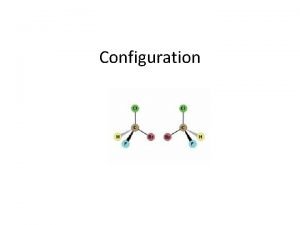
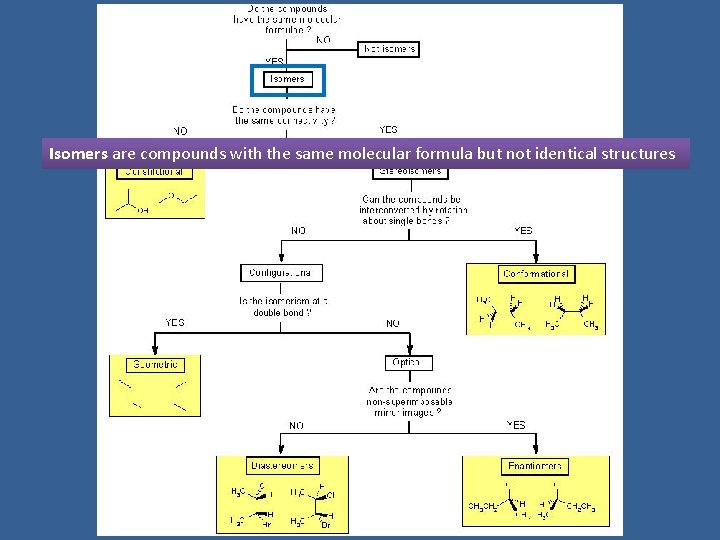
INTRODUCTION TO STEREOCHEMISTRY Tree of Stereochemistry Isomers are



- Slides: 52

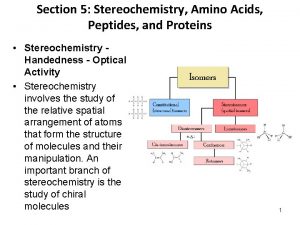
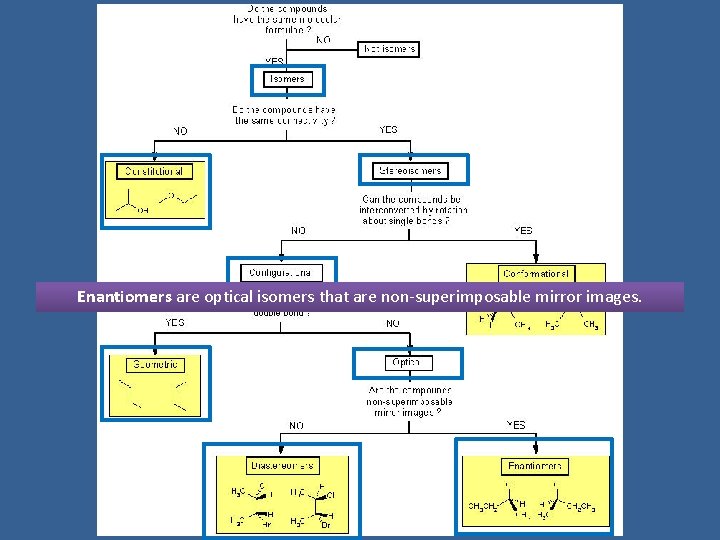
INTRODUCTION TO STEREOCHEMISTRY

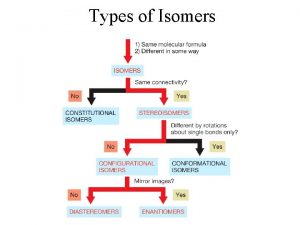
Tree of Stereochemistry

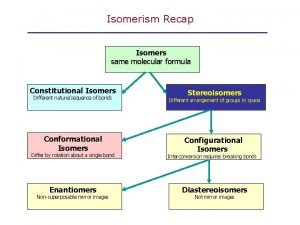
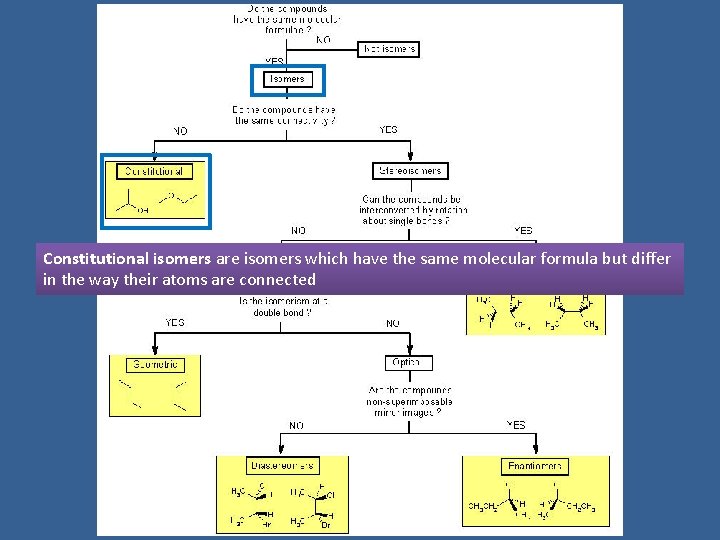
Isomers are compounds with the same molecular formula but not identical structures

