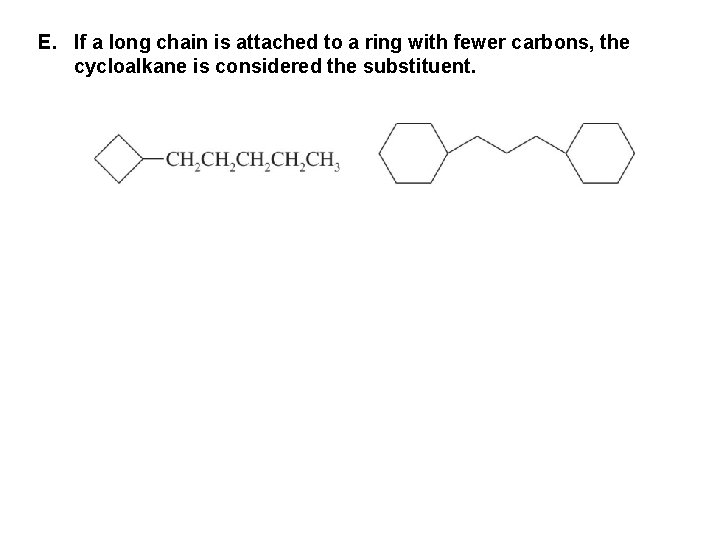
E. If a long chain is attached to a ring with fewer carbons, the cycloalkane is considered the substituent.

Bicyclic compounds q Bicyloalkanes contain 2 fused or bridged rings. q The alkane with the same number of total carbons is used as the parent and the prefix bicyclo- is used. Bicyclo[2. 2. 1]heptane

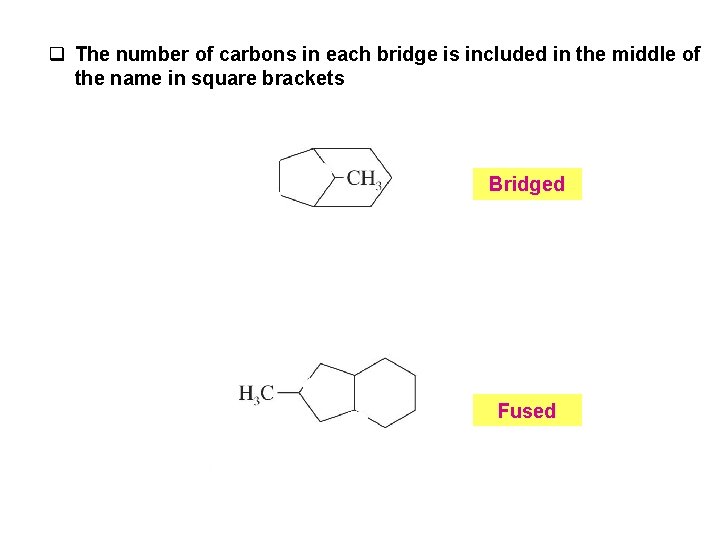
q The number of carbons in each bridge is included in the middle of the name in square brackets Bridged Fused
![Give the name of the following compounds 2 Clorobicyclo1 1 0butane bicyclo3 3 2decane Give the name of the following compounds: 2 -Clorobicyclo[1. 1. 0]butane bicyclo[3. 3. 2]decane](https://slidetodoc.com/presentation_image/e9ed4e859d4db498e686f9d4dfdc9d50/image-33.jpg)
Give the name of the following compounds: 2 -Clorobicyclo[1. 1. 0]butane bicyclo[3. 3. 2]decane bicyclo[3. 2. 1]octane 9 -Bromoobicyclo[4. 2. 1]nonane
![Write the structural formula of the following 7 7 Dichlorobicyclo2 2 1heptane bicyclo3 2 Write the structural formula of the following: 7, 7 -Dichlorobicyclo[2. 2. 1]heptane bicyclo[3. 2.](https://slidetodoc.com/presentation_image/e9ed4e859d4db498e686f9d4dfdc9d50/image-34.jpg)
Write the structural formula of the following: 7, 7 -Dichlorobicyclo[2. 2. 1]heptane bicyclo[3. 2. 0]heptane

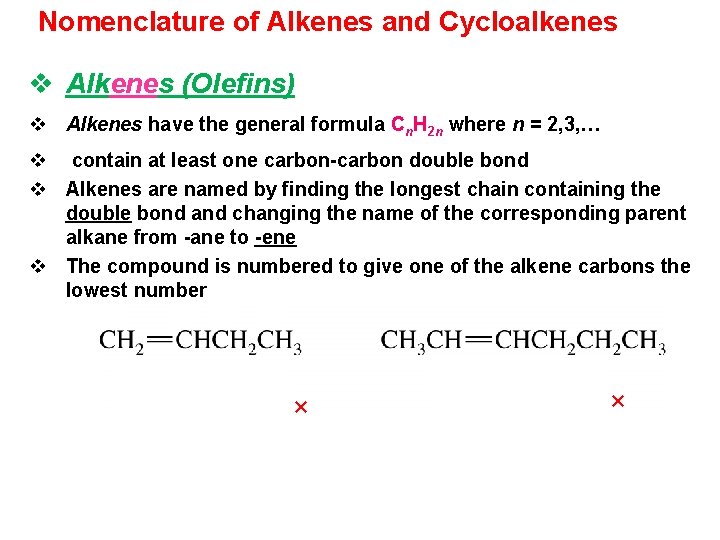
Nomenclature of Alkenes and Cycloalkenes v Alkenes (Olefins) v Alkenes have the general formula Cn. H 2 n where n = 2, 3, … v contain at least one carbon-carbon double bond v Alkenes are named by finding the longest chain containing the double bond and changing the name of the corresponding parent alkane from -ane to -ene v The compound is numbered to give one of the alkene carbons the lowest number × ×

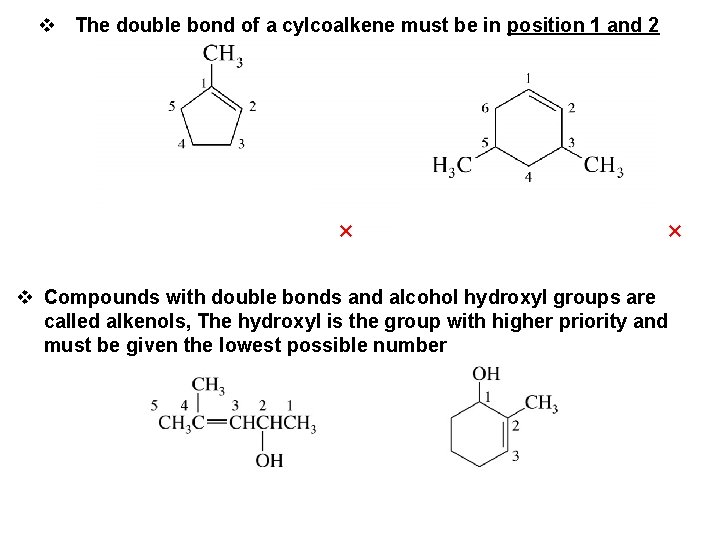
v The double bond of a cylcoalkene must be in position 1 and 2 × × v Compounds with double bonds and alcohol hydroxyl groups are called alkenols, The hydroxyl is the group with higher priority and must be given the lowest possible number

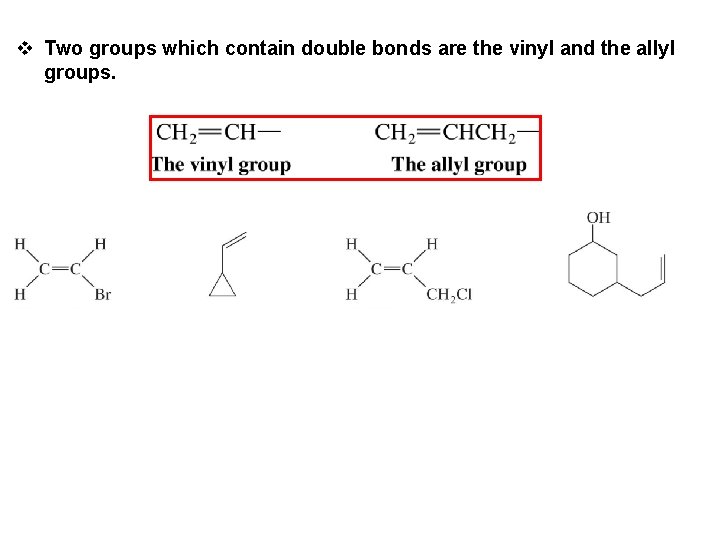
v Two groups which contain double bonds are the vinyl and the allyl groups.

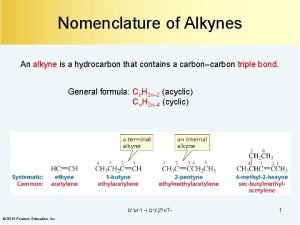
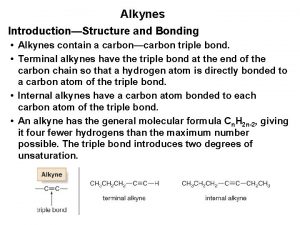
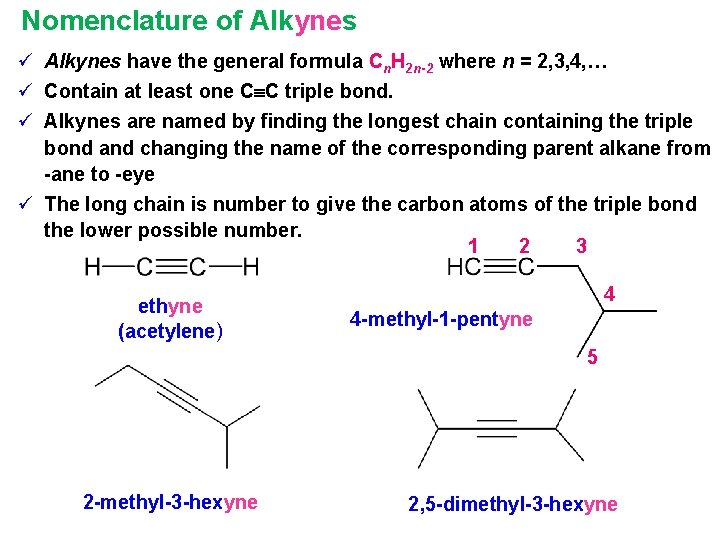
Nomenclature of Alkynes ü Alkynes have the general formula Cn. H 2 n-2 where n = 2, 3, 4, … ü Contain at least one C C triple bond. ü Alkynes are named by finding the longest chain containing the triple bond and changing the name of the corresponding parent alkane from -ane to -eye ü The long chain is number to give the carbon atoms of the triple bond the lower possible number. 1 2 3 ethyne (acetylene) 4 4 -methyl-1 -pentyne 5 2 -methyl-3 -hexyne 2, 5 -dimethyl-3 -hexyne

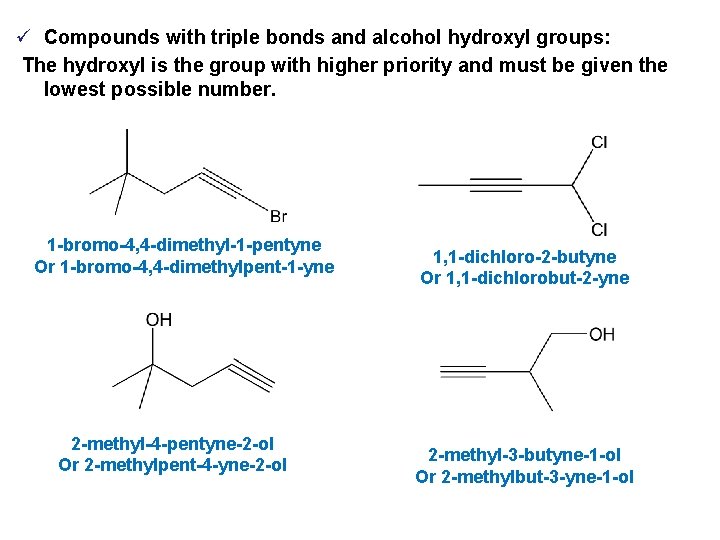
ü Compounds with triple bonds and alcohol hydroxyl groups: The hydroxyl is the group with higher priority and must be given the lowest possible number. 1 -bromo-4, 4 -dimethyl-1 -pentyne Or 1 -bromo-4, 4 -dimethylpent-1 -yne 2 -methyl-4 -pentyne-2 -ol Or 2 -methylpent-4 -yne-2 -ol 1, 1 -dichloro-2 -butyne Or 1, 1 -dichlorobut-2 -yne 2 -methyl-3 -butyne-1 -ol Or 2 -methylbut-3 -yne-1 -ol

