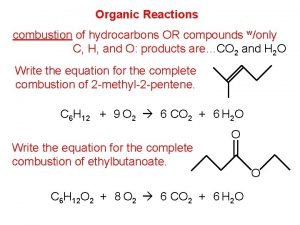
CH 3 Organic Compounds Alkanes and Their Stereochemistry











- Slides: 58

CH 3: Organic Compounds: Alkanes and Their Stereochemistry Renee Y. Becker CHM 2210 Valencia Community College 1

Why this Chapter • Alkanes are unreactive, but provide useful vehicle to introduce important ideas about organic compounds • Alkanes will be used to discuss basic approaches to naming organic compounds • We will take an initial look at 3 -D aspects of molecules 2

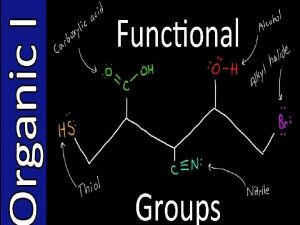
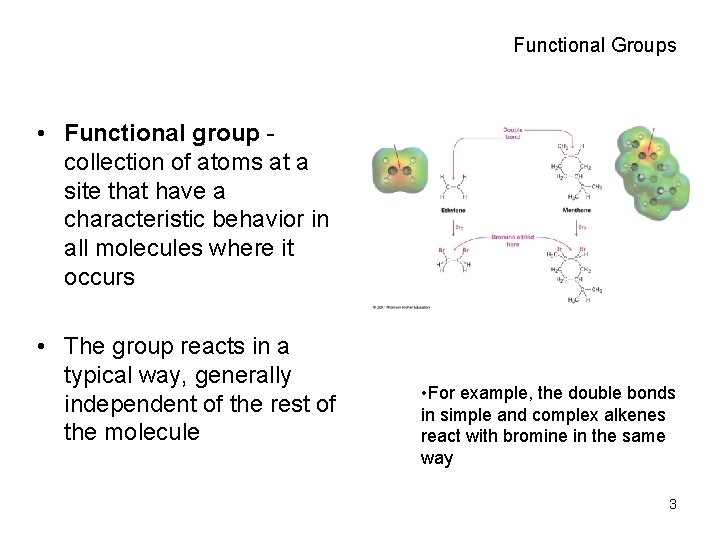
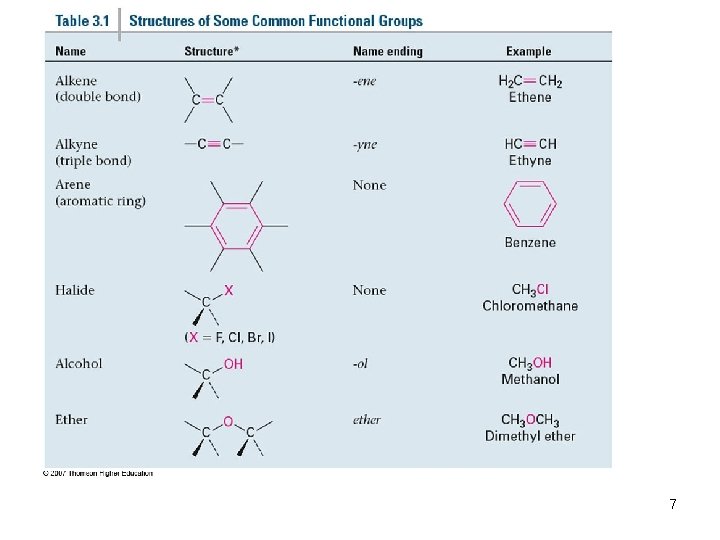
Functional Groups • Functional group collection of atoms at a site that have a characteristic behavior in all molecules where it occurs • The group reacts in a typical way, generally independent of the rest of the molecule • For example, the double bonds in simple and complex alkenes react with bromine in the same way 3

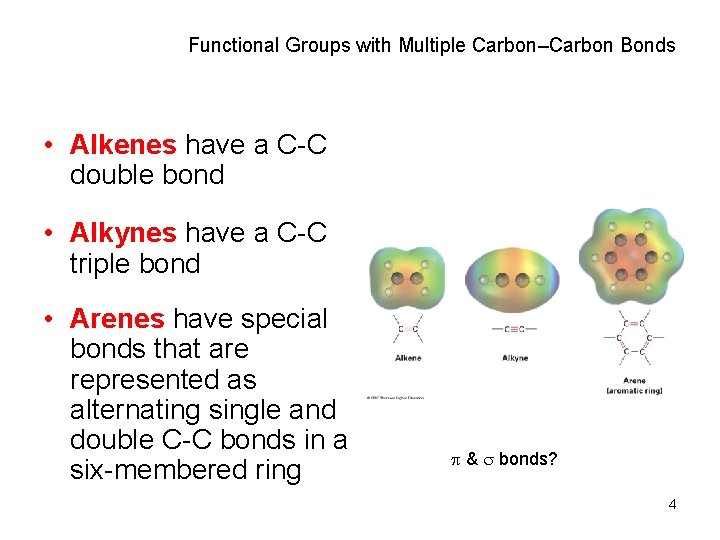
Functional Groups with Multiple Carbon–Carbon Bonds • Alkenes have a C-C double bond • Alkynes have a C-C triple bond • Arenes have special bonds that are represented as alternating single and double C-C bonds in a six-membered ring & bonds? 4

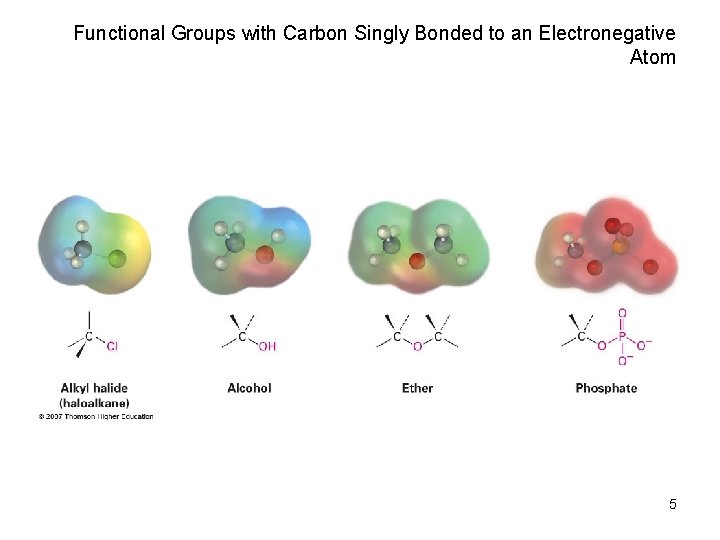
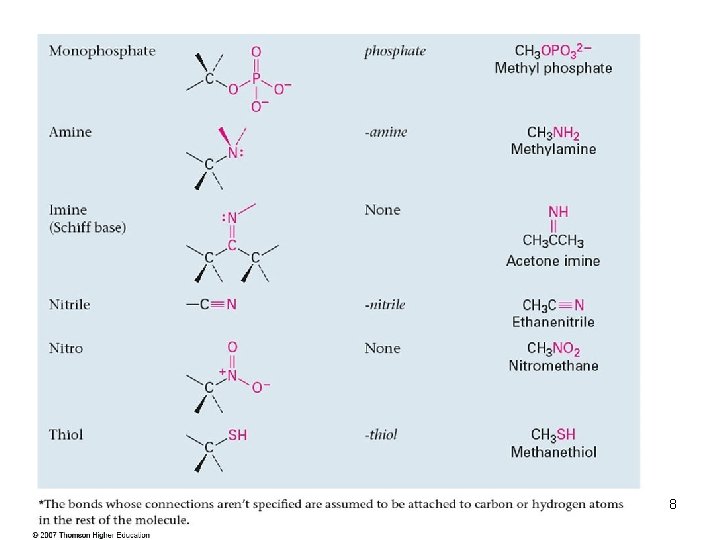
Functional Groups with Carbon Singly Bonded to an Electronegative Atom 5