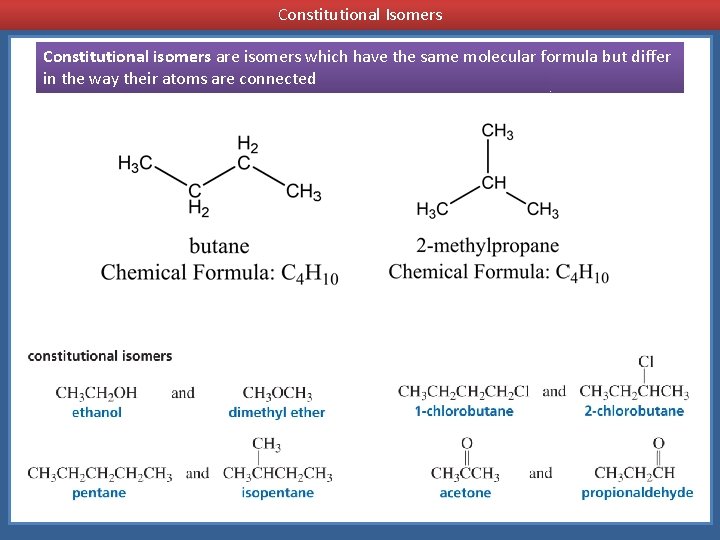
Constitutional isomers are isomers which have the same molecular formula but differ in the way their atoms are connected

Constitutional Isomers Constitutional isomers are isomers which have the same molecular formula but differ in the way their atoms are connected

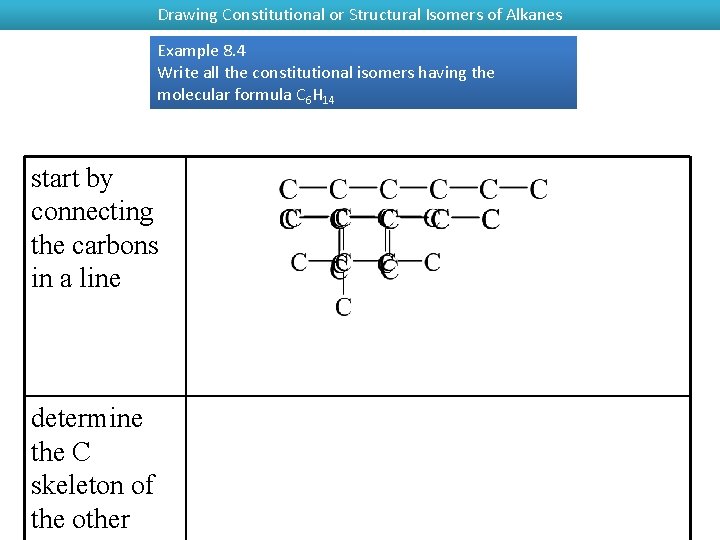
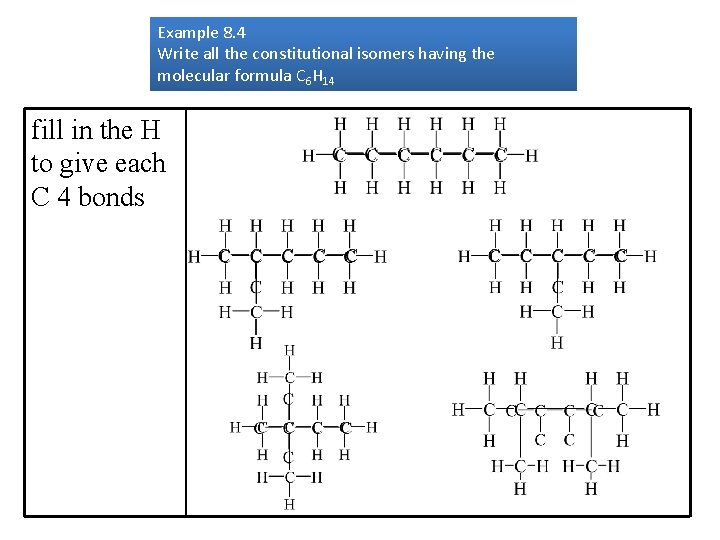
Drawing Constitutional or Structural Isomers of Alkanes Example 8. 4 Write all the constitutional isomers having the molecular formula C 6 H 14 start by connecting the carbons in a line determine the C skeleton of the other

Example 8. 4 Write all the constitutional isomers having the molecular formula C 6 H 14 fill in the H to give each C 4 bonds

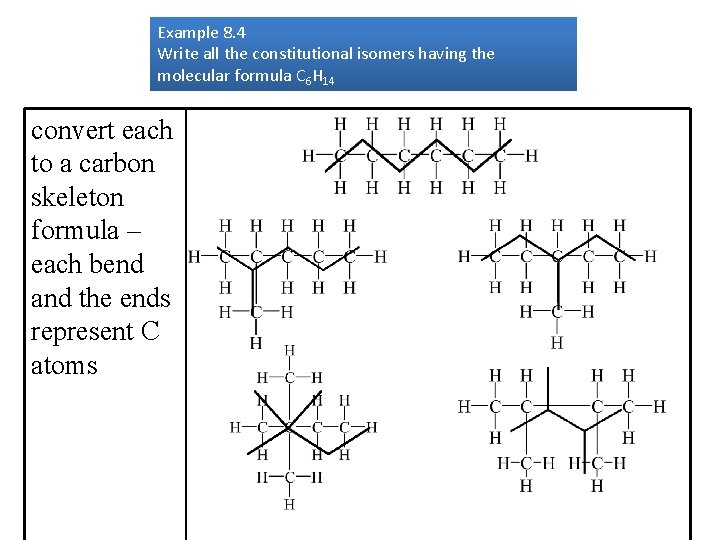
Example 8. 4 Write all the constitutional isomers having the molecular formula C 6 H 14 convert each to a carbon skeleton formula – each bend and the ends represent C atoms