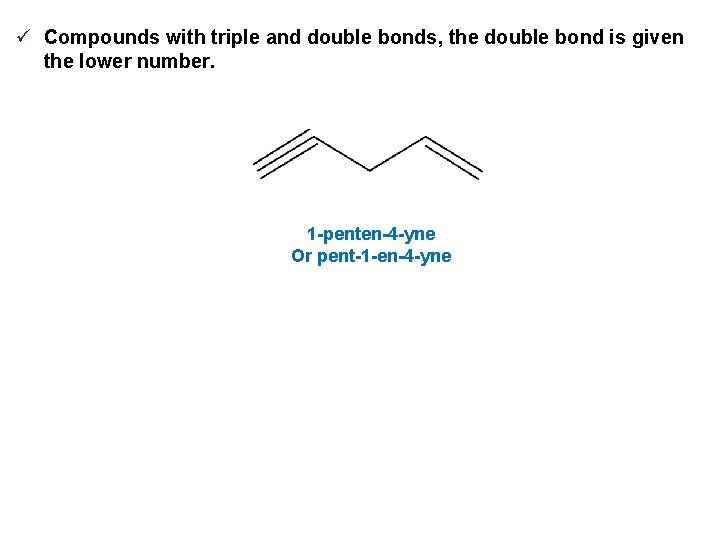
ü Compounds with triple and double bonds, the double bond is given the lower number. 1 -penten-4 -yne Or pent-1 -en-4 -yne

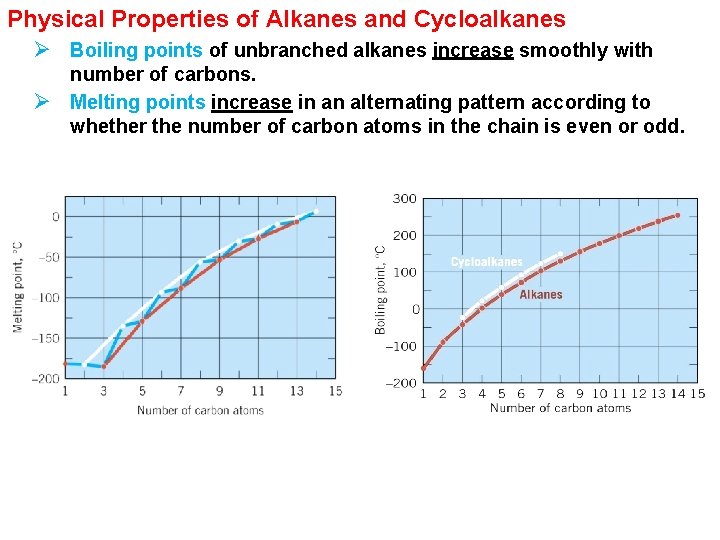
Physical Properties of Alkanes and Cycloalkanes Ø Boiling points of unbranched alkanes increase smoothly with number of carbons. Ø Melting points increase in an alternating pattern according to whether the number of carbon atoms in the chain is even or odd.

If we examine the unbranched alkenes in the latest figures, we notice that: § Each alkane differs from the preceding alkane by one methylene group (-CH 2). § Each member differs from the next one by a constant unit, is called a homologous series. § At room temperature (25°C) and (1 atm) pressure: • The C 1 -C 4 homologous series of unbranched alkanes are gases. • The C 5 -C 17 unbranched alkanes are liquids. • The C 18 -more unbranched alkanes are solids.

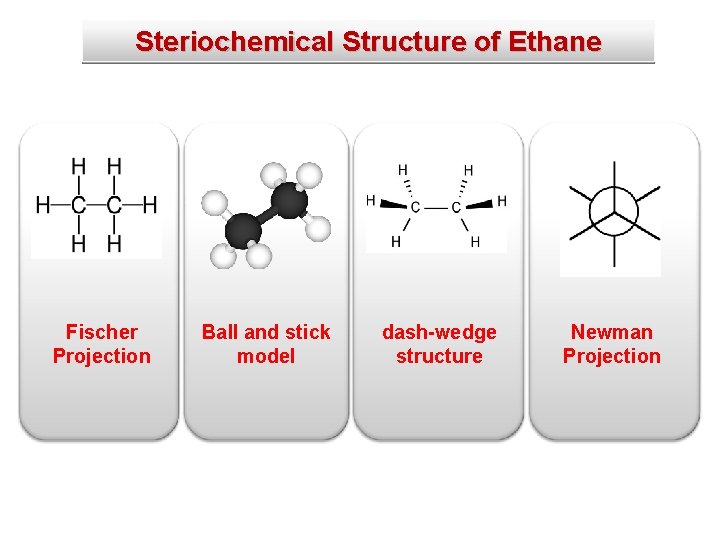
Steriochemical Structure of Ethane Fischer Projection Ball and stick model dash-wedge structure Newman Projection

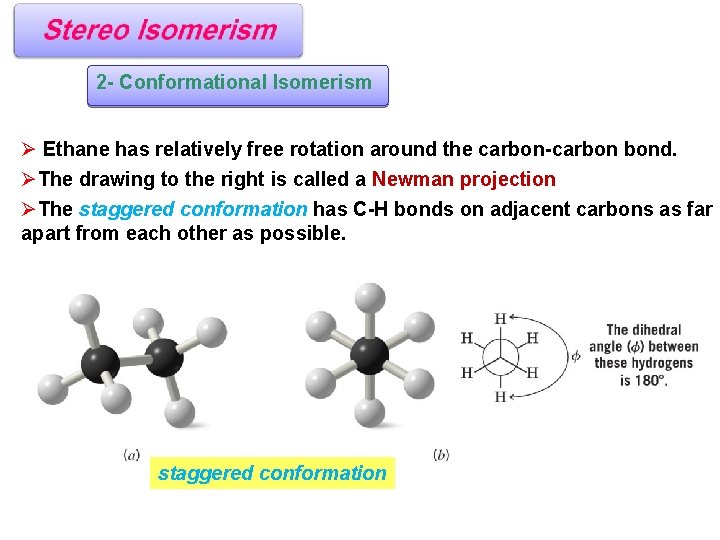
2 - Conformational Isomerism Ø Ethane has relatively free rotation around the carbon-carbon bond. ØThe drawing to the right is called a Newman projection ØThe staggered conformation has C-H bonds on adjacent carbons as far apart from each other as possible. staggered conformation

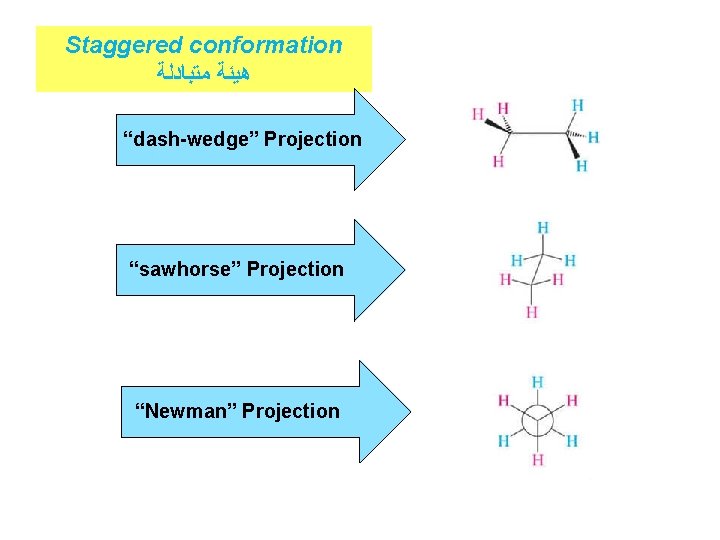
Staggered conformation ﻫﻴﺌﺔ ﻣﺘﺒﺎﺩﻟﺔ “dash-wedge” Projection “sawhorse” Projection “Newman” Projection

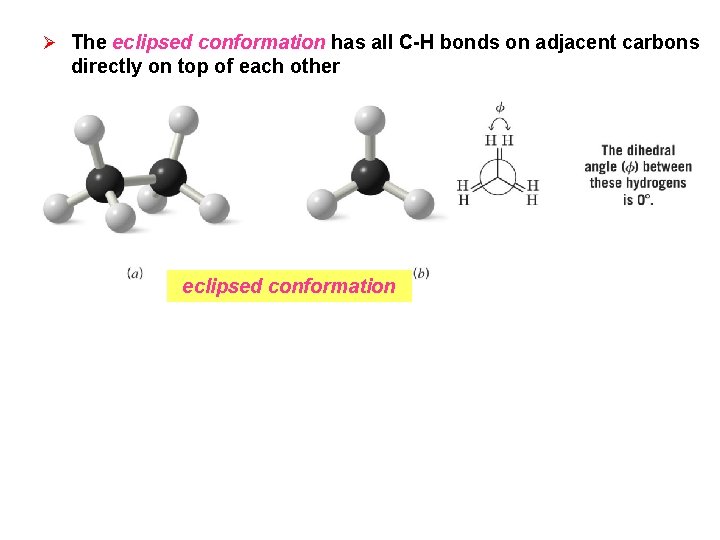
Ø The eclipsed conformation has all C-H bonds on adjacent carbons directly on top of each other eclipsed conformation

Eclipsed conformation ﻫﻴﺌﺔ ﻣﻨﻜﺴﻔﺔ “dash-wedge” Projection “Newman” Projection

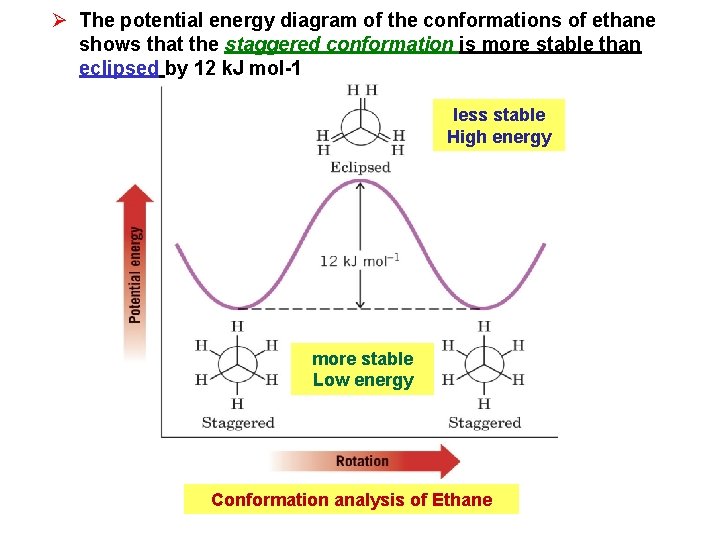
Ø The potential energy diagram of the conformations of ethane shows that the staggered conformation is more stable than eclipsed by 12 k. J mol-1 less stable High energy more stable Low energy Conformation analysis of Ethane

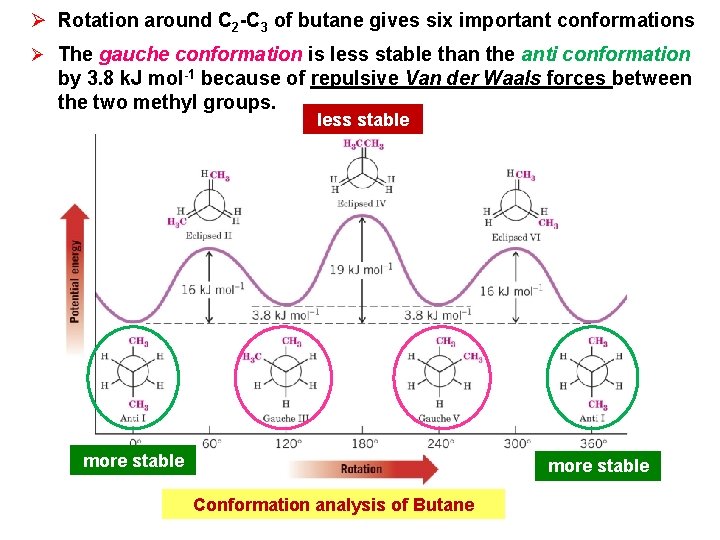
Ø Rotation around C 2 -C 3 of butane gives six important conformations Ø The gauche conformation is less stable than the anti conformation by 3. 8 k. J mol-1 because of repulsive Van der Waals forces between the two methyl groups. less stable more stable Conformation analysis of Butane