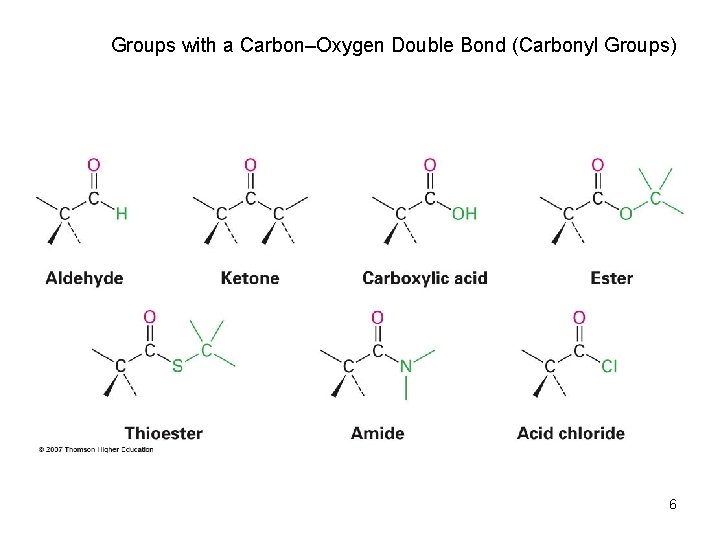
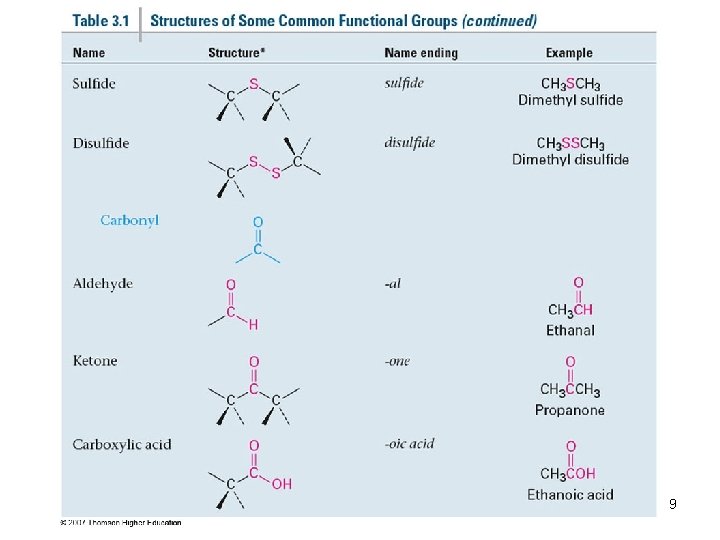
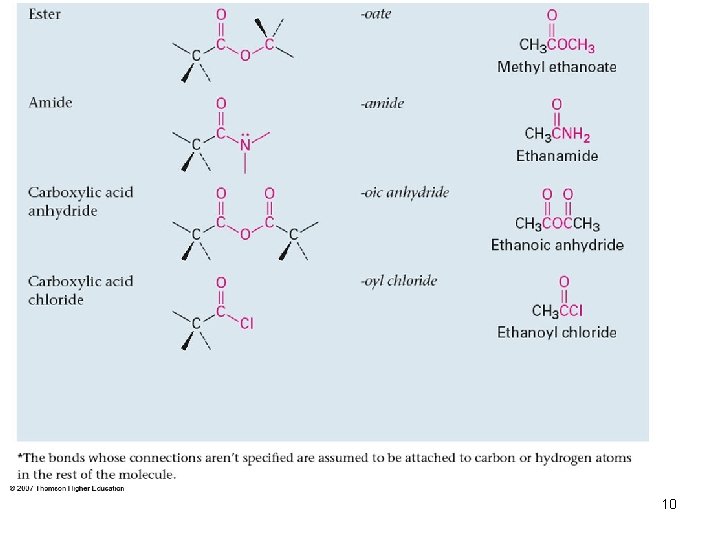
Groups with a Carbon–Oxygen Double Bond (Carbonyl Groups) 6

7

8

9

10

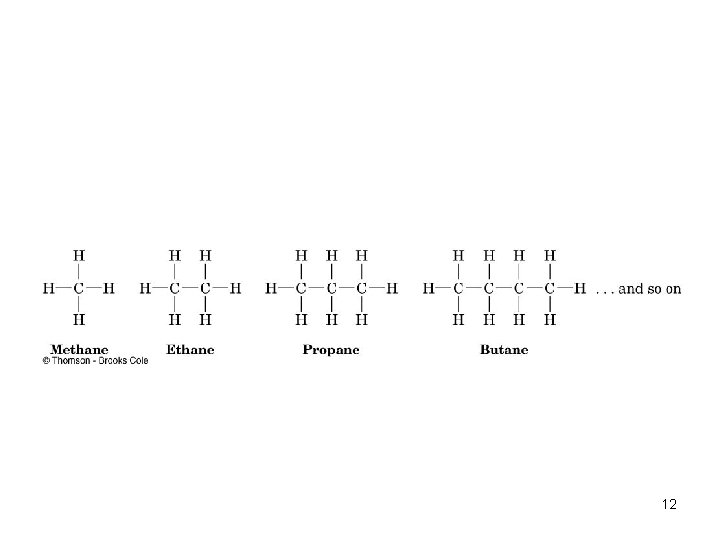
Alkanes • Alkanes: Compounds with C-C single bonds and C-H bonds only (no functional groups) • Connecting carbons can lead to large or small molecules • The formula for an alkane with no rings in it must be Cn. H 2 n+2 where the number of C’s is n • Alkanes are saturated with hydrogen (no more can be added • They are also called aliphatic compounds 11

12

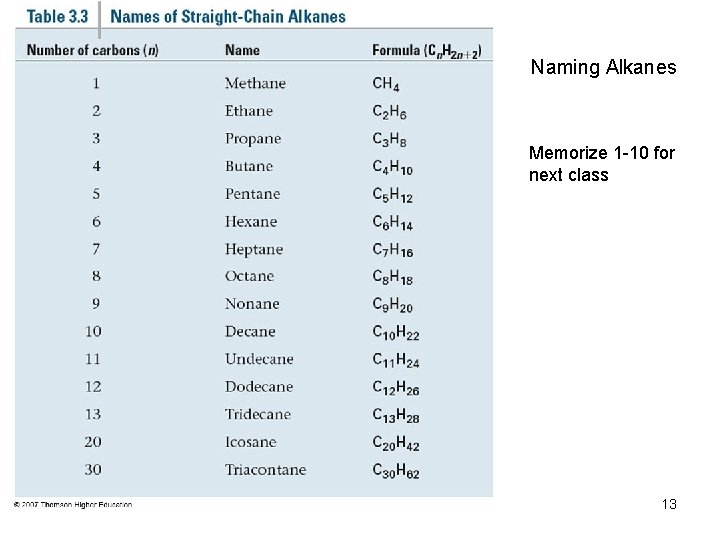
Naming Alkanes Memorize 1 -10 for next class 13

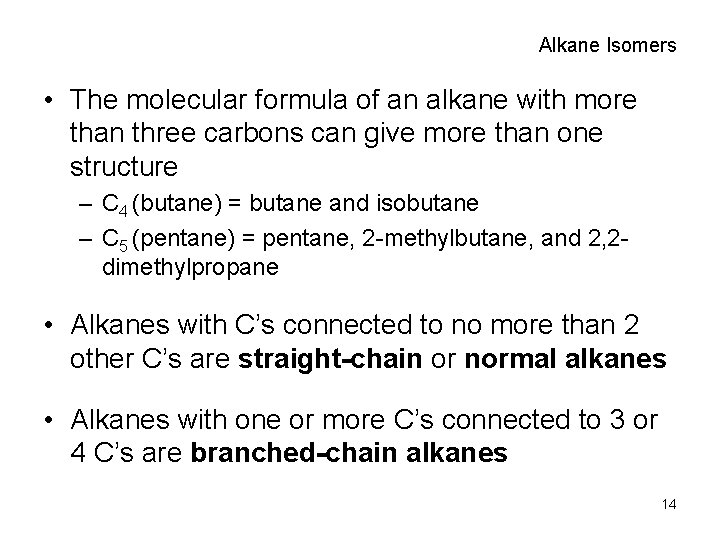
Alkane Isomers • The molecular formula of an alkane with more than three carbons can give more than one structure – C 4 (butane) = butane and isobutane – C 5 (pentane) = pentane, 2 -methylbutane, and 2, 2 dimethylpropane • Alkanes with C’s connected to no more than 2 other C’s are straight-chain or normal alkanes • Alkanes with one or more C’s connected to 3 or 4 C’s are branched-chain alkanes 14