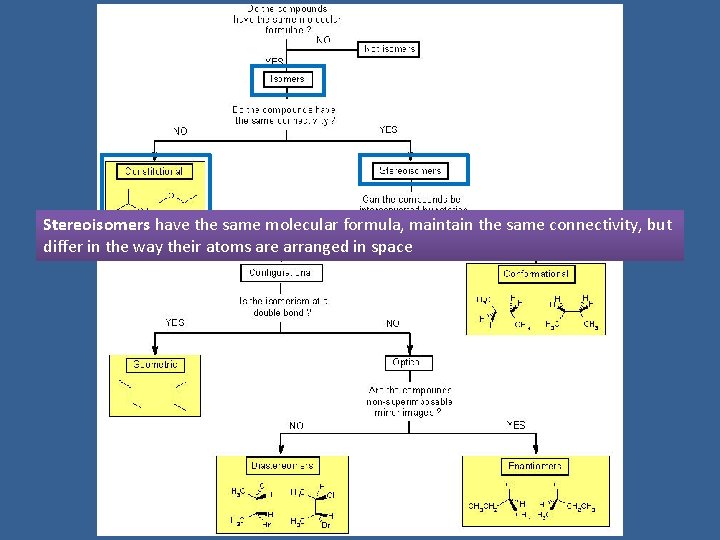
Stereoisomers have the same molecular formula, maintain the same connectivity, but differ in the way their atoms are arranged in space

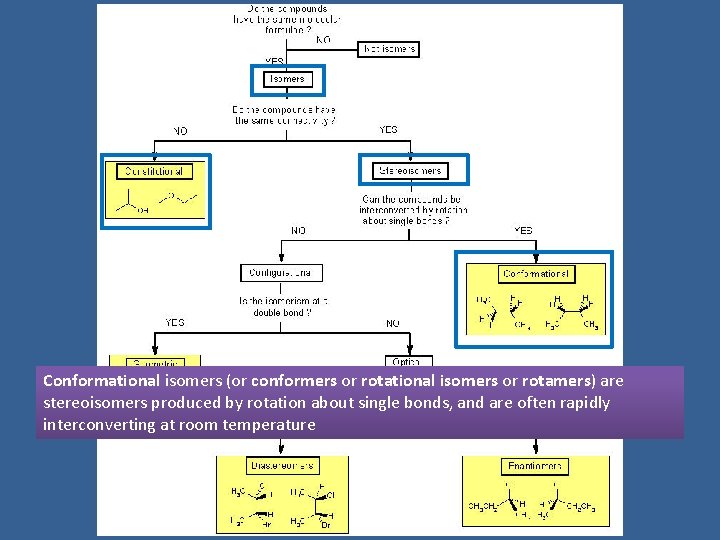
Conformational isomers (or conformers or rotational isomers or rotamers) are stereoisomers produced by rotation about single bonds, and are often rapidly interconverting at room temperature

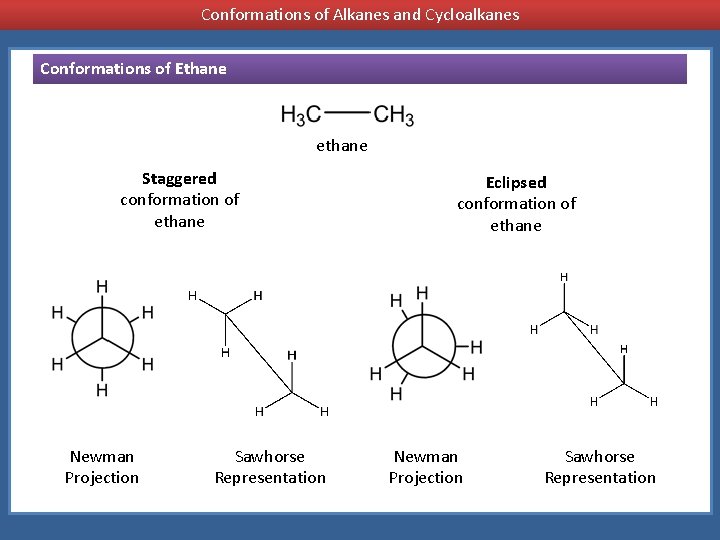
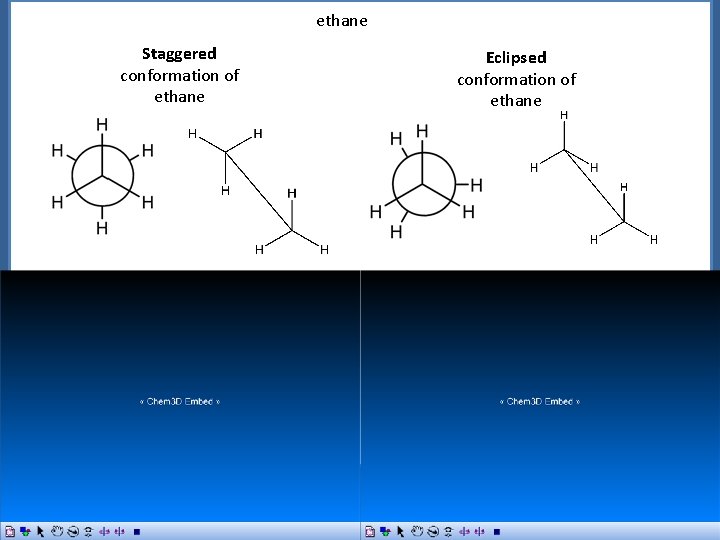
Conformations of Alkanes and Cycloalkanes Conformations of Ethane ethane Staggered conformation of ethane Newman Projection Sawhorse Representation Eclipsed conformation of ethane Newman Projection Sawhorse Representation

ethane Staggered conformation of ethane Newman Projection Sawhorse Representation Eclipsed conformation of ethane Newman Projection Sawhorse Representation