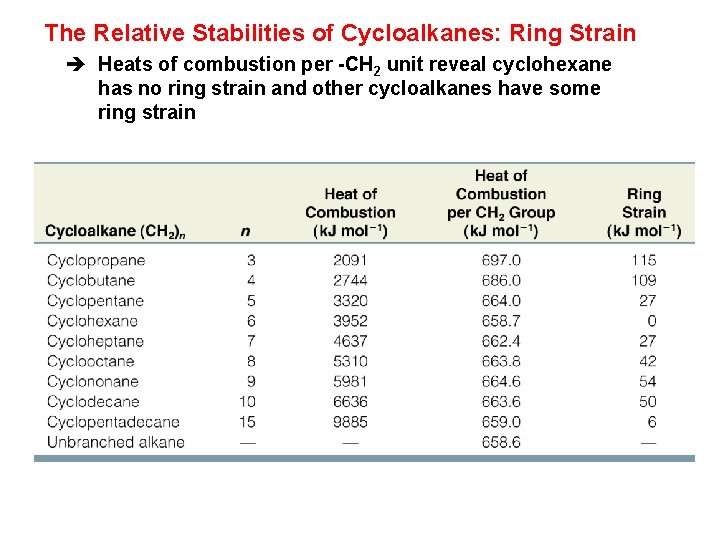
The Relative Stabilities of Cycloalkanes: Ring Strain è Heats of combustion per -CH 2 unit reveal cyclohexane has no ring strain and other cycloalkanes have some ring strain

The Relative Stabilities of Cycloalkanes: Ring Strain in Cyclopropane and Cyclobutane § Experiment have shown that cyclohexane is the most stable cycolalkane. § Cyclopropane and cyclobutane are much less stable. § This difference in relative stability is due to ring strain, which comprises by: § Angle strain is caused by bond angles different from 109. 5 o § Torsional strain is caused by eclipsing C-H bonds on adjacent carbons

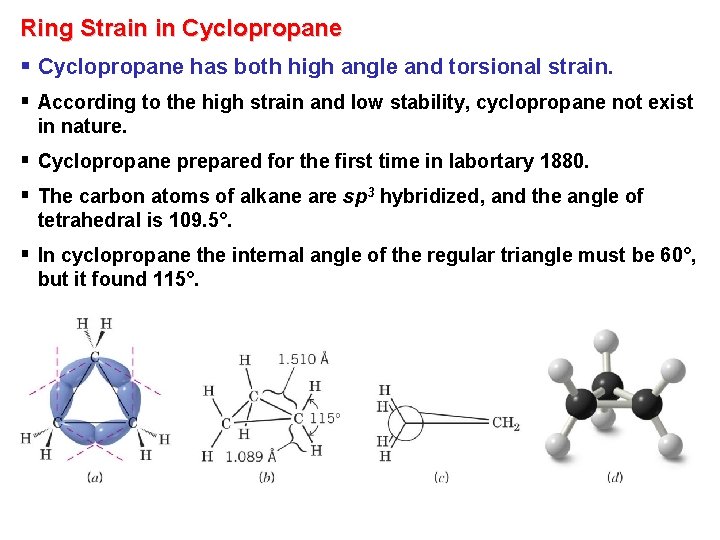
Ring Strain in Cyclopropane § Cyclopropane has both high angle and torsional strain. § According to the high strain and low stability, cyclopropane not exist in nature. § Cyclopropane prepared for the first time in labortary 1880. § The carbon atoms of alkane are sp 3 hybridized, and the angle of tetrahedral is 109. 5°. § In cyclopropane the internal angle of the regular triangle must be 60°, but it found 115°.

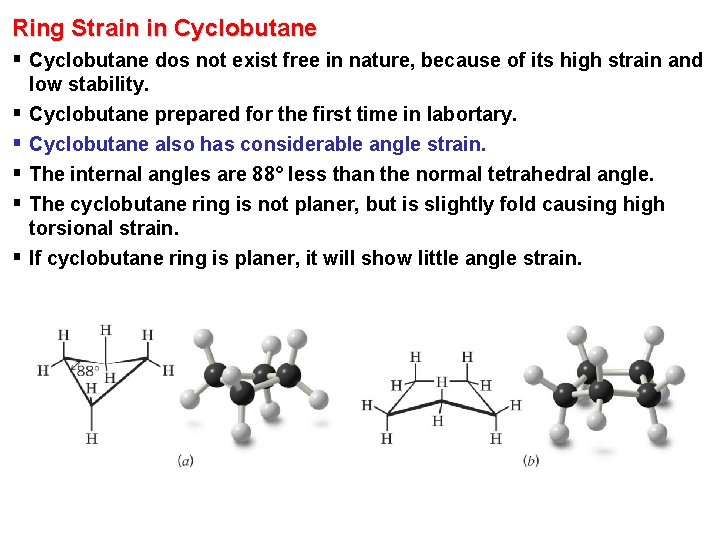
Ring Strain in Cyclobutane § Cyclobutane dos not exist free in nature, because of its high strain and § § § low stability. Cyclobutane prepared for the first time in labortary. Cyclobutane also has considerable angle strain. The internal angles are 88° less than the normal tetrahedral angle. The cyclobutane ring is not planer, but is slightly fold causing high torsional strain. If cyclobutane ring is planer, it will show little angle strain.

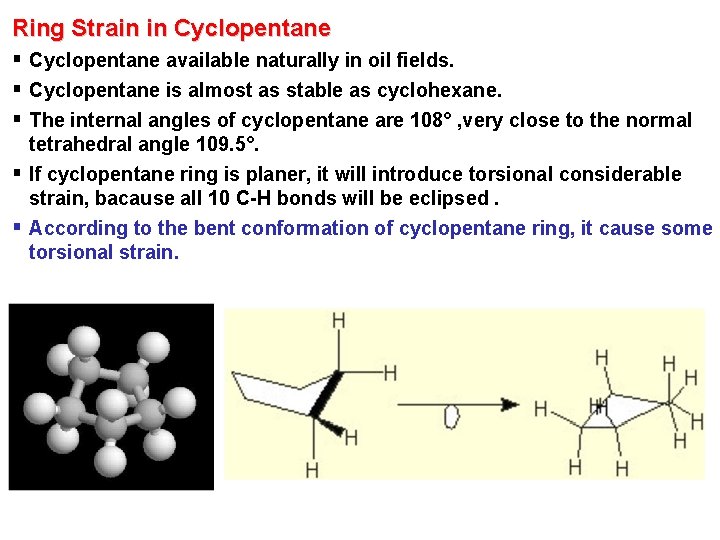
Ring Strain in Cyclopentane § Cyclopentane available naturally in oil fields. § Cyclopentane is almost as stable as cyclohexane. § The internal angles of cyclopentane are 108° , very close to the normal tetrahedral angle 109. 5°. § If cyclopentane ring is planer, it will introduce torsional considerable strain, bacause all 10 C-H bonds will be eclipsed. § According to the bent conformation of cyclopentane ring, it cause some torsional strain.

Ring Strain in Cyclopentane § Because of the non-planer conformation of cyclopentane, slightly twisting of C-C bond occur with little change in energy. § Therefore, the molecule is flexible and shift rapidly from one conformation to the another as shown. § Cyclopentane has little torsional and angle strain. Envelope Half-chair

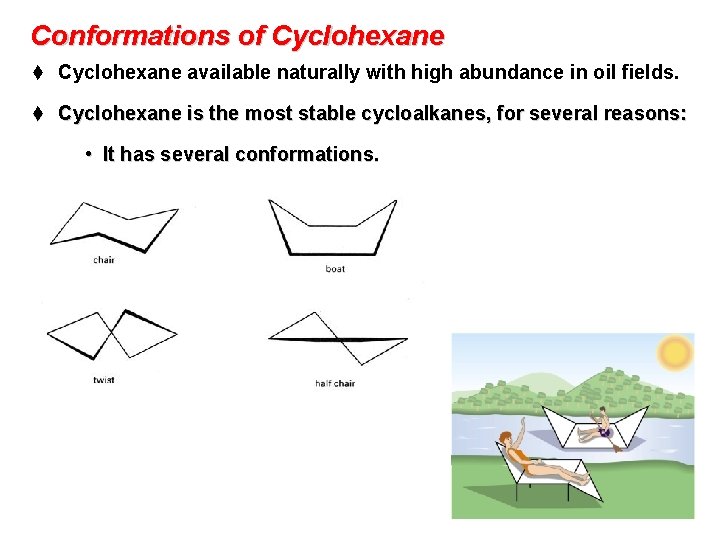
Conformations of Cyclohexane t Cyclohexane available naturally with high abundance in oil fields. t Cyclohexane is the most stable cycloalkanes, for several reasons: • It has several conformations.

• • • The most stable of cyclohexane is the chair conformation. The chair conformation has no ring strain (angle and torsional strain). All bond angles are 109. 5° and all C-H bonds are perfectly staggered. Chair Conformation Newman Projection of Chair Conformation

t heat of combustion suggests that angle strain is unimportant in cyclohexane. t tetrahedral bond angles require nonplanar geometries.

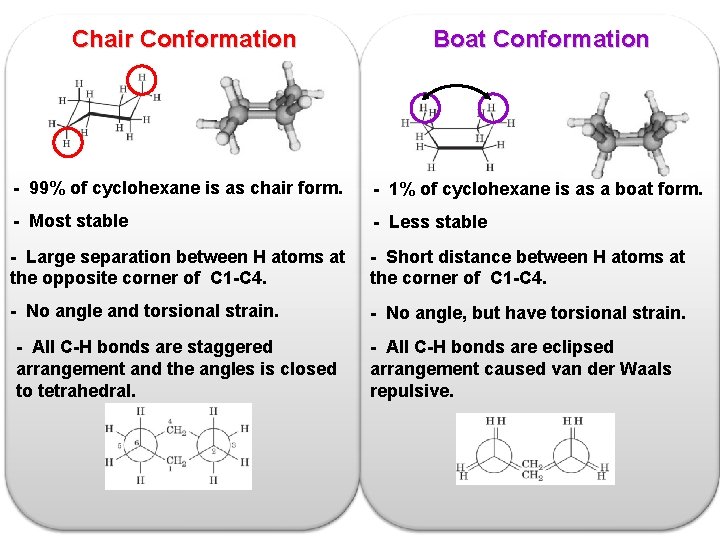
Chair Conformation Boat Conformation - 99% of cyclohexane is as chair form. - 1% of cyclohexane is as a boat form. - Most stable - Less stable - Large separation between H atoms at the opposite corner of C 1 -C 4. - Short distance between H atoms at the corner of C 1 -C 4. - No angle and torsional strain. - No angle, but have torsional strain. - All C-H bonds are staggered arrangement and the angles is closed to tetrahedral. - All C-H bonds are eclipsed arrangement caused van der Waals repulsive.

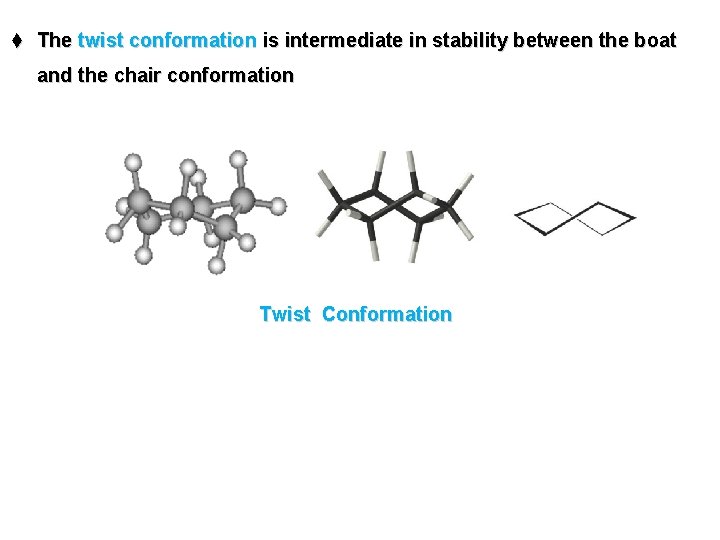
t The twist conformation is intermediate in stability between the boat and the chair conformation Twist Conformation

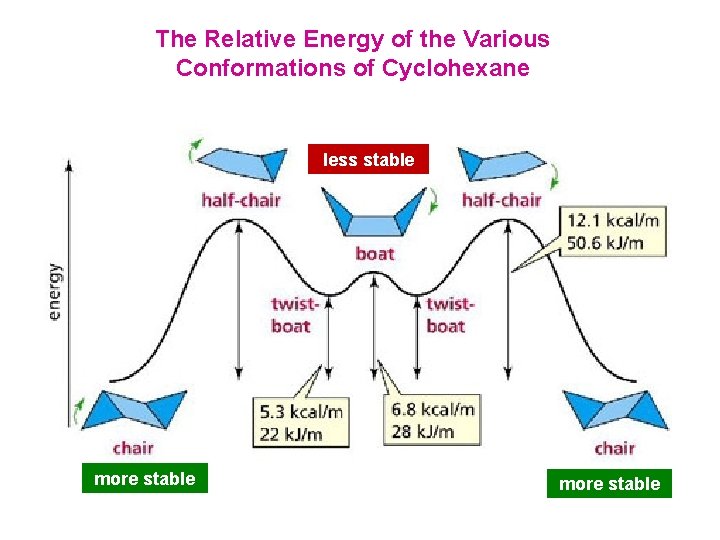
The Relative Energy of the Various Conformations of Cyclohexane less stable more stable

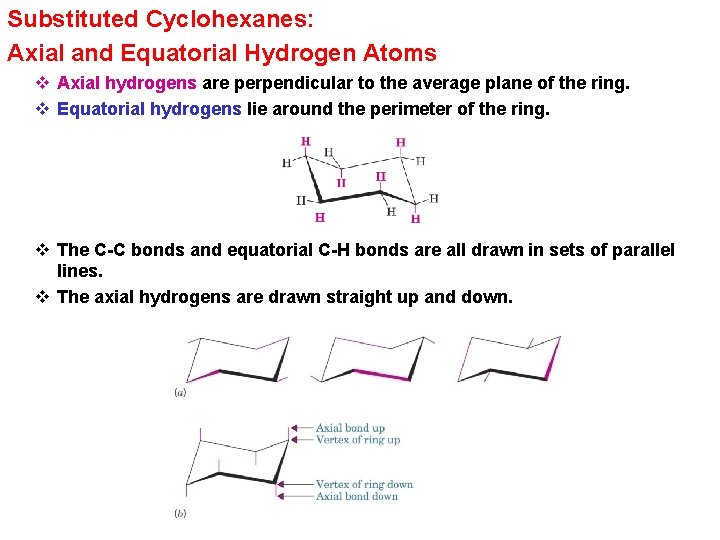
Substituted Cyclohexanes: Axial and Equatorial Hydrogen Atoms v Axial hydrogens are perpendicular to the average plane of the ring. v Equatorial hydrogens lie around the perimeter of the ring. v The C-C bonds and equatorial C-H bonds are all drawn in sets of parallel lines. v The axial hydrogens are drawn straight up and down.