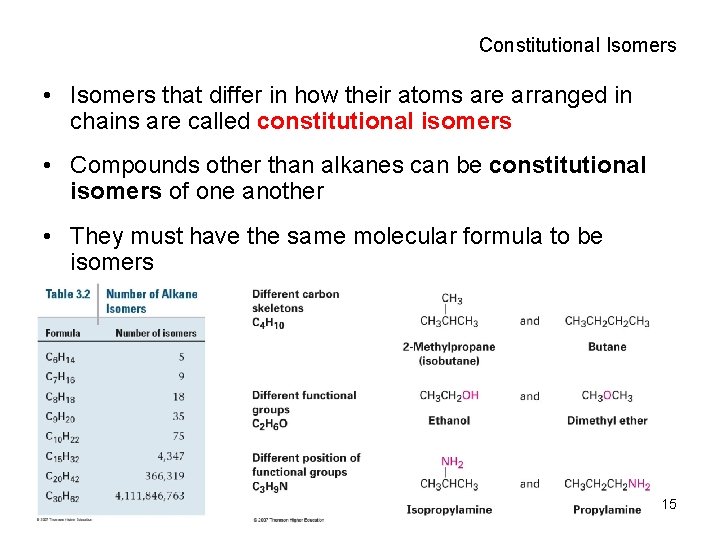
Constitutional Isomers • Isomers that differ in how their atoms are arranged in chains are called constitutional isomers • Compounds other than alkanes can be constitutional isomers of one another • They must have the same molecular formula to be isomers 15

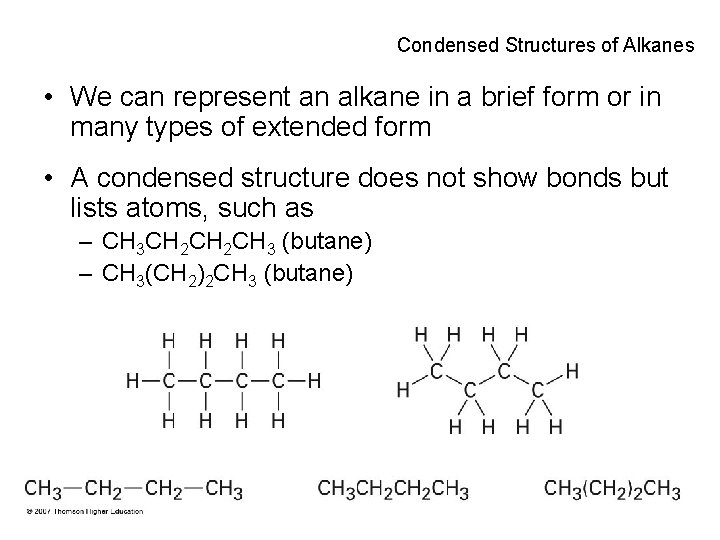
Condensed Structures of Alkanes • We can represent an alkane in a brief form or in many types of extended form • A condensed structure does not show bonds but lists atoms, such as – CH 3 CH 2 CH 3 (butane) – CH 3(CH 2)2 CH 3 (butane) 16
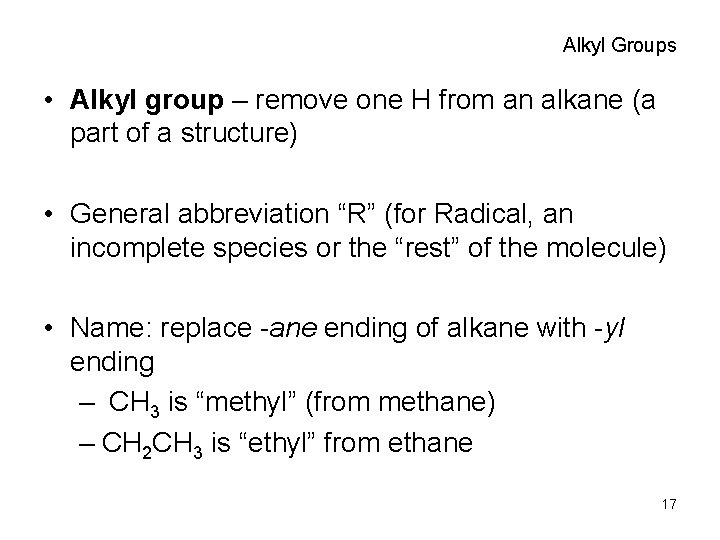
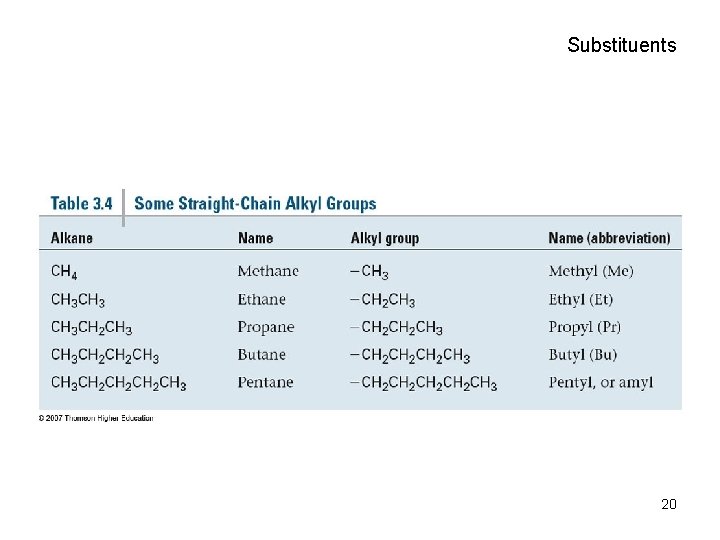
Alkyl Groups • Alkyl group – remove one H from an alkane (a part of a structure) • General abbreviation “R” (for Radical, an incomplete species or the “rest” of the molecule) • Name: replace -ane ending of alkane with -yl ending – CH 3 is “methyl” (from methane) – CH 2 CH 3 is “ethyl” from ethane 17

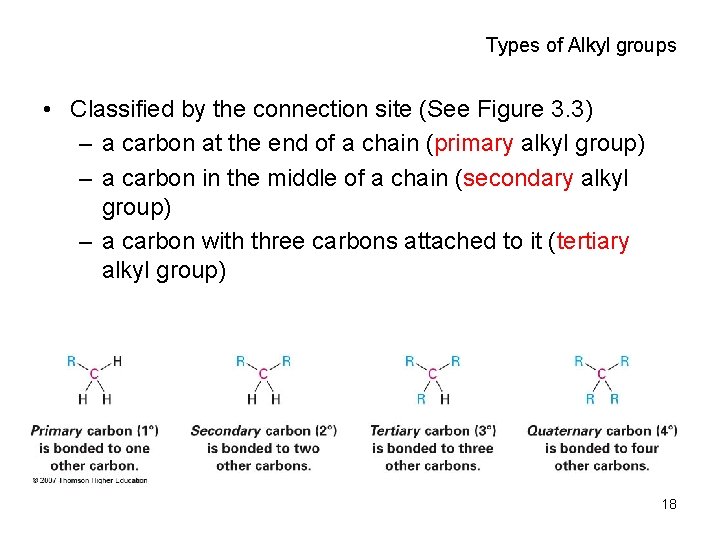
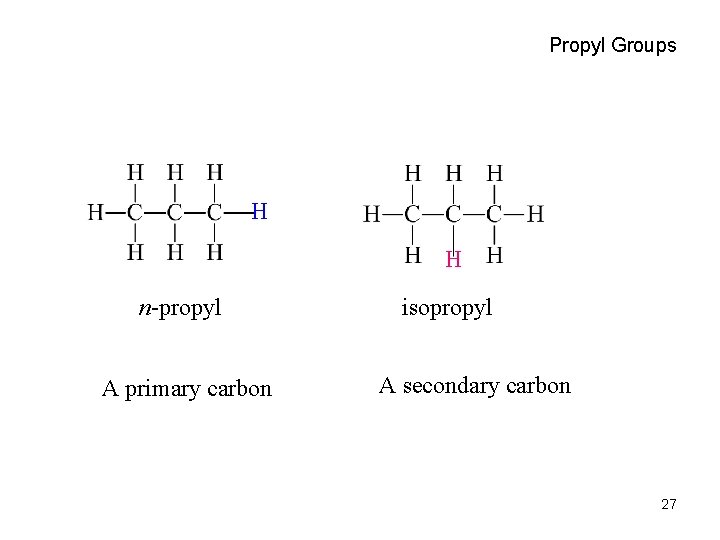
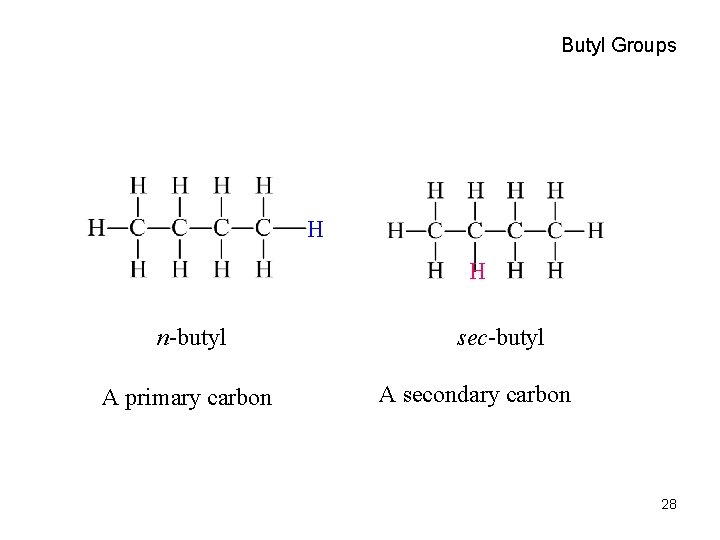
Types of Alkyl groups • Classified by the connection site (See Figure 3. 3) – a carbon at the end of a chain (primary alkyl group) – a carbon in the middle of a chain (secondary alkyl group) – a carbon with three carbons attached to it (tertiary alkyl group) 18