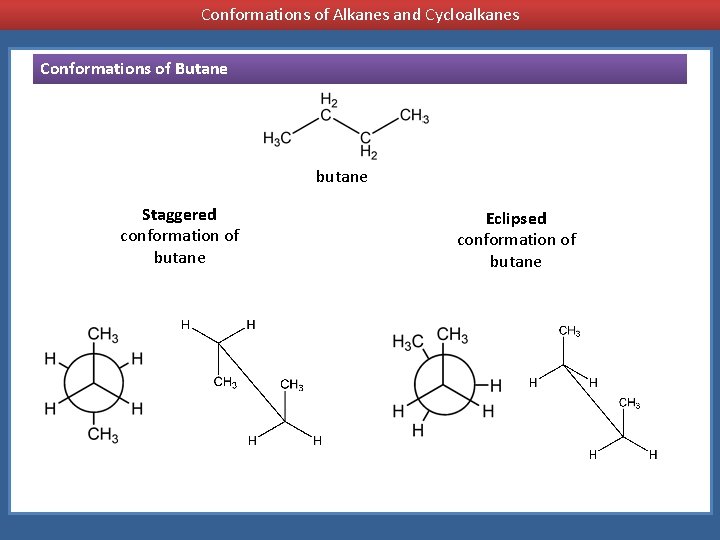
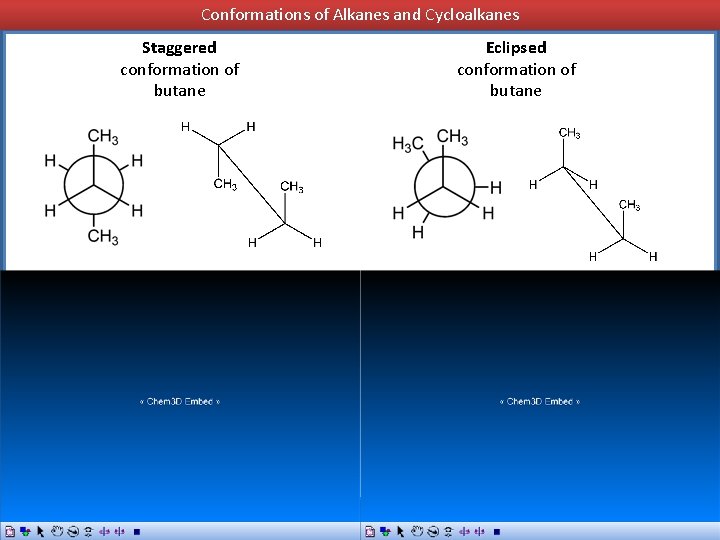
Conformations of Alkanes and Cycloalkanes Conformations of Butane butane Staggered conformation of butane Eclipsed conformation of butane

Conformations of Alkanes and Cycloalkanes Staggered conformation of butane Eclipsed conformation of butane

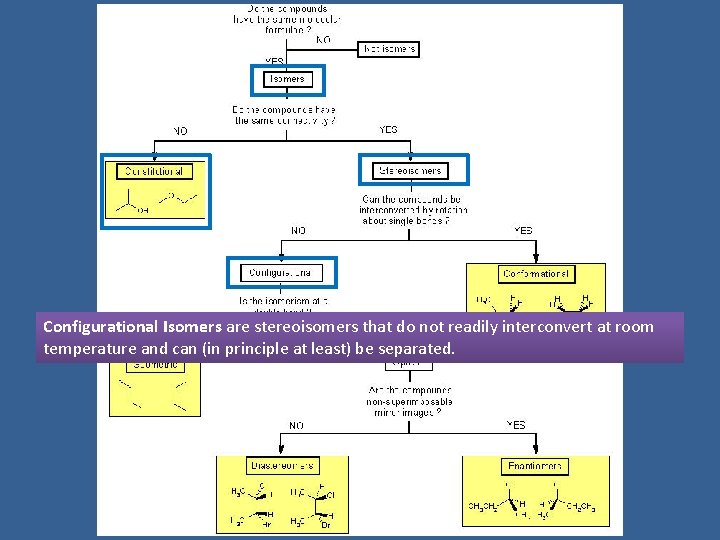
Configurational Isomers are stereoisomers that do not readily interconvert at room temperature and can (in principle at least) be separated.

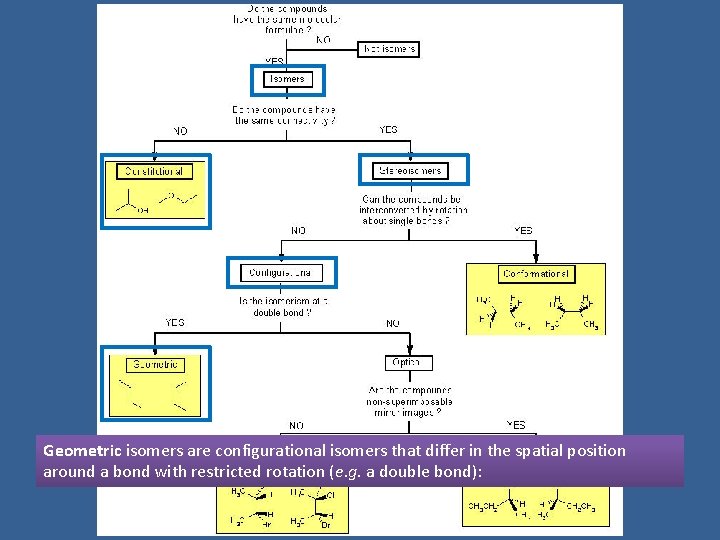
Geometric isomers are configurational isomers that differ in the spatial position around a bond with restricted rotation (e. g. a double bond):

Geometric (Cis and Trans) Isomers result from restriction rotation Compounds with double bonds cis isomer – have same substituents on the same side of the double bond (= Z with more complex molecules having high priority groups on the same side) trans isomer – have the same substituents on the opposite side of the double bond (= E with more complex molecules having high priority groups on opposite sides) Compounds with bonds in a ring: cis isomer – have the same substituents on the same side of the ring trans isomer - have the same substituents on the opposite side of the ring