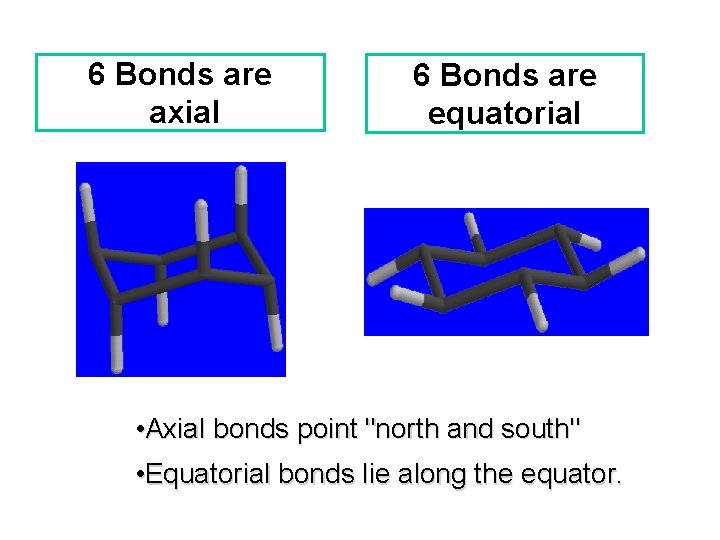
6 Bonds are axial 6 Bonds are equatorial • Axial bonds point "north and south" • Equatorial bonds lie along the equator.

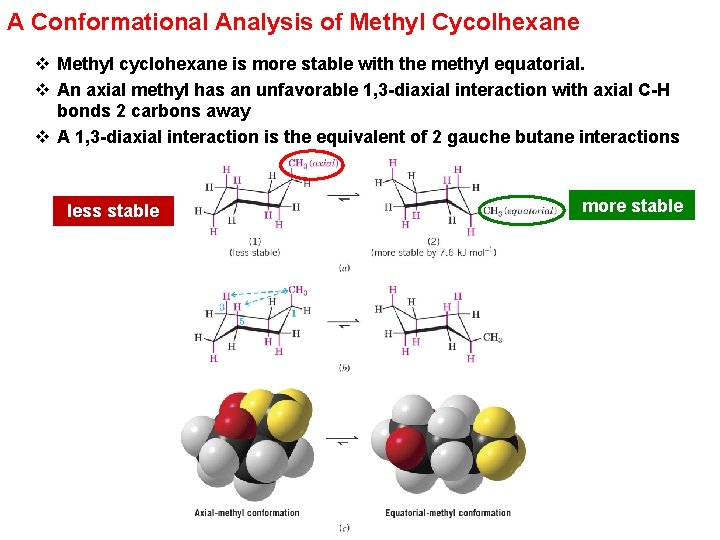
A Conformational Analysis of Methyl Cycolhexane v Methyl cyclohexane is more stable with the methyl equatorial. v An axial methyl has an unfavorable 1, 3 -diaxial interaction with axial C-H bonds 2 carbons away v A 1, 3 -diaxial interaction is the equivalent of 2 gauche butane interactions less stable more stable

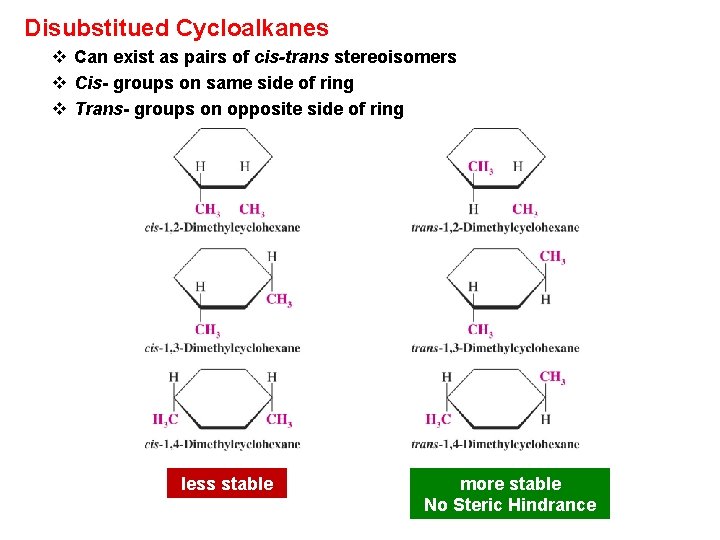
Disubstitued Cycloalkanes v Can exist as pairs of cis-trans stereoisomers v Cis- groups on same side of ring v Trans- groups on opposite side of ring less stable more stable No Steric Hindrance

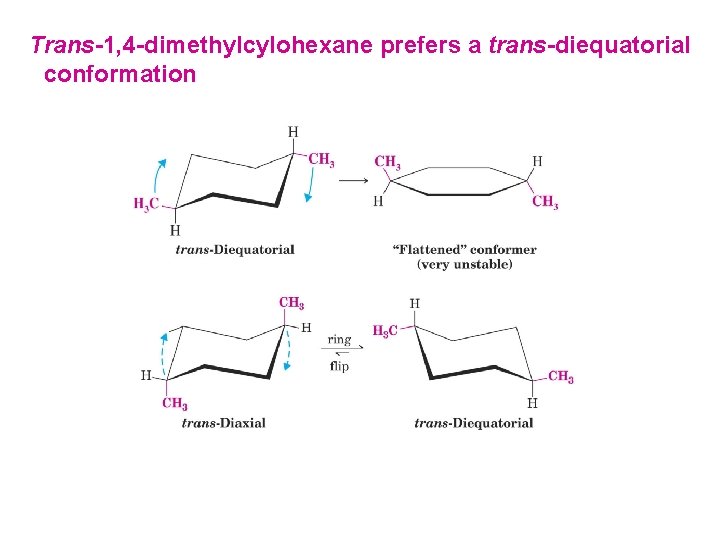
Trans-1, 4 -dimethylcylohexane prefers a trans-diequatorial conformation

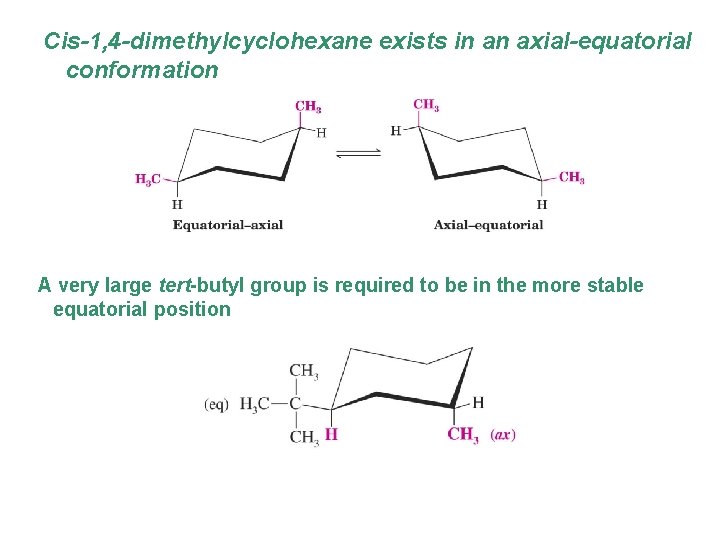
Cis-1, 4 -dimethylcyclohexane exists in an axial-equatorial conformation A very large tert-butyl group is required to be in the more stable equatorial position

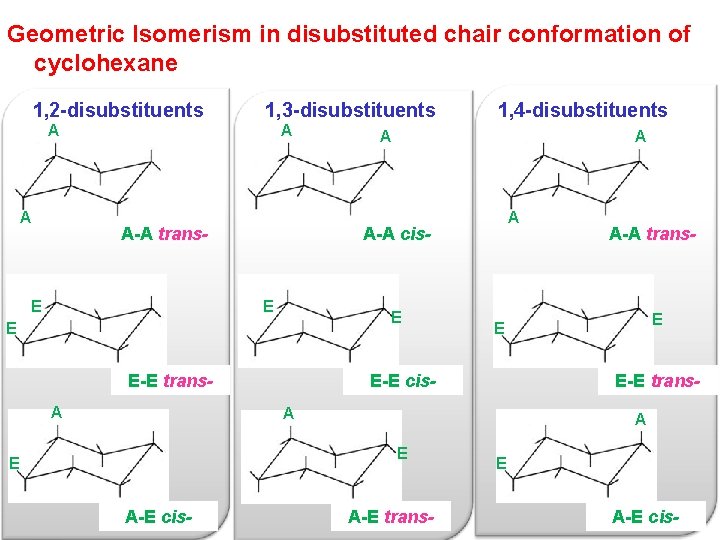
Geometric Isomerism in disubstituted chair conformation of cyclohexane 1, 2 -disubstituents 1, 3 -disubstituents A A-A trans- E A A-A cis- E E-E trans. A 1, 4 -disubstituents E-E cis- E-E trans. A E A-E cis- E E A-A trans- A-E trans- E A-E cis-

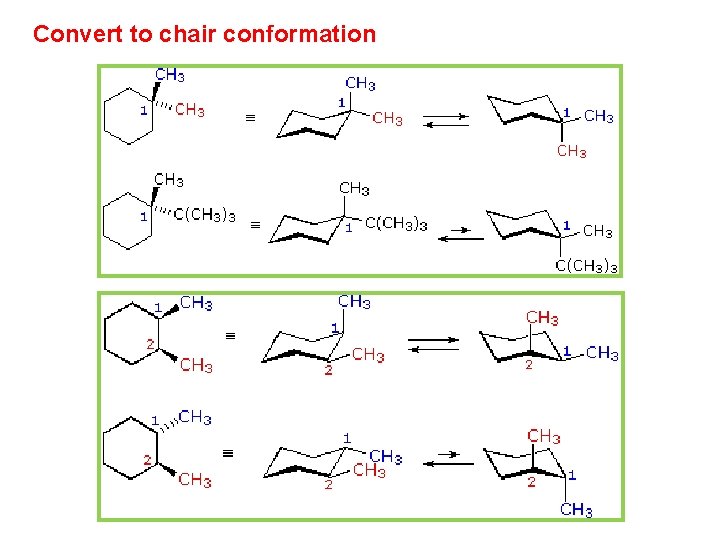
Convert to chair conformation

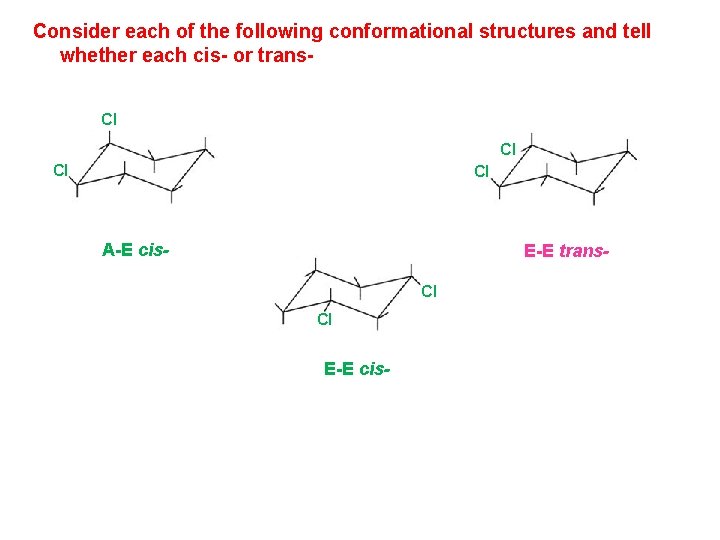
Consider each of the following conformational structures and tell whether each cis- or trans. Cl Cl A-E cis- E-E trans. Cl Cl E-E cis-