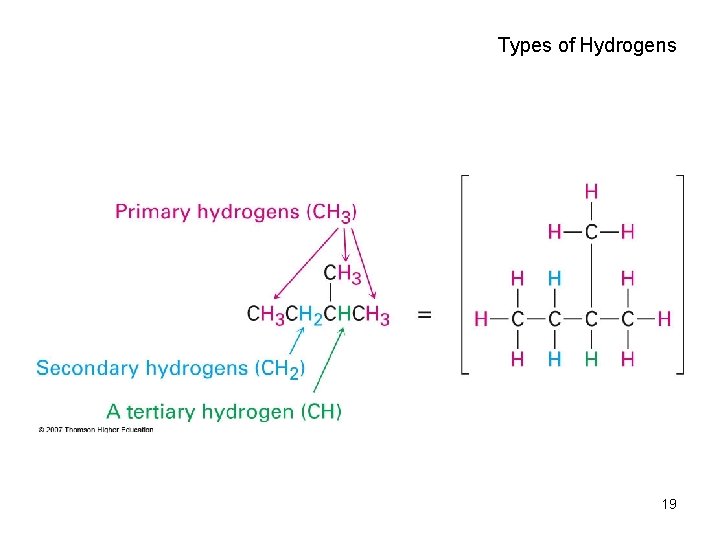
Types of Hydrogens 19

Substituents 20

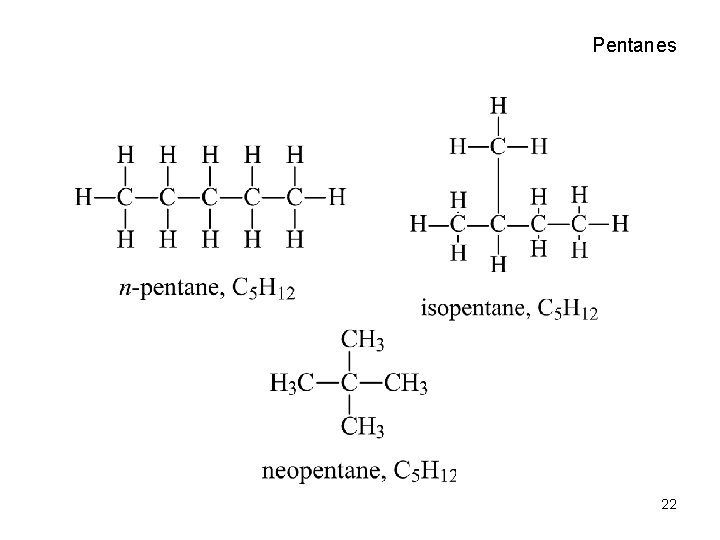
Common Names • Isobutane, “isomer of butane” • Isopentane, isohexane, etc. , methyl branch on next-to-last carbon in chain. • Neopentane, most highly branched • Five possible isomers of hexane, 18 isomers of octane and 75 for decane! 21

Pentanes 22

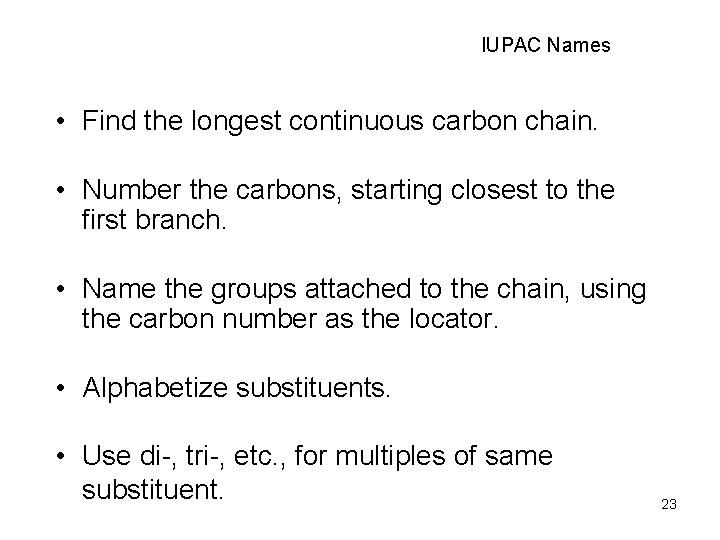
IUPAC Names • Find the longest continuous carbon chain. • Number the carbons, starting closest to the first branch. • Name the groups attached to the chain, using the carbon number as the locator. • Alphabetize substituents. • Use di-, tri-, etc. , for multiples of same substituent. 23

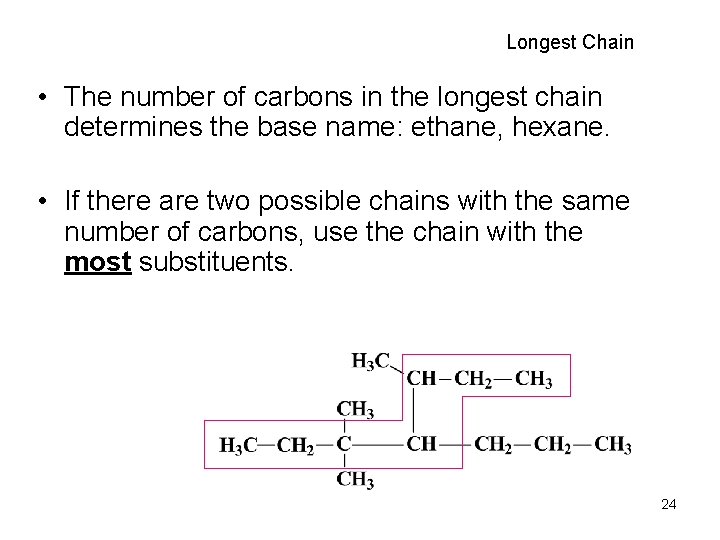
Longest Chain • The number of carbons in the longest chain determines the base name: ethane, hexane. • If there are two possible chains with the same number of carbons, use the chain with the most substituents. 24

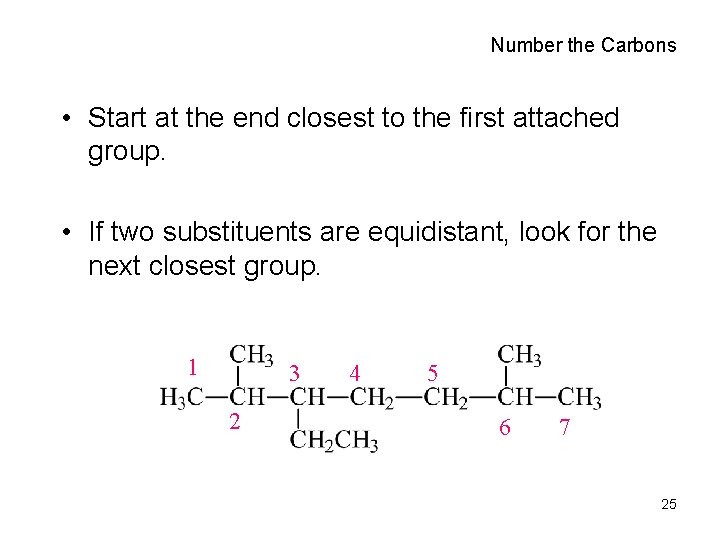
Number the Carbons • Start at the end closest to the first attached group. • If two substituents are equidistant, look for the next closest group. 1 3 2 4 5 6 7 25