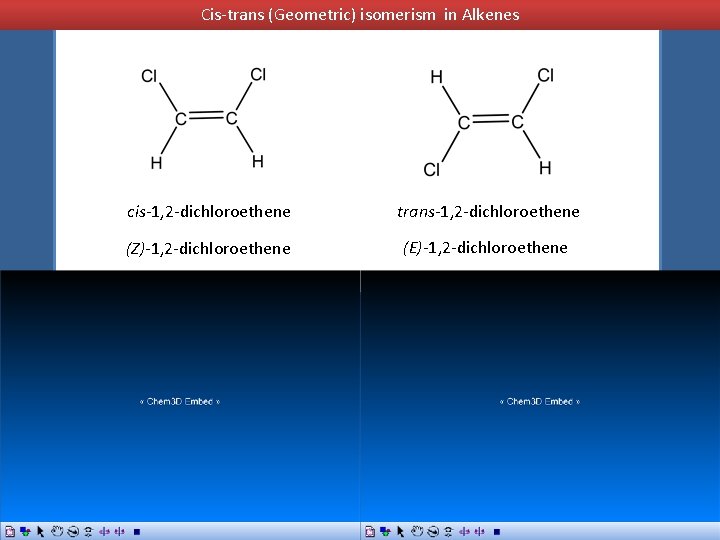
Cis-trans (Geometric) isomerism in Alkenes cis-1, 2 -dichloroethene trans-1, 2 -dichloroethene (Z)-1, 2 -dichloroethene (E)-1, 2 -dichloroethene

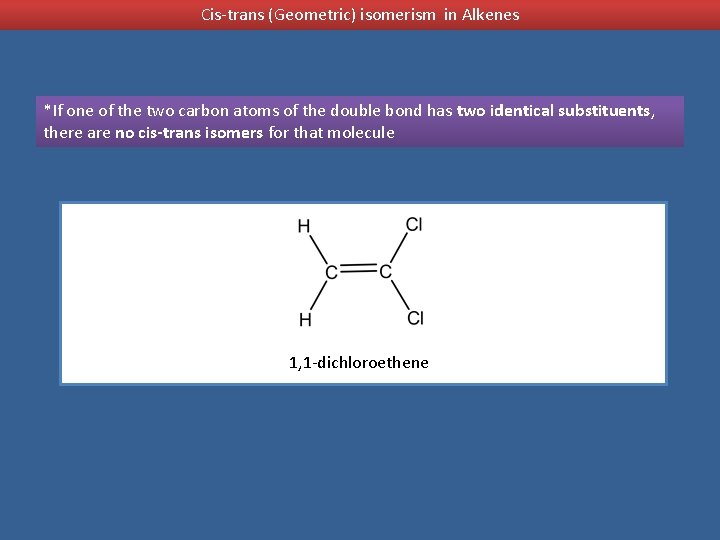
Cis-trans (Geometric) isomerism in Alkenes *If one of the two carbon atoms of the double bond has two identical substituents, there are no cis-trans isomers for that molecule 1, 1 -dichloroethene

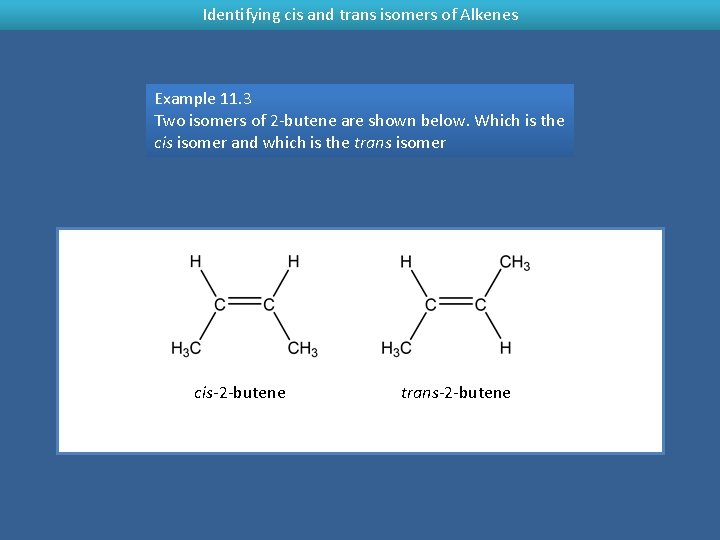
Identifying cis and trans isomers of Alkenes Example 11. 3 Two isomers of 2 -butene are shown below. Which is the cis isomer and which is the trans isomer cis-2 -butene trans-2 -butene

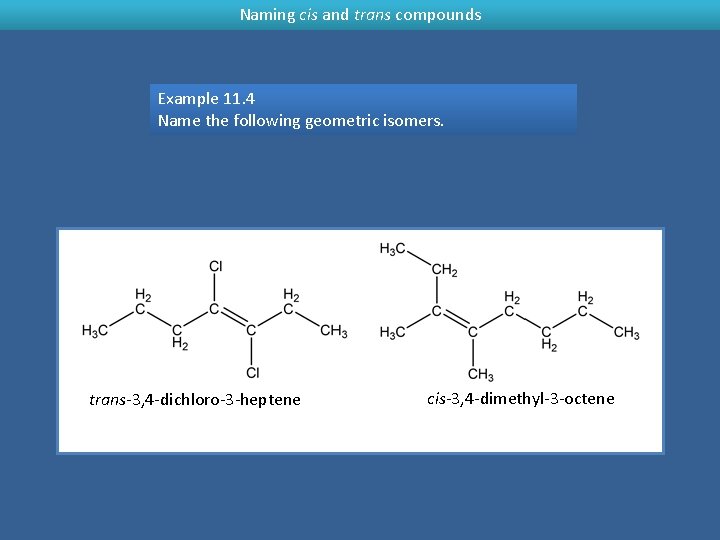
Naming cis and trans compounds Example 11. 4 Name the following geometric isomers. trans-3, 4 -dichloro-3 -heptene cis-3, 4 -dimethyl-3 -octene