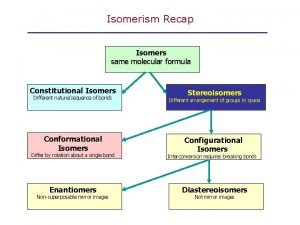
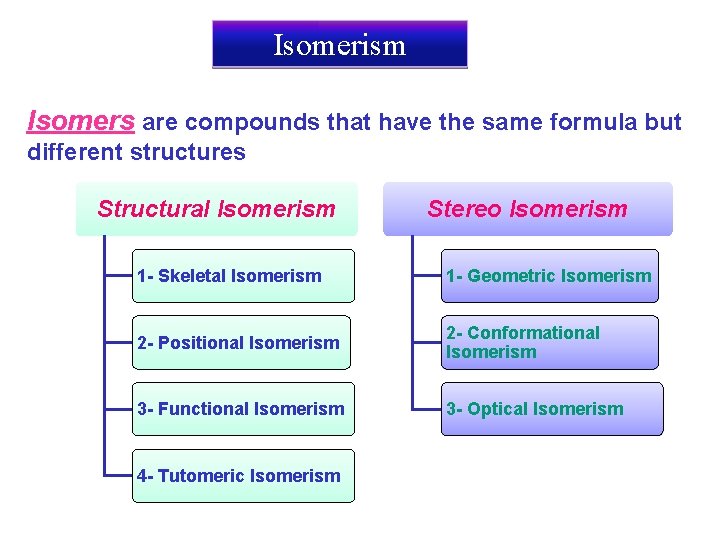
Isomerism Isomers are compounds that have the same formula but different structures Structural Isomerism Stereo Isomerism 1 - Skeletal Isomerism 1 - Geometric Isomerism 2 - Positional Isomerism 2 - Conformational Isomerism 3 - Functional Isomerism 3 - Optical Isomerism 4 - Tutomeric Isomerism

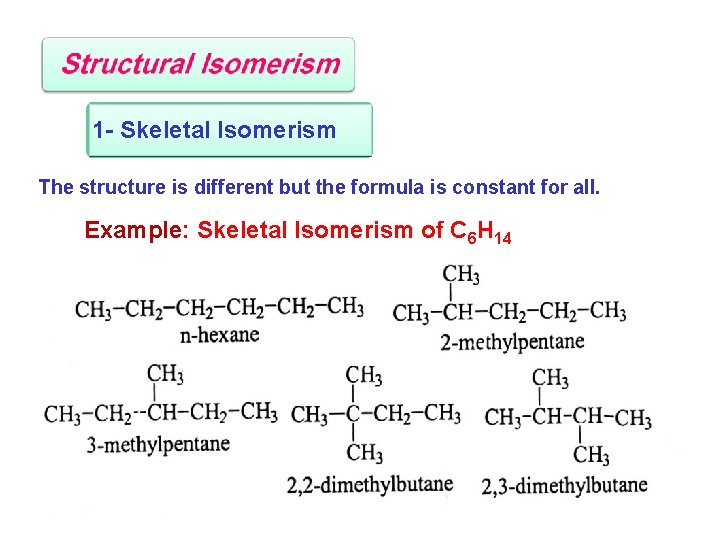
1 - Skeletal Isomerism The structure is different but the formula is constant for all. Example: Skeletal Isomerism of C 6 H 14

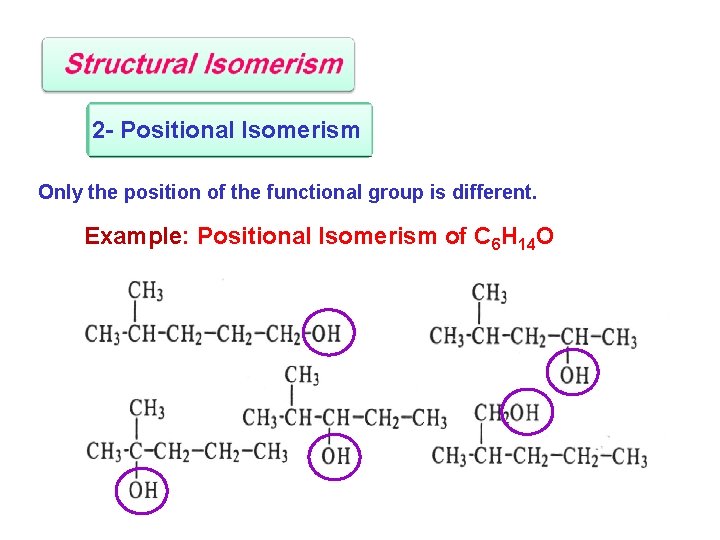
2 - Positional Isomerism Only the position of the functional group is different. Example: Positional Isomerism of C 6 H 14 O

3 - Functional Isomerism The type of the functional group is different. Example 1: Functional Isomerism of C 3 H 6 O Ketone Aldehyde Example 2: Functional Isomerism of C 2 H 6 O Alcohol Ether

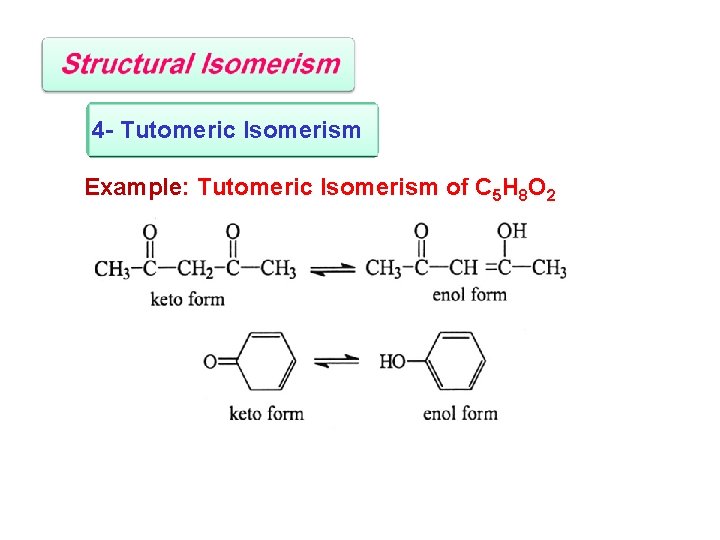
4 - Tutomeric Isomerism Example: Tutomeric Isomerism of C 5 H 8 O 2

Isomerism Constitutional (Structural) Isomerism Stereo Isomerism 1 - Skeletal Isomerism 1 - Geometric Isomerism 2 - Positional Isomerism 2 - Conformational Isomerism 3 - Functional Isomerism 3 - Optical Isomerism 4 - Tutomeric Isomerism

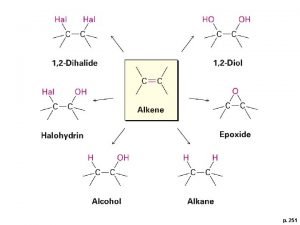
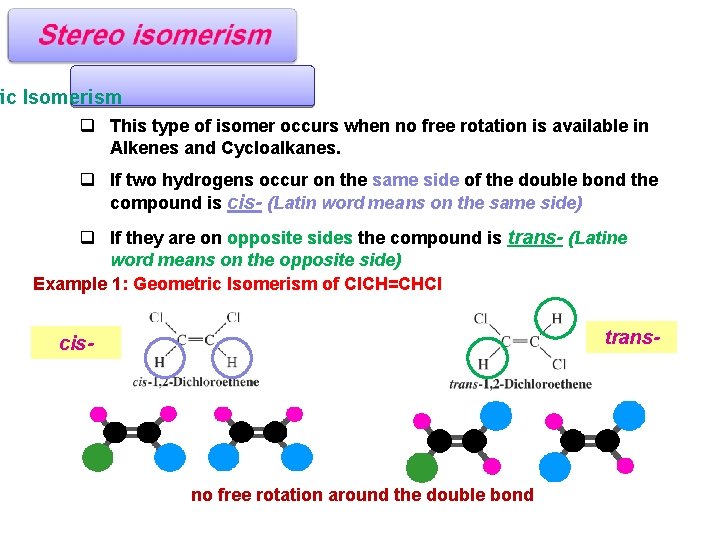
ric Isomerism q This type of isomer occurs when no free rotation is available in Alkenes and Cycloalkanes. q If two hydrogens occur on the same side of the double bond the compound is cis- (Latin word means on the same side) q If they are on opposite sides the compound is trans- (Latine word means on the opposite side) Example 1: Geometric Isomerism of Cl. CH=CHCl trans- cis- no free rotation around the double bond

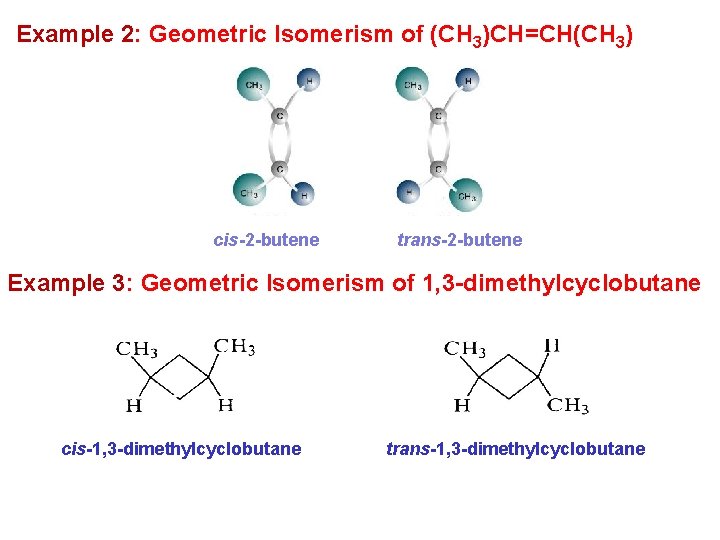
Example 2: Geometric Isomerism of (CH 3)CH=CH(CH 3) cis-2 -butene trans-2 -butene Example 3: Geometric Isomerism of 1, 3 -dimethylcyclobutane cis-1, 3 -dimethylcyclobutane trans-1, 3 -dimethylcyclobutane

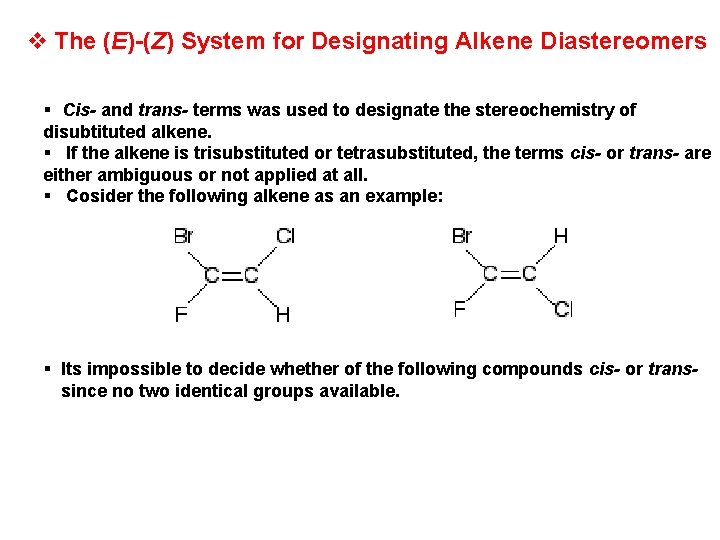
v The (E)-(Z) System for Designating Alkene Diastereomers § Cis- and trans- terms was used to designate the stereochemistry of disubtituted alkene. § If the alkene is trisubstituted or tetrasubstituted, the terms cis- or trans- are either ambiguous or not applied at all. § Cosider the following alkene as an example: § Its impossible to decide whether of the following compounds cis- or transsince no two identical groups available.