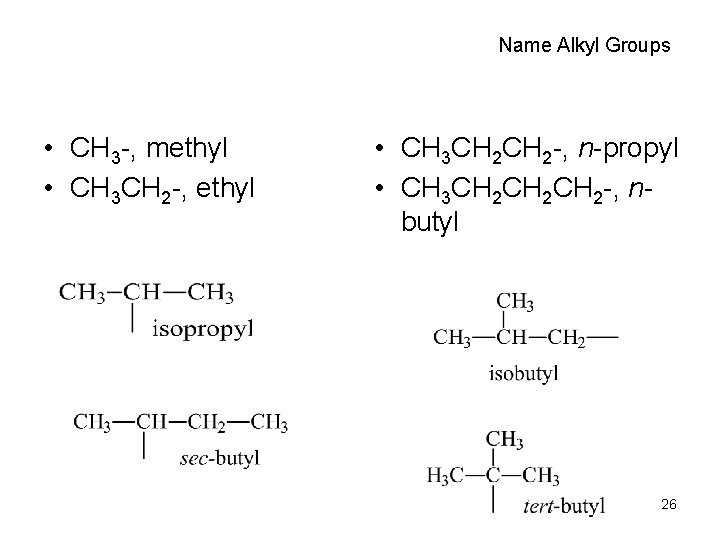
Name Alkyl Groups • CH 3 -, methyl • CH 3 CH 2 -, ethyl • CH 3 CH 2 -, n-propyl • CH 3 CH 2 CH 2 -, nbutyl 26

Propyl Groups H H n-propyl A primary carbon isopropyl A secondary carbon 27

Butyl Groups H H n-butyl A primary carbon sec-butyl A secondary carbon 28

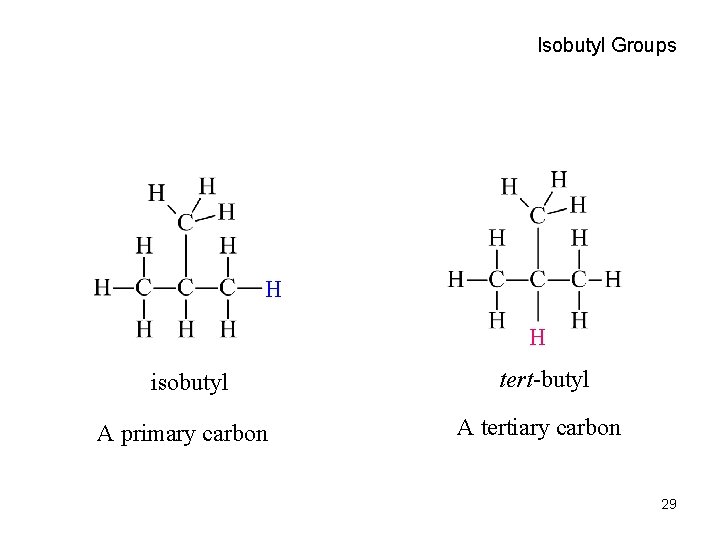
Isobutyl Groups H H isobutyl A primary carbon tert-butyl A tertiary carbon 29

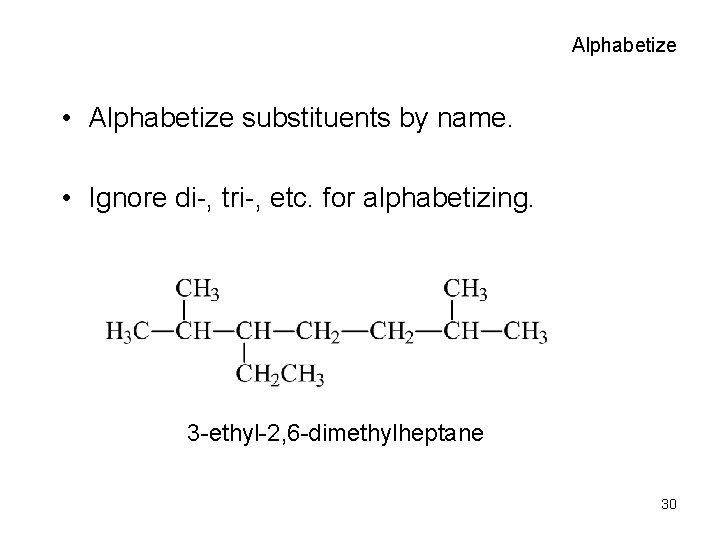
Alphabetize • Alphabetize substituents by name. • Ignore di-, tri-, etc. for alphabetizing. 3 -ethyl-2, 6 -dimethylheptane 30

Example 1 Write structures for the following: a) 3 -ethyl-3 -methylpentane b) 4 -t-butyl-2 -methylheptane c) 5 -isopropyl-3, 3, 4 -trimethyloctane d) 3 -ethyl-2, 4, 5 -trimethylheptane 31

Example 2 Provide IUPAC & common names (1 -3) 32

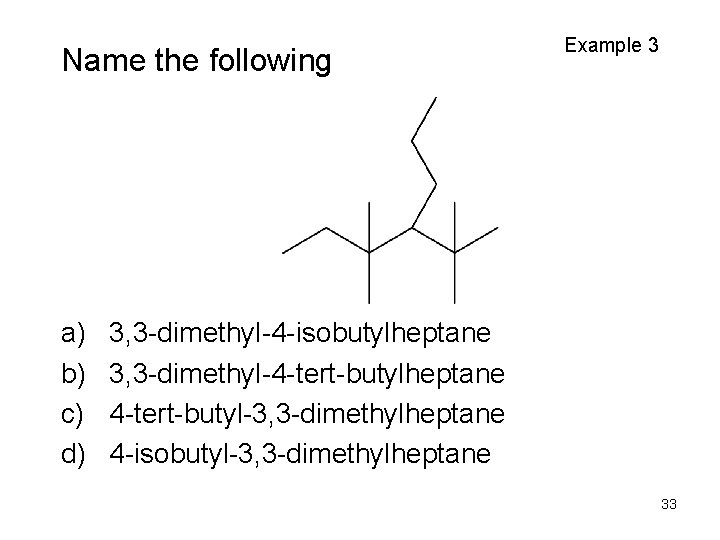
Name the following a) b) c) d) Example 3 3, 3 -dimethyl-4 -isobutylheptane 3, 3 -dimethyl-4 -tert-butylheptane 4 -tert-butyl-3, 3 -dimethylheptane 4 -isobutyl-3, 3 -dimethylheptane 33

Complex Substituents • If the branch has a branch, number the carbons from the point of attachment. • Name the branch off the branch using a locator number. • Parentheses are used around the complex branch name. • For alphabetizing use the first letter of the complex sub. Even if it is a numerical (di, tri, etc) 34

Complex Examples 35

Example 4 Draw the structures and give their more common names a) (1 -methyl) group b) (2 -methylpropyl) group c) (1 -methylpropyl) group d) (1, 1 -dimethyl) group 36

Example 5 Draw the structures a) 4 -(1 -methyl)heptane b) 5 -(1, 2, 2 -trimethylpropyl)nonane 37

Assignment • For next class draw the 9 isomers of heptane and name them 38

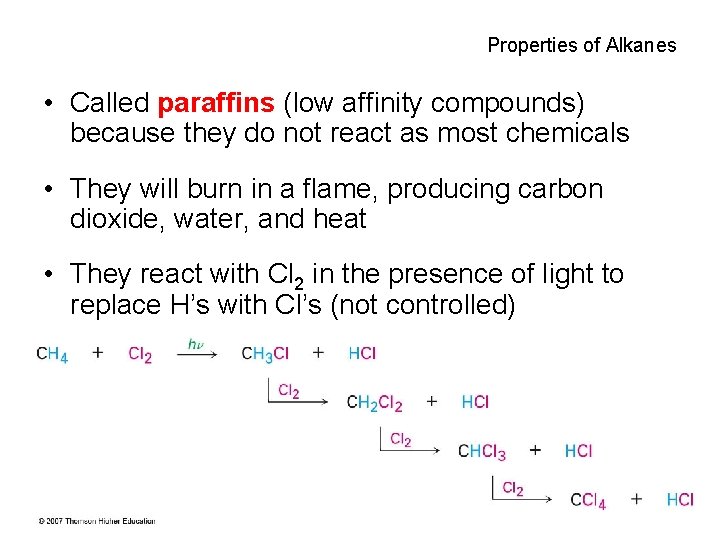
Properties of Alkanes • Called paraffins (low affinity compounds) because they do not react as most chemicals • They will burn in a flame, producing carbon dioxide, water, and heat • They react with Cl 2 in the presence of light to replace H’s with Cl’s (not controlled) 39

Physical Properties: Alkanes • Solubility: hydrophobic • Density: less than 1 g/m. L • Boiling points increase with increasing carbons (little less for branched chains). • Melting points increase with increasing carbons (less for odd-number of carbons). 40