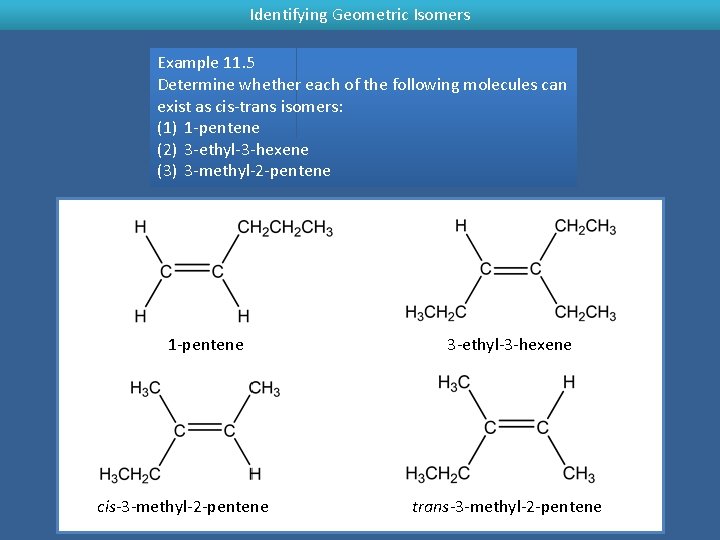
Identifying Geometric Isomers Example 11. 5 Determine whether each of the following molecules can exist as cis-trans isomers: (1) 1 -pentene (2) 3 -ethyl-3 -hexene (3) 3 -methyl-2 -pentene 1 -pentene cis-3 -methyl-2 -pentene 3 -ethyl-3 -hexene trans-3 -methyl-2 -pentene

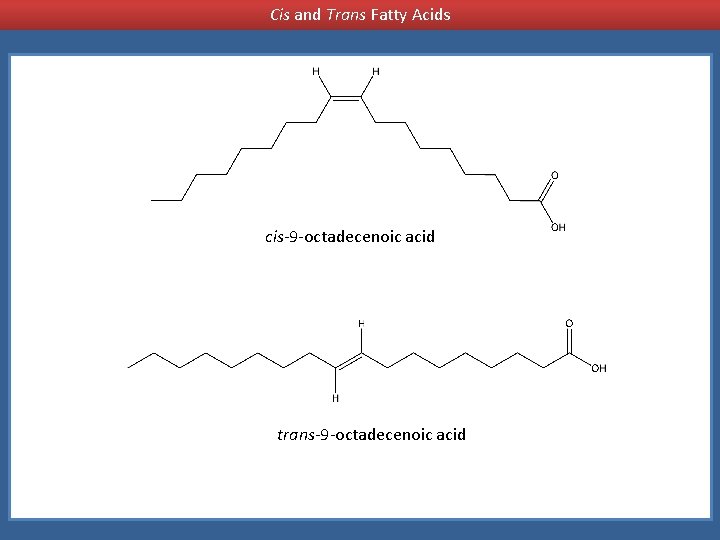
Cis and Trans Fatty Acids cis-9 -octadecenoic acid trans-9 -octadecenoic acid

Cis and Trans Fatty Acids

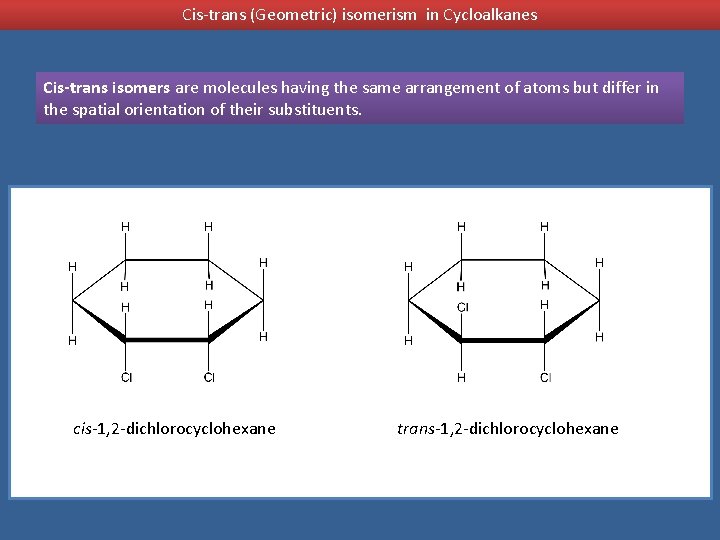
Cis-trans (Geometric) isomerism in Cycloalkanes Cis-trans isomers are molecules having the same arrangement of atoms but differ in the spatial orientation of their substituents. cis-1, 2 -dichlorocyclohexane trans-1, 2 -dichlorocyclohexane

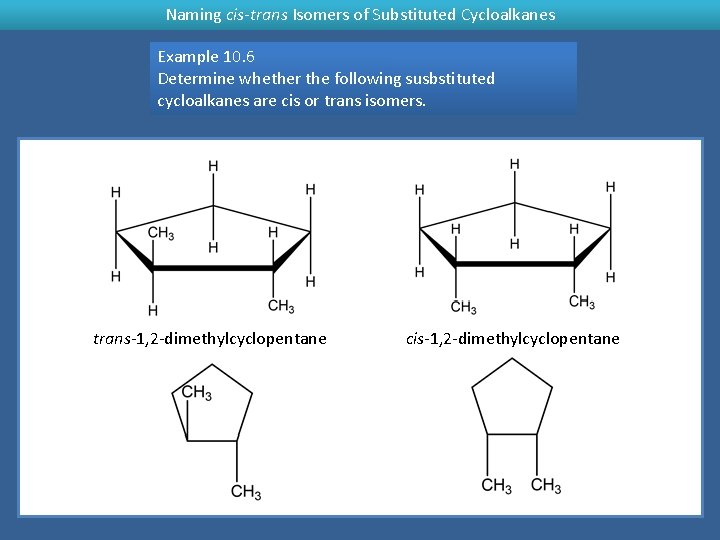
Naming cis-trans Isomers of Substituted Cycloalkanes Example 10. 6 Determine whether the following susbstituted cycloalkanes are cis or trans isomers. trans-1, 2 -dimethylcyclopentane cis-1, 2 -dimethylcyclopentane