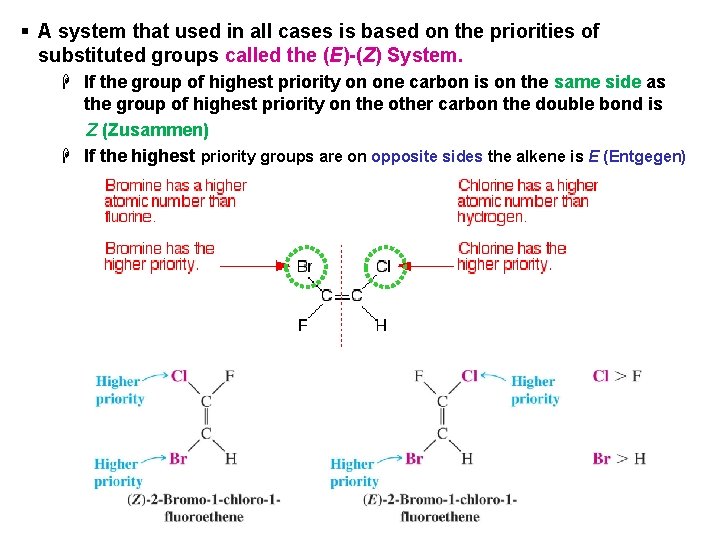
§ A system that used in all cases is based on the priorities of substituted groups called the (E)-(Z) System. H If the group of highest priority on one carbon is on the same side as the group of highest priority on the other carbon the double bond is Z (Zusammen) H If the highest priority groups are on opposite sides the alkene is E (Entgegen)

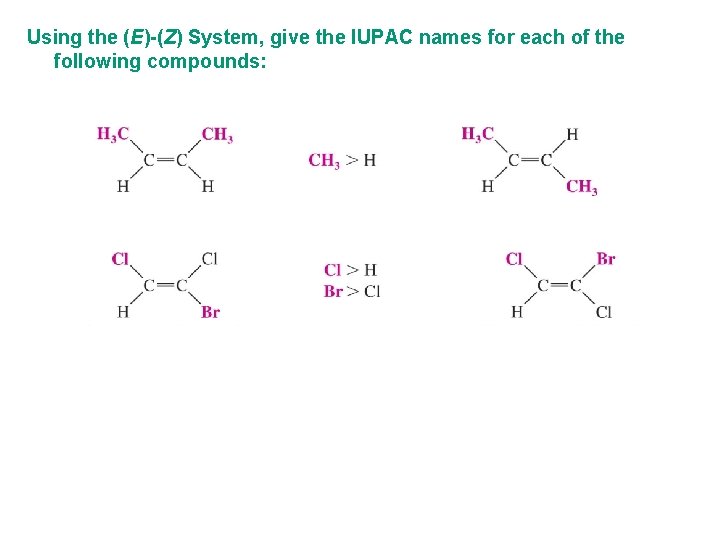
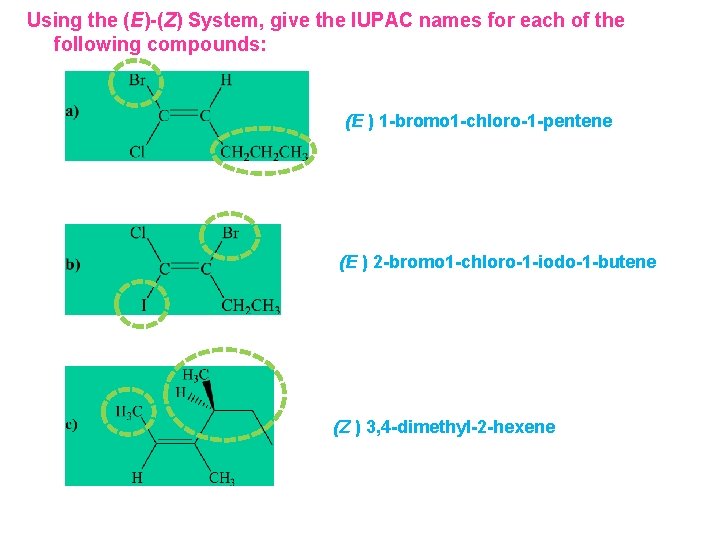
Using the (E)-(Z) System, give the IUPAC names for each of the following compounds:

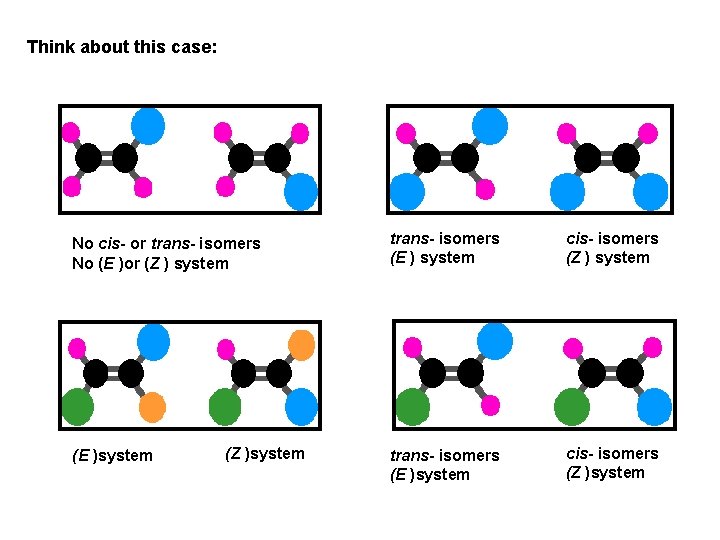
Think about this case: No cis- or trans- isomers No (E )or (Z ) system (E )system (Z )system trans- isomers (E ) system cis- isomers (Z ) system trans- isomers (E )system cis- isomers (Z )system

Using the (E)-(Z) System, give the IUPAC names for each of the following compounds: (E ) 1 -bromo 1 -chloro-1 -pentene (E ) 2 -bromo 1 -chloro-1 -iodo-1 -butene (Z ) 3, 4 -dimethyl-2 -hexene

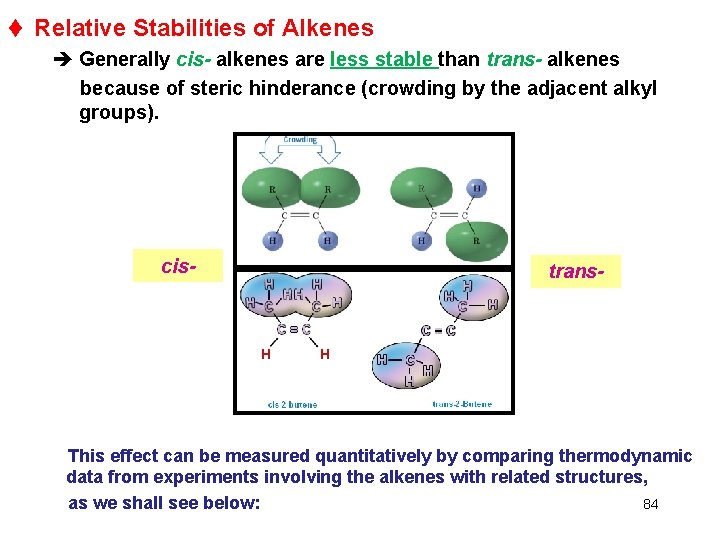
t Relative Stabilities of Alkenes è Generally cis- alkenes are less stable than trans- alkenes because of steric hinderance (crowding by the adjacent alkyl groups). cis- trans- This effect can be measured quantitatively by comparing thermodynamic data from experiments involving the alkenes with related structures, 84 as we shall see below:

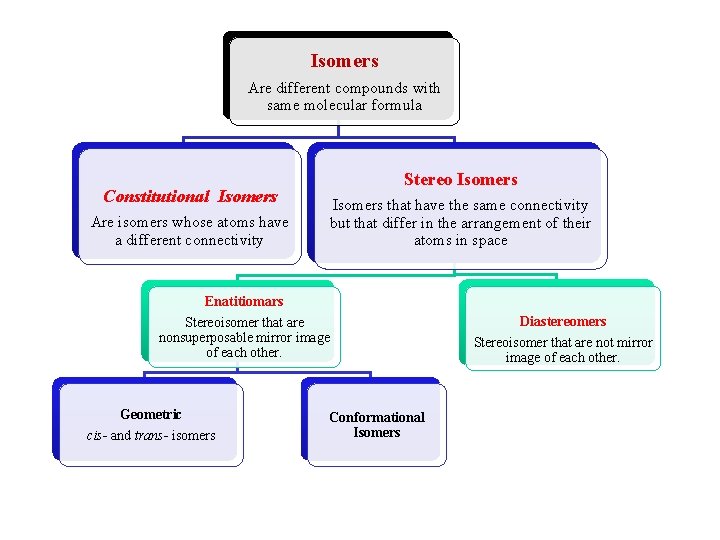
Isomers Are different compounds with same molecular formula Constitutional Isomers Are isomers whose atoms have a different connectivity Stereo Isomers that have the same connectivity but that differ in the arrangement of their atoms in space Enatitiomars Stereoisomer that are nonsuperposable mirror image of each other. Geometric cis- and trans- isomers Conformational Isomers Diastereomers Stereoisomer that are not mirror image of each other.


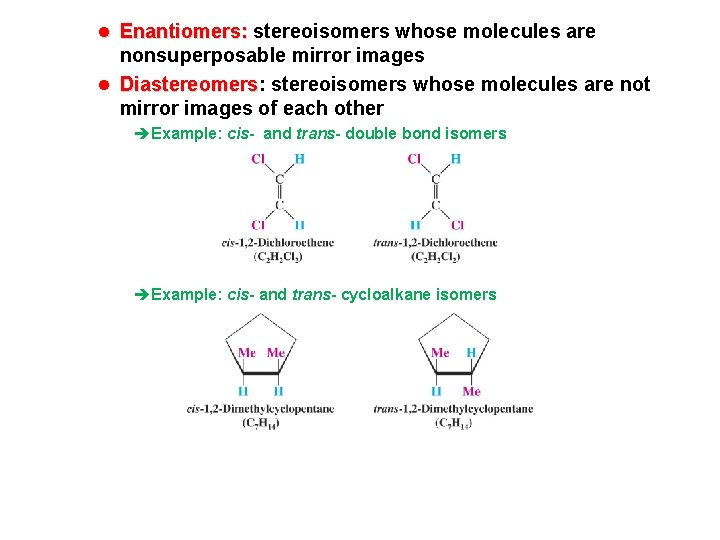
l Enantiomers: stereoisomers whose molecules are nonsuperposable mirror images l Diastereomers: Diastereomers stereoisomers whose molecules are not mirror images of each other èExample: cis- and trans- double bond isomers èExample: cis- and trans- cycloalkane isomers

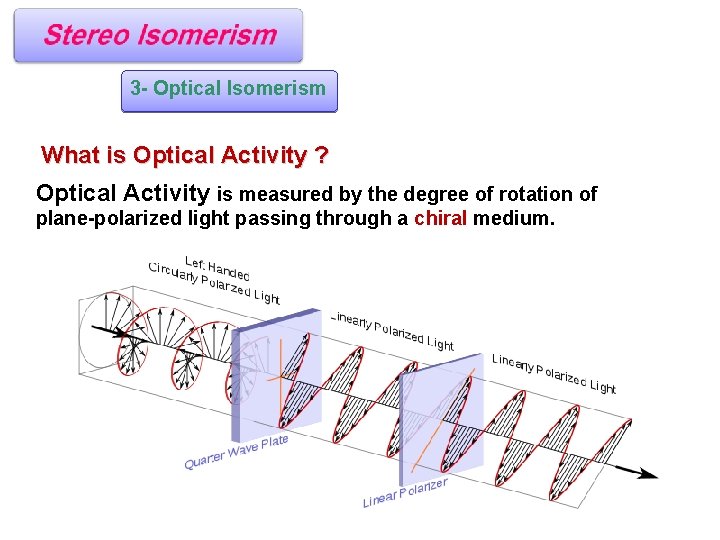
3 - Optical Isomerism What is Optical Activity ? Optical Activity is measured by the degree of rotation of plane-polarized light passing through a chiral medium.