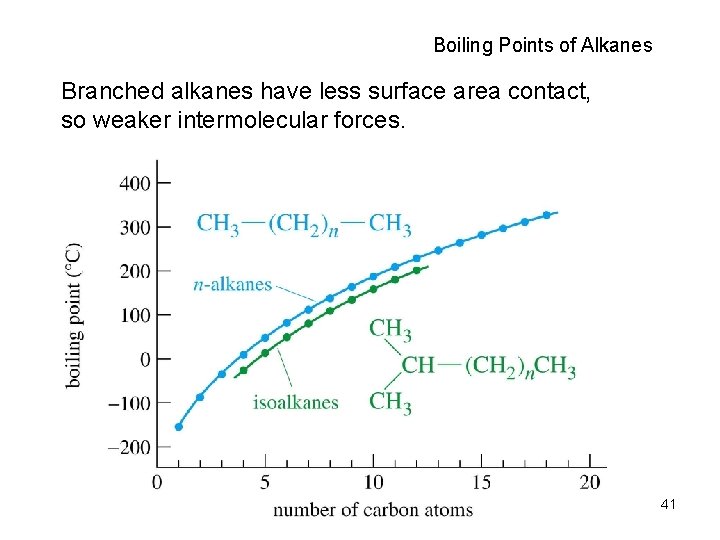
Boiling Points of Alkanes Branched alkanes have less surface area contact, so weaker intermolecular forces. 41

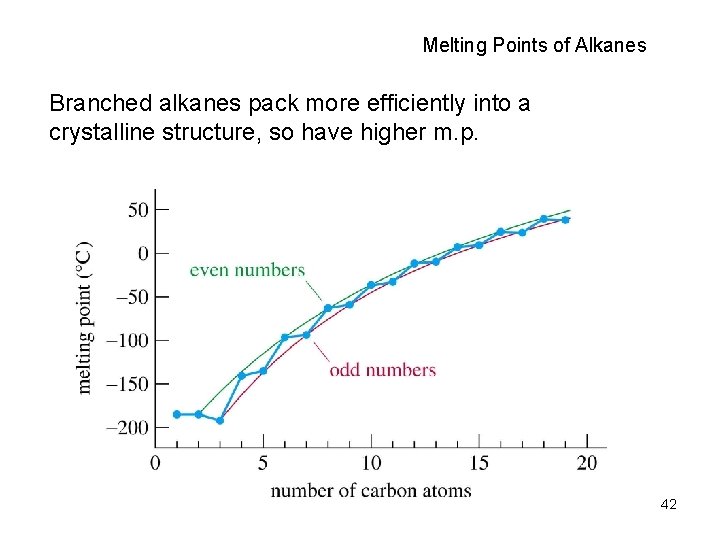
Melting Points of Alkanes Branched alkanes pack more efficiently into a crystalline structure, so have higher m. p. 42

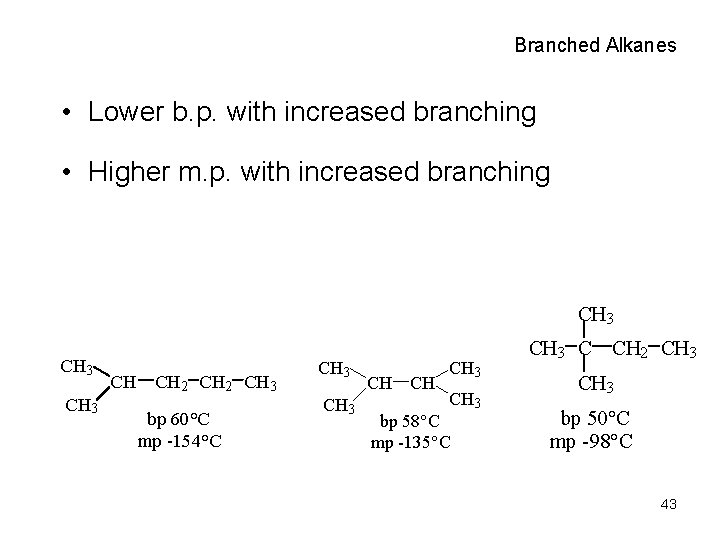
Branched Alkanes • Lower b. p. with increased branching • Higher m. p. with increased branching CH 3 CH CH 2 CH 3 bp 60°C mp -154°C CH 3 CH CH CH 3 bp 58°C mp -135°C CH 3 C CH 2 CH 3 bp 50°C mp -98°C 43

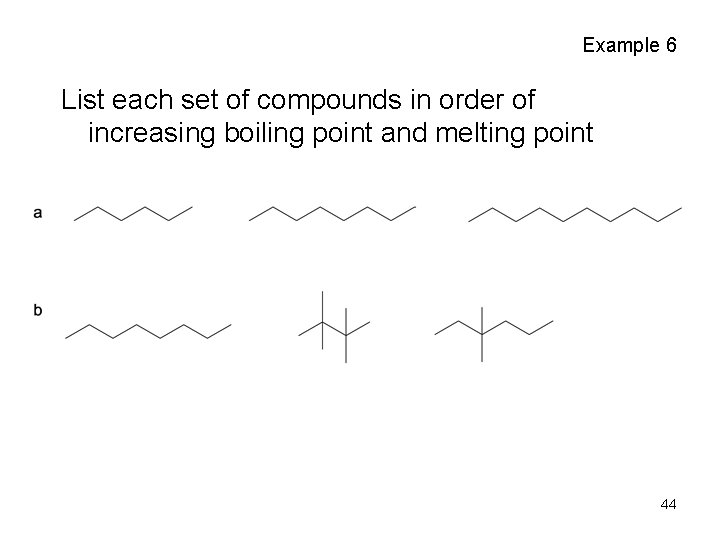
Example 6 List each set of compounds in order of increasing boiling point and melting point 44

Example 7 Which of the following has the highest boiling point? a) 3 -methylpentane b) 2, 2 -dimethylbutane c) Hexane d) Methane 45

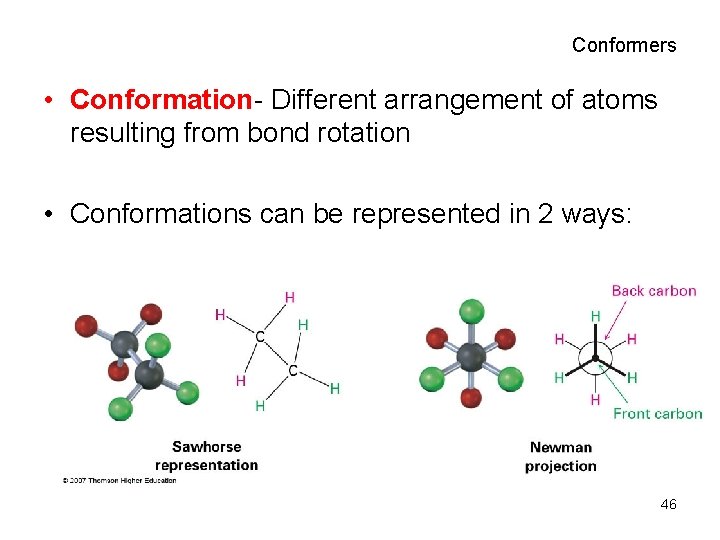
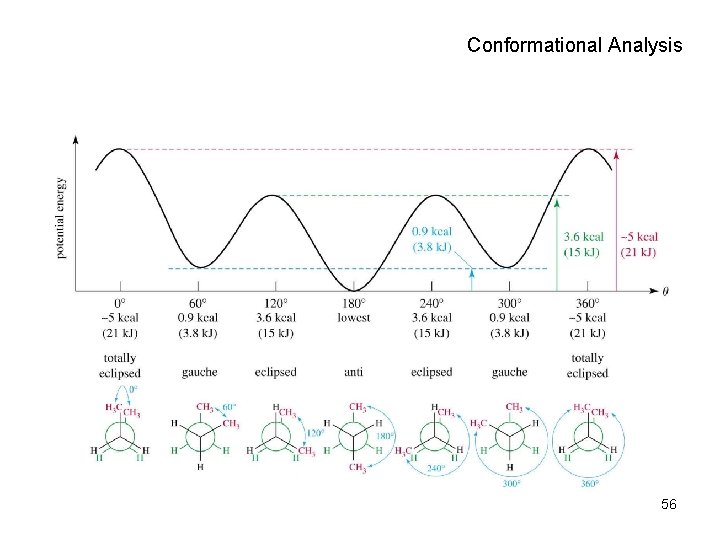
Conformers • Conformation- Different arrangement of atoms resulting from bond rotation • Conformations can be represented in 2 ways: 46