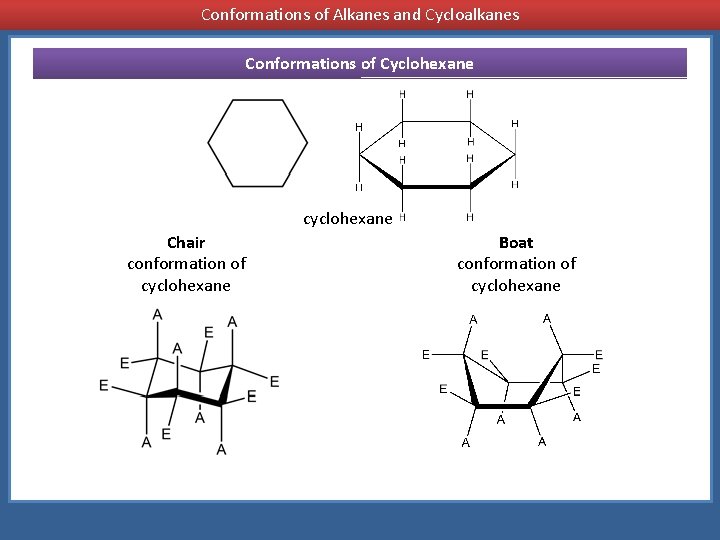
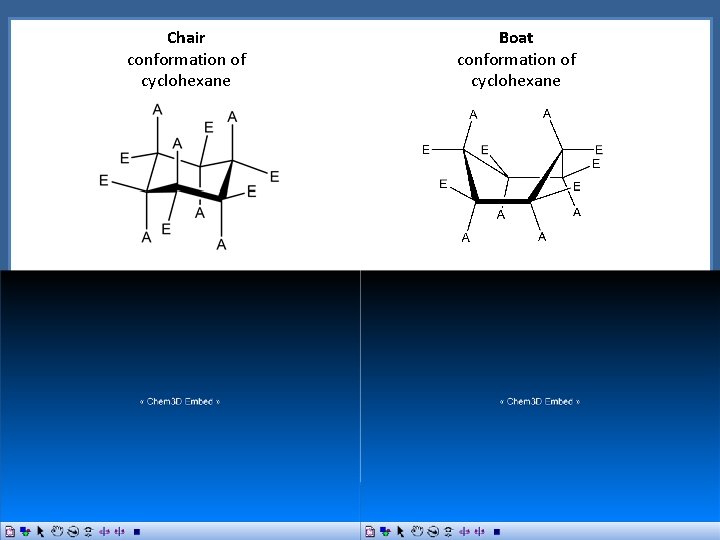
Conformations of Alkanes and Cycloalkanes Conformations of Cyclohexane cyclohexane Chair conformation of cyclohexane Boat conformation of cyclohexane

Chair conformation of cyclohexane Boat conformation of cyclohexane

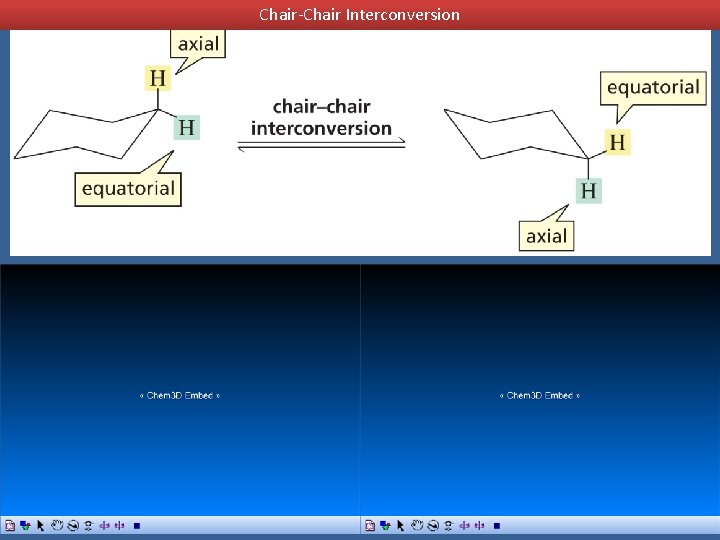
Chair-Chair Interconversion

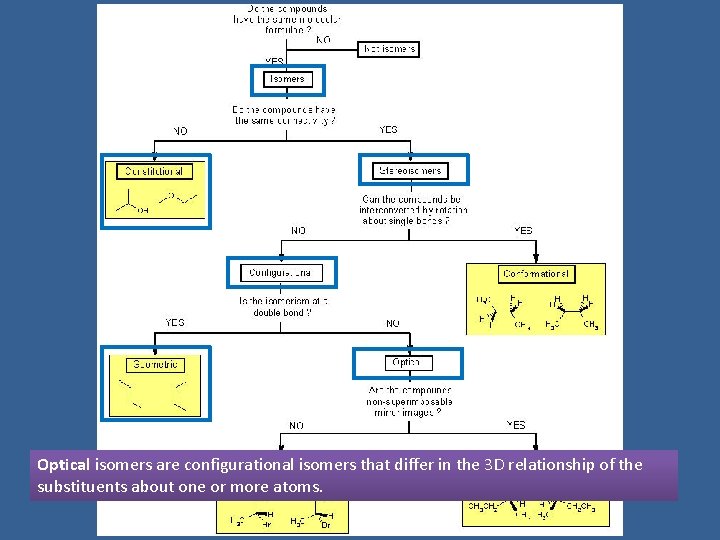
Optical isomers are configurational isomers that differ in the 3 D relationship of the substituents about one or more atoms.