Polarimeter is an instrument used to measure the optical rotation of various substances on plane-polarized light. optical active substances in Polarimeter tube Analyzer prism polarized light unpolarized light polarizer light source rotated polarized light

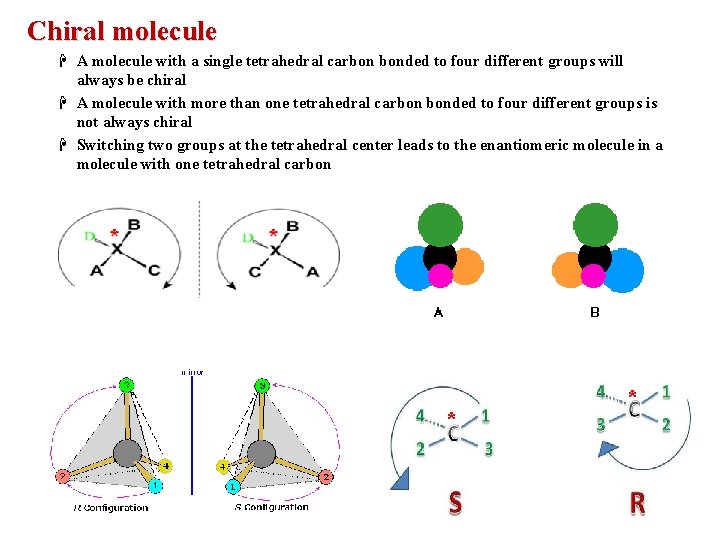
Chiral molecule H A molecule with a single tetrahedral carbon bonded to four different groups will always be chiral H A molecule with more than one tetrahedral carbon bonded to four different groups is not always chiral H Switching two groups at the tetrahedral center leads to the enantiomeric molecule in a molecule with one tetrahedral carbon * *

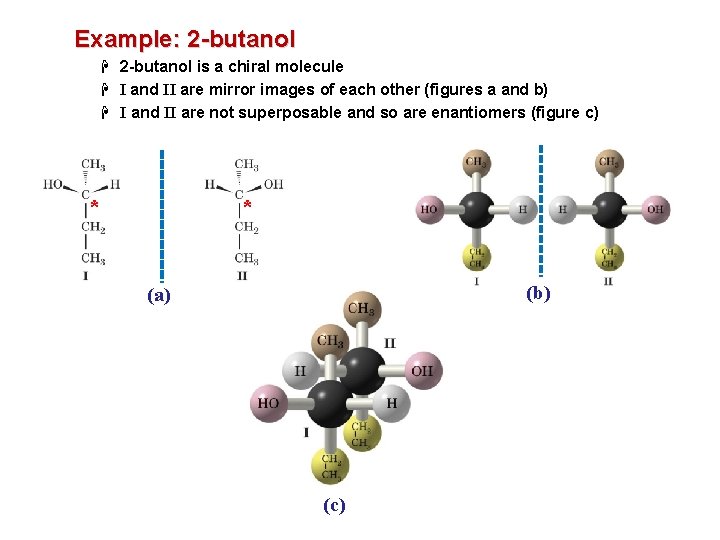
Example: 2 -butanol H 2 -butanol is a chiral molecule H I and II are mirror images of each other (figures a and b) H I and II are not superposable and so are enantiomers (figure c) * * (b) (a) (c)

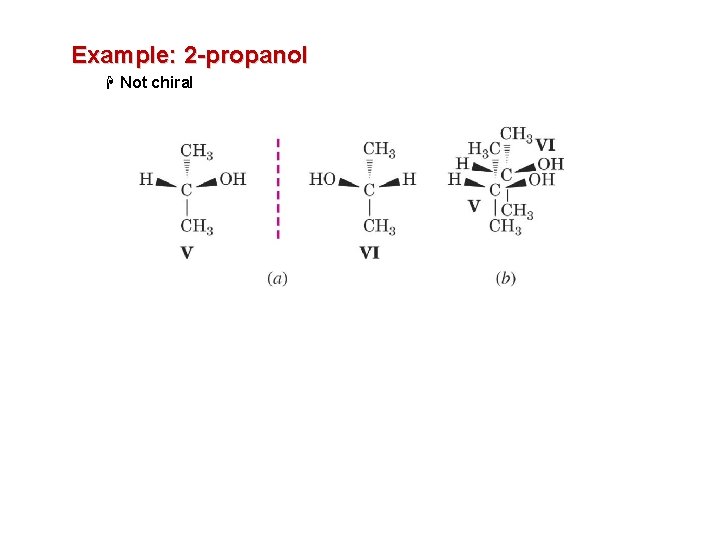
Example: 2 -propanol H Not chiral

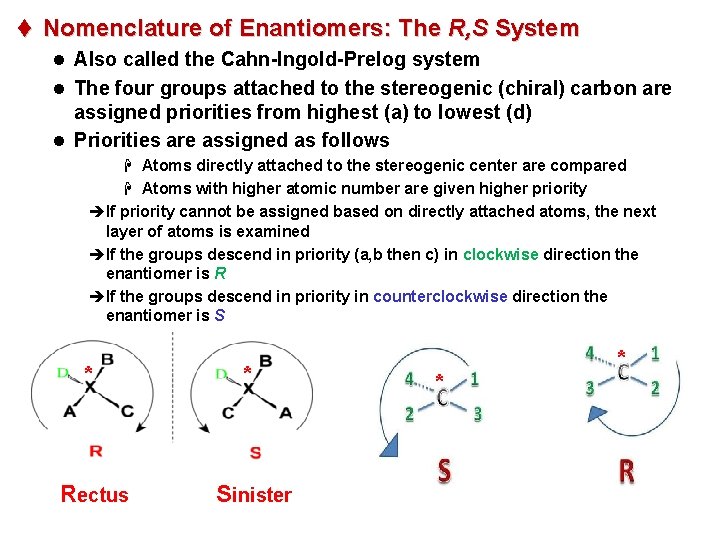
t Nomenclature of Enantiomers: The R, S System l Also called the Cahn-Ingold-Prelog system l The four groups attached to the stereogenic (chiral) carbon are assigned priorities from highest (a) to lowest (d) l Priorities are assigned as follows H Atoms directly attached to the stereogenic center are compared H Atoms with higher atomic number are given higher priority èIf priority cannot be assigned based on directly attached atoms, the next layer of atoms is examined èIf the groups descend in priority (a, b then c) in clockwise direction the enantiomer is R èIf the groups descend in priority in counterclockwise direction the enantiomer is S * Rectus * Sinister * *

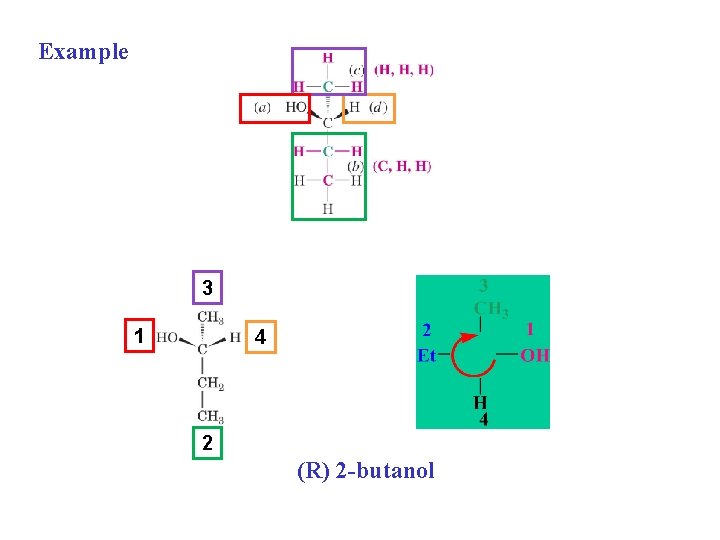
Example 3 1 4 2 (R) 2 -butanol

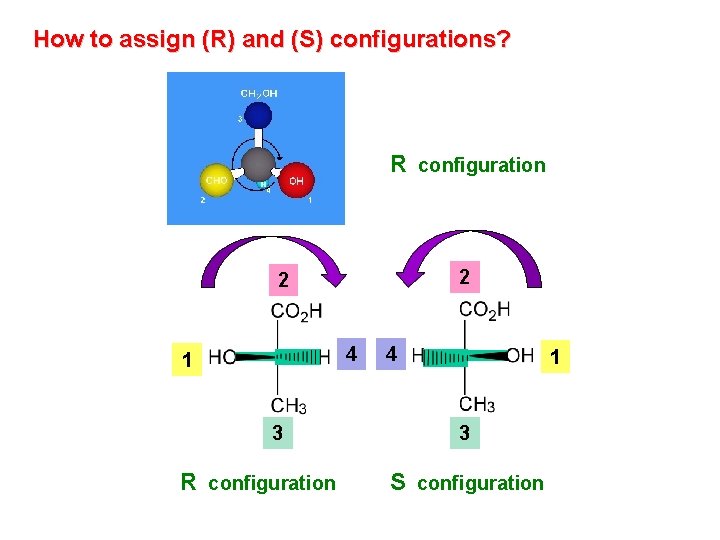
How to assign (R) and (S) configurations? R configuration 2 2 4 1 3 R configuration 4 1 3 S configuration

Indicate the R or S configuration

What is the meaning of (+) or (-) and d- l- ? ? Ø Rotation of the plane of polarized light in the clockwise sense is taken as positive (+) Ø Rotation in the counterclockwise sense is taken as a negative (-) rotation. Ø Older terms for positive and negative rotations were dextrorotatory and levorotatory, from the Latin prefixes dextro- ("to the right") and levo- ("to the left"), respectively

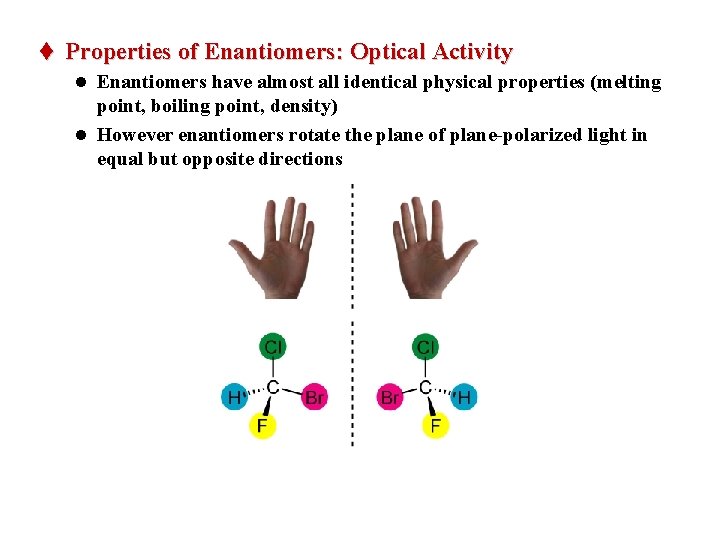
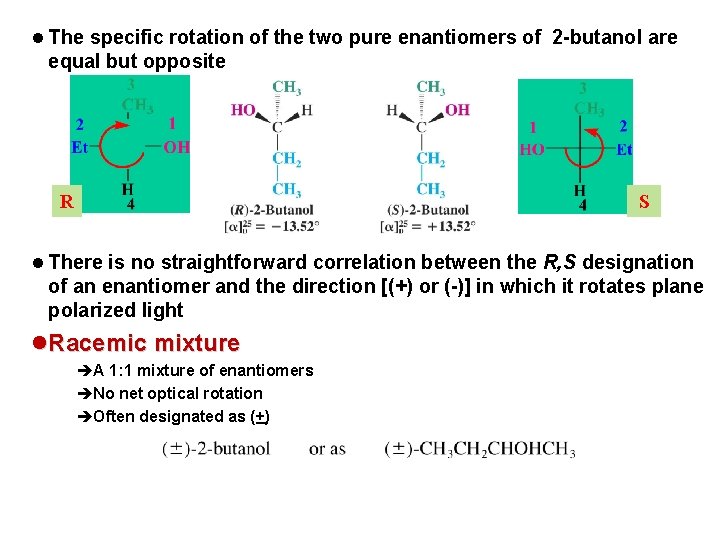
t Properties of Enantiomers: Optical Activity l Enantiomers have almost all identical physical properties (melting point, boiling point, density) l However enantiomers rotate the plane of plane-polarized light in equal but opposite directions

l The specific rotation of the two pure enantiomers of 2 -butanol are equal but opposite R S l There is no straightforward correlation between the R, S designation of an enantiomer and the direction [(+) or (-)] in which it rotates plane polarized light l Racemic mixture èA 1: 1 mixture of enantiomers èNo net optical rotation èOften designated as (+)

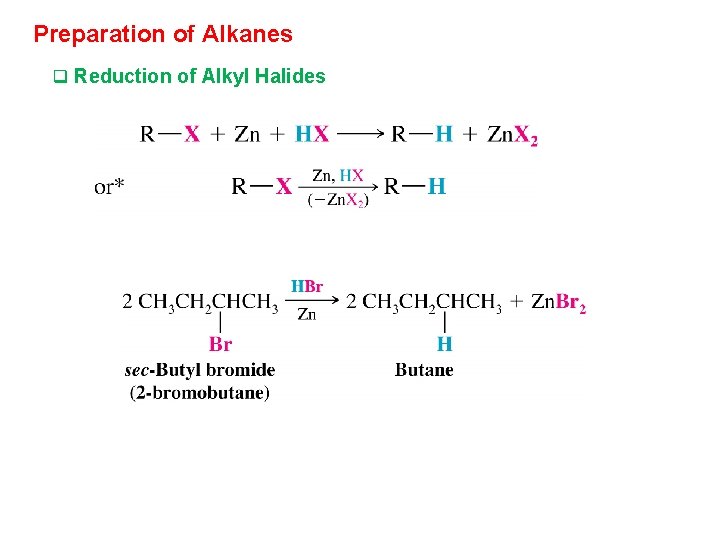
Preparation of Alkanes q Reduction of Alkyl Halides