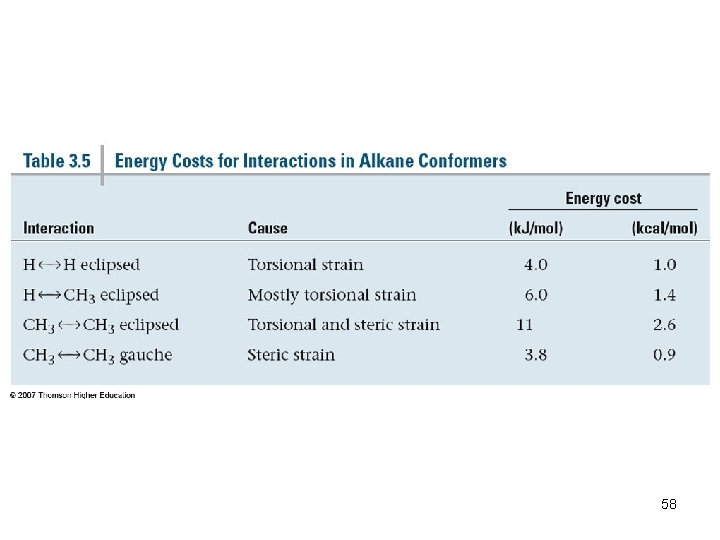
Torsional Strain • We do not observe perfectly free rotation • There is a barrier to rotation, and some conformers are more stable than others • Staggered- most stable: all 6 C-H bonds are as far away as possible • Eclipsed- least stable: all 6 C-H bonds are as close as possible to each other 47

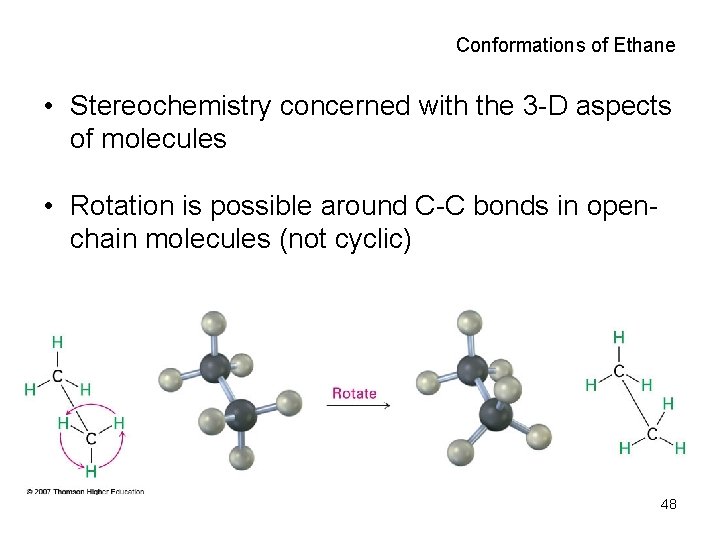
Conformations of Ethane • Stereochemistry concerned with the 3 -D aspects of molecules • Rotation is possible around C-C bonds in openchain molecules (not cyclic) 48

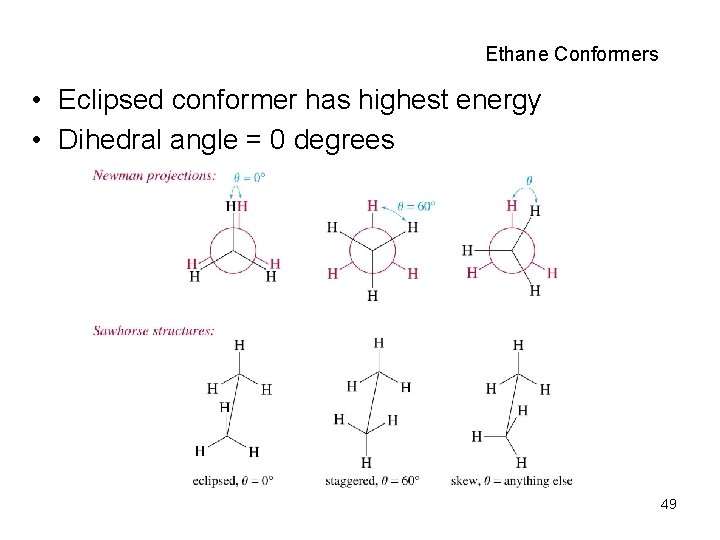
Ethane Conformers • Eclipsed conformer has highest energy • Dihedral angle = 0 degrees 49

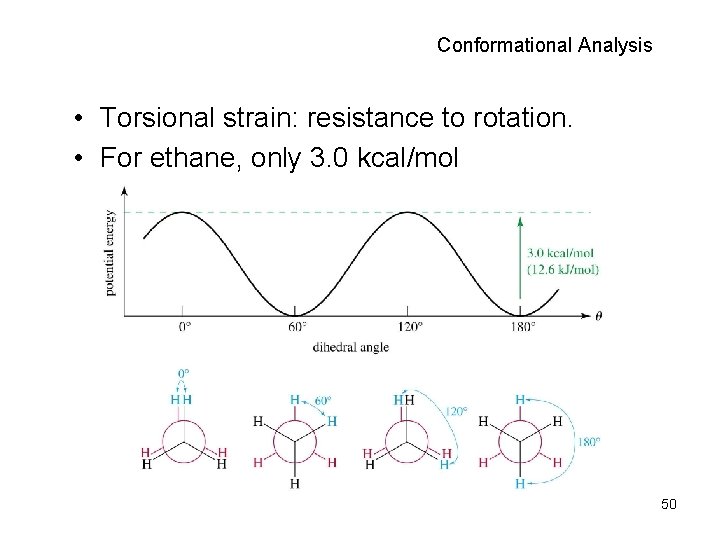
Conformational Analysis • Torsional strain: resistance to rotation. • For ethane, only 3. 0 kcal/mol 50

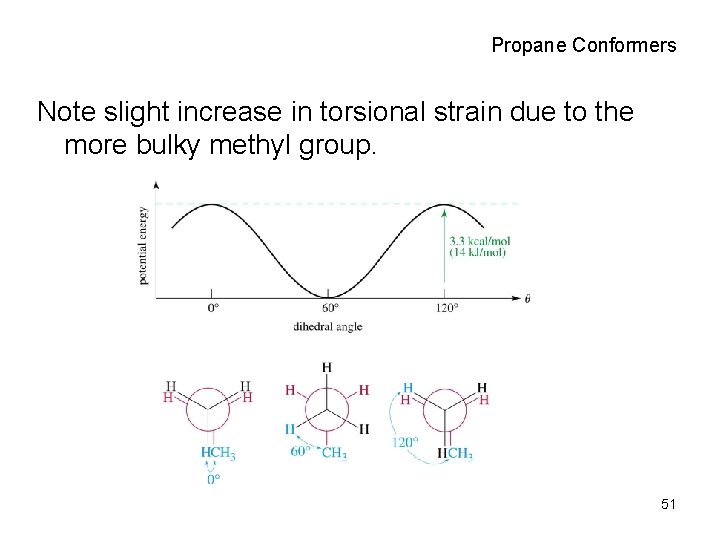
Propane Conformers Note slight increase in torsional strain due to the more bulky methyl group. 51

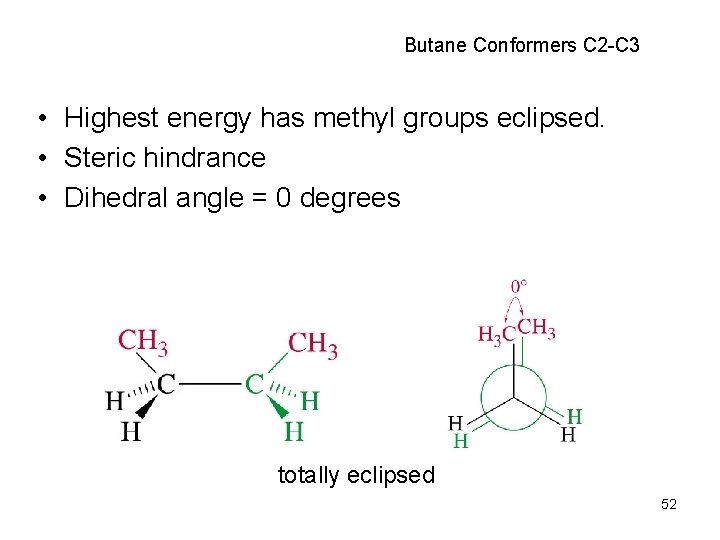
Butane Conformers C 2 -C 3 • Highest energy has methyl groups eclipsed. • Steric hindrance • Dihedral angle = 0 degrees totally eclipsed 52