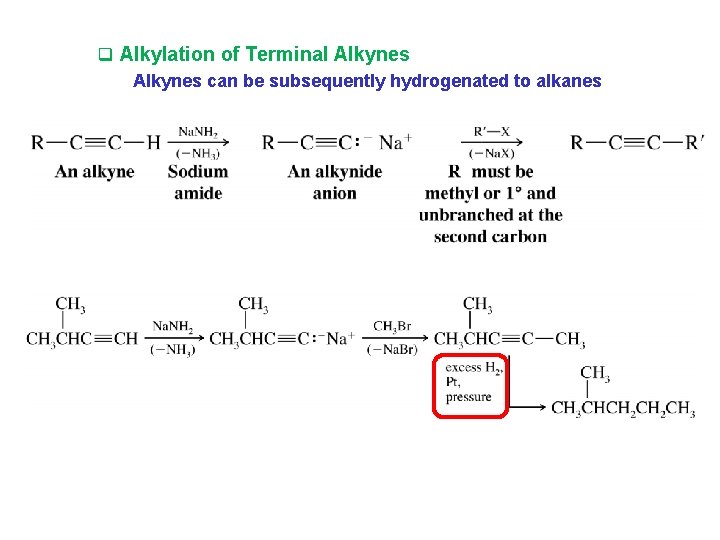
q Alkylation of Terminal Alkynes can be subsequently hydrogenated to alkanes


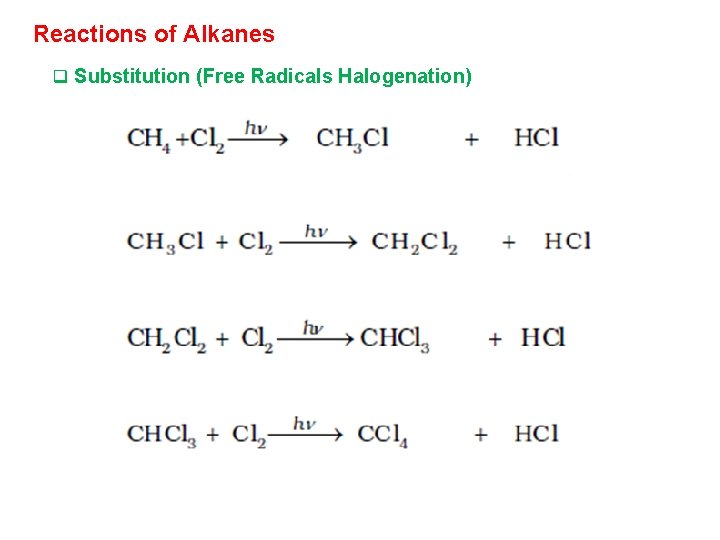
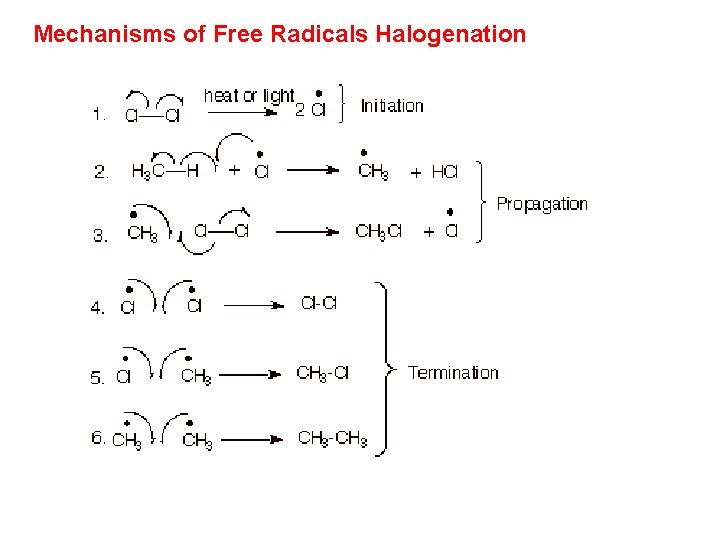
Reactions of Alkanes q Substitution (Free Radicals Halogenation)

Mechanisms of Free Radicals Halogenation

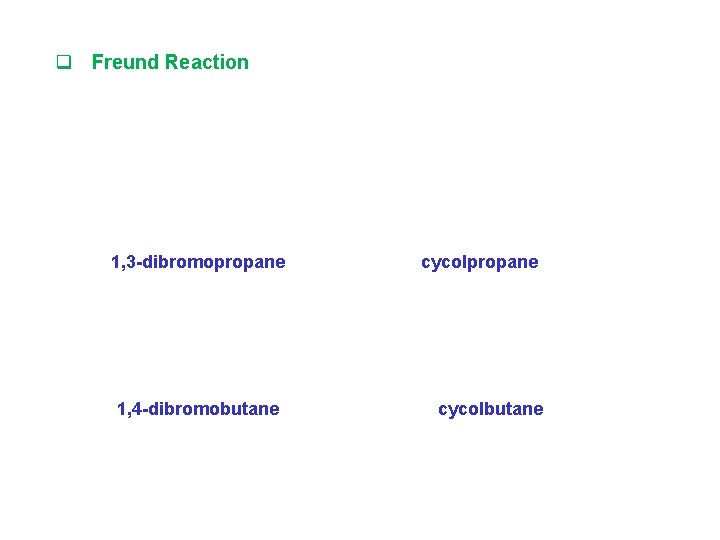
q Freund Reaction 1, 3 -dibromopropane 1, 4 -dibromobutane cycolpropane cycolbutane

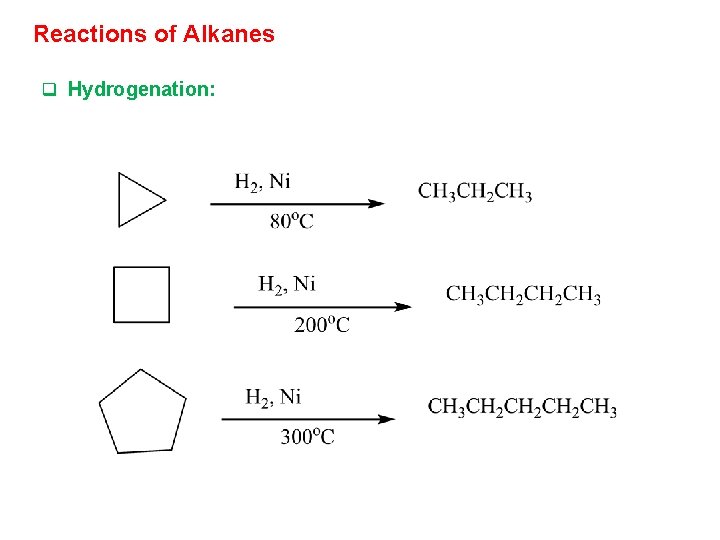
Reactions of Alkanes q Hydrogenation:
 Alkanes alkenes alkynes
Alkanes alkenes alkynes How to name compounds in organic chemistry
How to name compounds in organic chemistry Constitutional isomer vs stereoisomer
Constitutional isomer vs stereoisomer Propane conformers
Propane conformers Non superimposable mirror images are
Non superimposable mirror images are Ethane
Ethane Alkenes reactions and synthesis
Alkenes reactions and synthesis Halogenation of alkynes
Halogenation of alkynes Triple bond nomenclature
Triple bond nomenclature Is acid catalyzed hydration syn or anti
Is acid catalyzed hydration syn or anti Addition of hydrogen halides to alkynes
Addition of hydrogen halides to alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Mercury catalyzed hydration of alkynes
Mercury catalyzed hydration of alkynes Alkenes general formula
Alkenes general formula Hybridization of alkynes
Hybridization of alkynes Alkynes structural formula
Alkynes structural formula Preparation of alkynes
Preparation of alkynes Alkynes
Alkynes Dihydroxylation
Dihydroxylation Alkyne to alcohol
Alkyne to alcohol First 10 members of alkynes
First 10 members of alkynes Enolate
Enolate Combustion reaction of alkanes
Combustion reaction of alkanes Alkanes solubility
Alkanes solubility Saturated bond
Saturated bond Shortened structural formula
Shortened structural formula Uses of alkanes
Uses of alkanes Alkanes list
Alkanes list General formula for alkyl group
General formula for alkyl group 3-butyl-3-propyl-1-pentyne
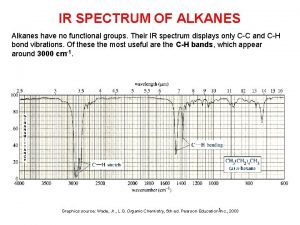
3-butyl-3-propyl-1-pentyne Ir spectra of alkanes
Ir spectra of alkanes Complete combustion of octane
Complete combustion of octane Alkanes def
Alkanes def Alkylation of alkanes
Alkylation of alkanes Alkane viscosity
Alkane viscosity Relative stability of isomeric alkanes
Relative stability of isomeric alkanes Iupac stands for
Iupac stands for Test for alkenes
Test for alkenes Eth met prop
Eth met prop Uses of alkanes
Uses of alkanes Addition polymerisation animation
Addition polymerisation animation Incomplete combustion of alkene
Incomplete combustion of alkene Reactions of alkenes 2 chemsheets
Reactions of alkenes 2 chemsheets Hobr addition to alkene
Hobr addition to alkene Properties of alkenes
Properties of alkenes Hybridization of alkenes
Hybridization of alkenes Name the following alkenes
Name the following alkenes Naming alkenes
Naming alkenes Alkene family
Alkene family What is ozonolysis give an example
What is ozonolysis give an example Alkenes introduction
Alkenes introduction What are the seven strong acids
What are the seven strong acids Taser 7 nomenclature
Taser 7 nomenclature Nomenclature of bolts
Nomenclature of bolts Oblique cutting example
Oblique cutting example Basic organic nomenclature packet
Basic organic nomenclature packet 3,5,8,11,16,19
3,5,8,11,16,19 Spring nomenclature
Spring nomenclature Army skl
Army skl E rigging supply
E rigging supply Remington 870 nomenclature
Remington 870 nomenclature Nucleotide nomenclature
Nucleotide nomenclature Pentose sugar structure in dna
Pentose sugar structure in dna Nomenclature of coordination compounds
Nomenclature of coordination compounds Ester nomenclature
Ester nomenclature Amine functional group
Amine functional group End mill nomenclature
End mill nomenclature Difference between up milling and down milling
Difference between up milling and down milling Entretoise echelle a crochet
Entretoise echelle a crochet Spiral cam
Spiral cam Handcuffing positions
Handcuffing positions Nomenclature poteau incendie
Nomenclature poteau incendie Discrete igbts
Discrete igbts Rules of binomial nomenclature
Rules of binomial nomenclature Taxonomic hierarchy of lion
Taxonomic hierarchy of lion Binomial nomenclature examples
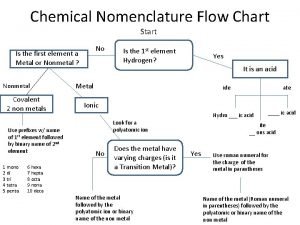
Binomial nomenclature examples Nomenclature flowchart
Nomenclature flowchart Naming amides
Naming amides Mixed nomenclature
Mixed nomenclature Naming acids
Naming acids Aerodynamic shape vs aerofoil shape
Aerodynamic shape vs aerofoil shape Taxonomic hierarchy mnemonic
Taxonomic hierarchy mnemonic Nomenclature of amide
Nomenclature of amide Alloy nomenclature
Alloy nomenclature Palindrome and mirror repeat
Palindrome and mirror repeat Alkene alcohol naming
Alkene alcohol naming Ester nomenclature
Ester nomenclature Cam nomenclature
Cam nomenclature Nomenclature of broaching tool
Nomenclature of broaching tool Binomial nomenclature examples
Binomial nomenclature examples Binomial nomenclature practice worksheet
Binomial nomenclature practice worksheet Nomenclature revit
Nomenclature revit Carbon nomenclature
Carbon nomenclature Example of alkanone
Example of alkanone Nomenclature acide carboxylique
Nomenclature acide carboxylique Acid nomenclature
Acid nomenclature Xxx police report
Xxx police report Honeywell gas valve nomenclature
Honeywell gas valve nomenclature Stairs nomenclature
Stairs nomenclature Gmdn udi
Gmdn udi Fisher-race nomenclature
Fisher-race nomenclature Pepperball training manual
Pepperball training manual Nomenclature in taxonomy
Nomenclature in taxonomy Heat exchanger duty
Heat exchanger duty Unsaturated hydrocarbon
Unsaturated hydrocarbon La nomenclature budgétaire
La nomenclature budgétaire Classification scheme of fungus and bacterium
Classification scheme of fungus and bacterium