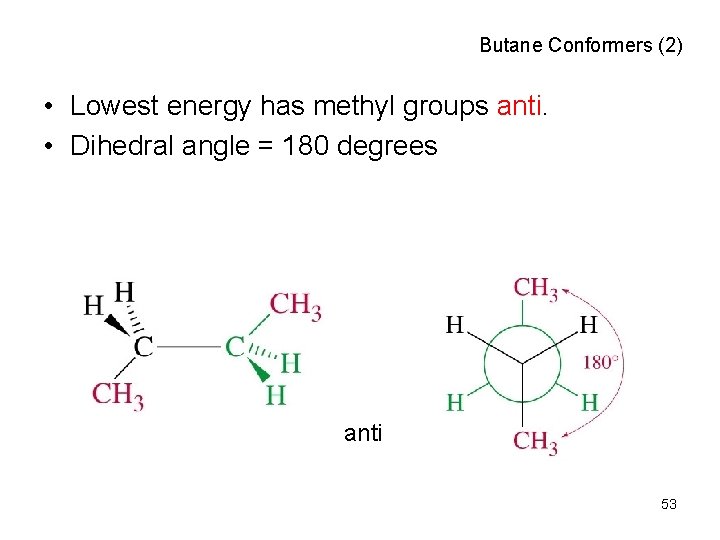
Butane Conformers (2) • Lowest energy has methyl groups anti. • Dihedral angle = 180 degrees anti 53

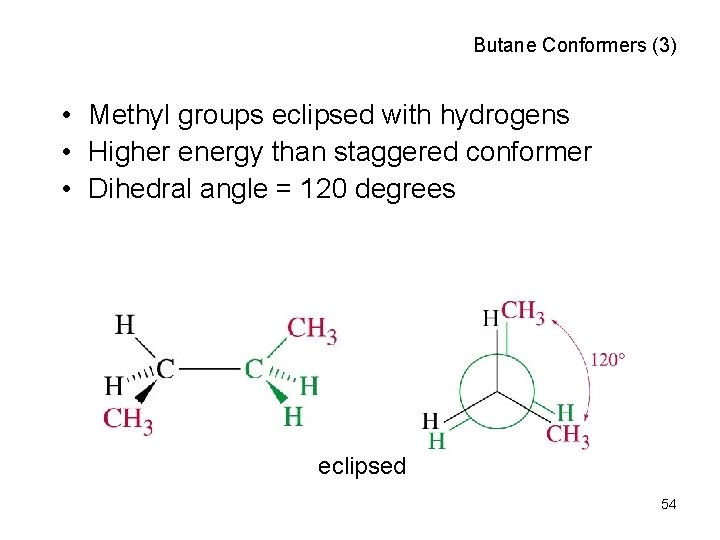
Butane Conformers (3) • Methyl groups eclipsed with hydrogens • Higher energy than staggered conformer • Dihedral angle = 120 degrees eclipsed 54

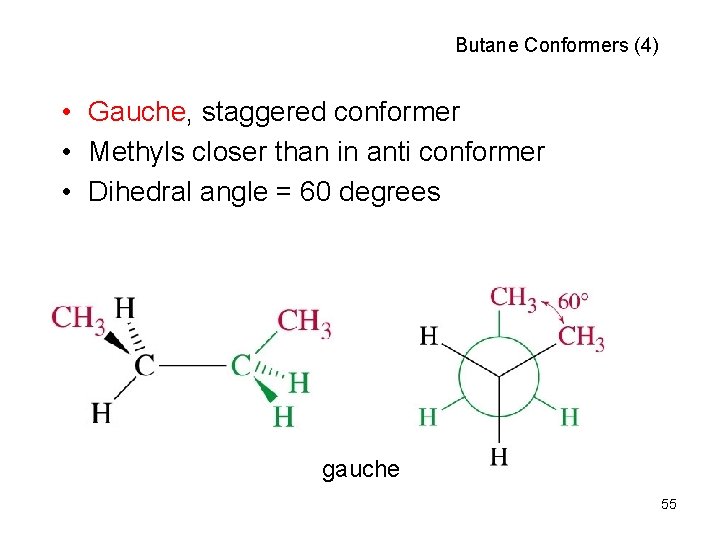
Butane Conformers (4) • Gauche, staggered conformer • Methyls closer than in anti conformer • Dihedral angle = 60 degrees gauche 55

Conformational Analysis 56

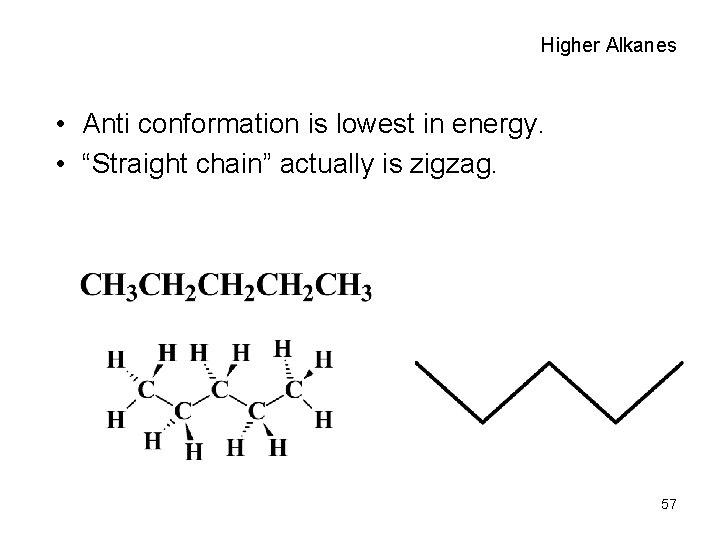
Higher Alkanes • Anti conformation is lowest in energy. • “Straight chain” actually is zigzag. 57

58
 Stereochemistry of alkanes and cycloalkanes
Stereochemistry of alkanes and cycloalkanes Cyclopentane cis trans isomers
Cyclopentane cis trans isomers Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Purification and characterization of organic compounds
Purification and characterization of organic compounds Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Metabolic changes of drugs and related organic compounds
Metabolic changes of drugs and related organic compounds All organic compounds contain carbon and ________.
All organic compounds contain carbon and ________. Covalent bond and ionic bond venn diagram
Covalent bond and ionic bond venn diagram What is protien
What is protien R vs s configuration
R vs s configuration Indicate the relationship of the pair of molecules shown.
Indicate the relationship of the pair of molecules shown. Father of stereochemistry
Father of stereochemistry Chiral vs achiral
Chiral vs achiral Stereochemistry
Stereochemistry Stereochemistry of cephalosporin
Stereochemistry of cephalosporin Introduction to stereochemistry
Introduction to stereochemistry How are vitamins absorbed
How are vitamins absorbed Vitamins classification chart
Vitamins classification chart The four types of organic compounds
The four types of organic compounds Decomposition of organic compounds
Decomposition of organic compounds Combustion reaction
Combustion reaction Organic compounds made by living things
Organic compounds made by living things All organic compounds must contain the element
All organic compounds must contain the element Organic compounds must contain
Organic compounds must contain In iupac naming priority order
In iupac naming priority order Organic vs inorganic compounds
Organic vs inorganic compounds Whats the difference between organic and inorganic
Whats the difference between organic and inorganic Organic vs inorganic compounds
Organic vs inorganic compounds What is the classification of organic compounds
What is the classification of organic compounds Carbon compound
Carbon compound Thin layer chromatography principle
Thin layer chromatography principle Pyranoses
Pyranoses Organic compounds grade 10 life science
Organic compounds grade 10 life science Organic halogen compounds
Organic halogen compounds Vitamins are organic compounds
Vitamins are organic compounds Families of organic compounds
Families of organic compounds Quantitative analysis of organic compounds ppt
Quantitative analysis of organic compounds ppt Type of organic compounds
Type of organic compounds Classification of organic compounds
Classification of organic compounds Prop but pent hex hept oct
Prop but pent hex hept oct Celiac beri beri
Celiac beri beri Organic compounds
Organic compounds Organic chemistry
Organic chemistry What is inorganic matter
What is inorganic matter Four types of organic compounds
Four types of organic compounds Alkanes solubility
Alkanes solubility Unbranched carbon chain
Unbranched carbon chain Alkane molecular formula
Alkane molecular formula Uses of alkanes
Uses of alkanes Alkanes list
Alkanes list General formula for alkyl group
General formula for alkyl group Alkanes alkenes alkynes
Alkanes alkenes alkynes Alkanes are also called
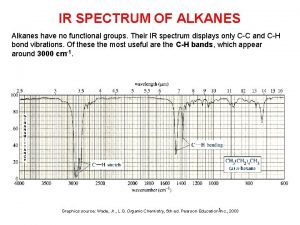
Alkanes are also called Ir spectra of alkanes
Ir spectra of alkanes Combustion reaction of alkanes
Combustion reaction of alkanes Combustion complete and incomplete
Combustion complete and incomplete Covalently def
Covalently def Alkylation of alkanes
Alkylation of alkanes Alkane viscosity
Alkane viscosity