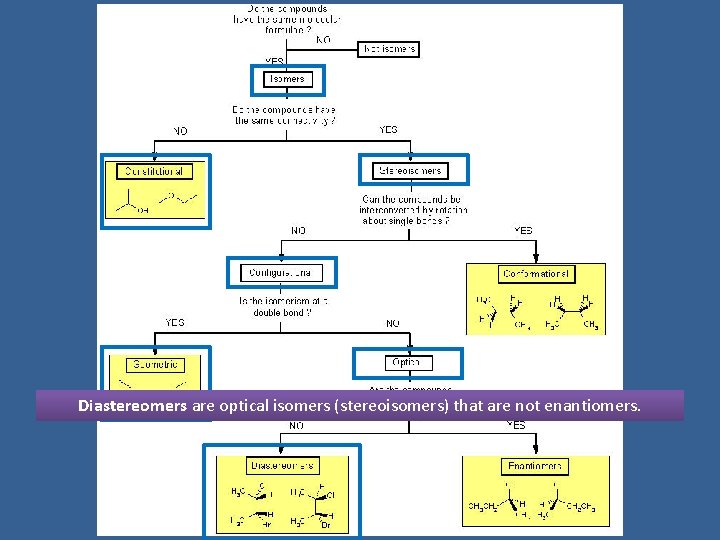
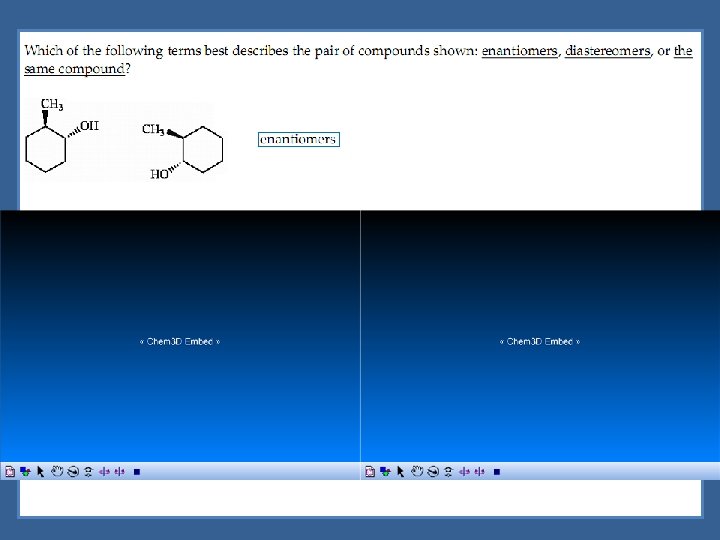
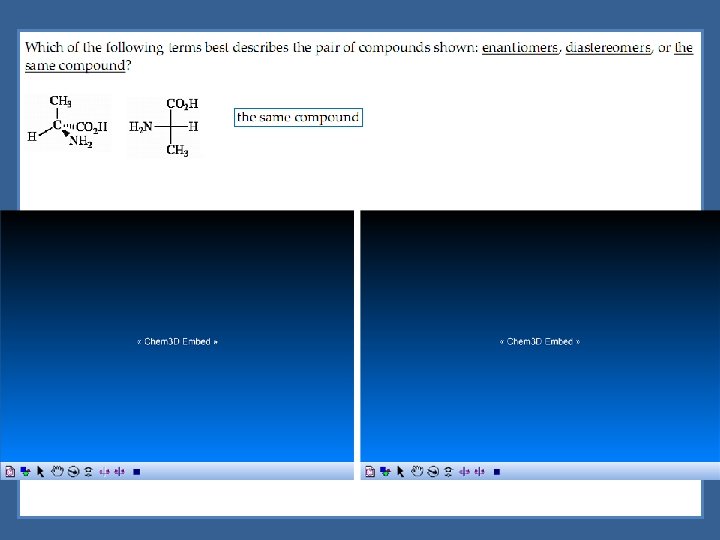
Diastereomers are optical isomers (stereoisomers) that are not enantiomers.

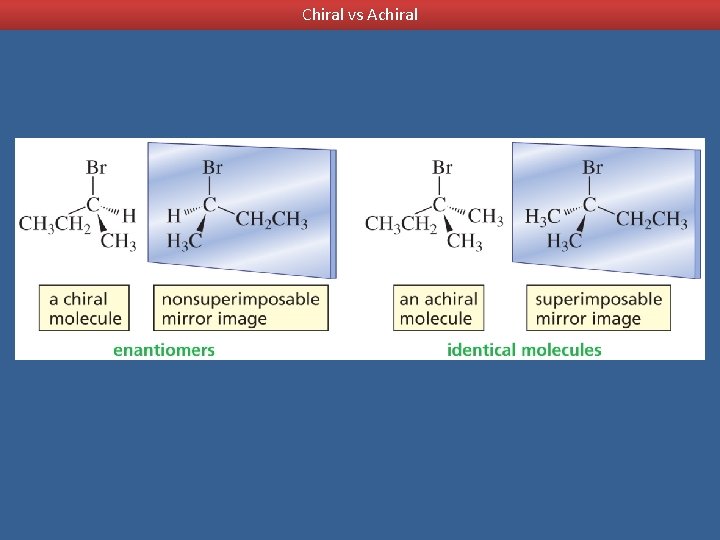
Enantiomers are optical isomers that are non-superimposable mirror images.

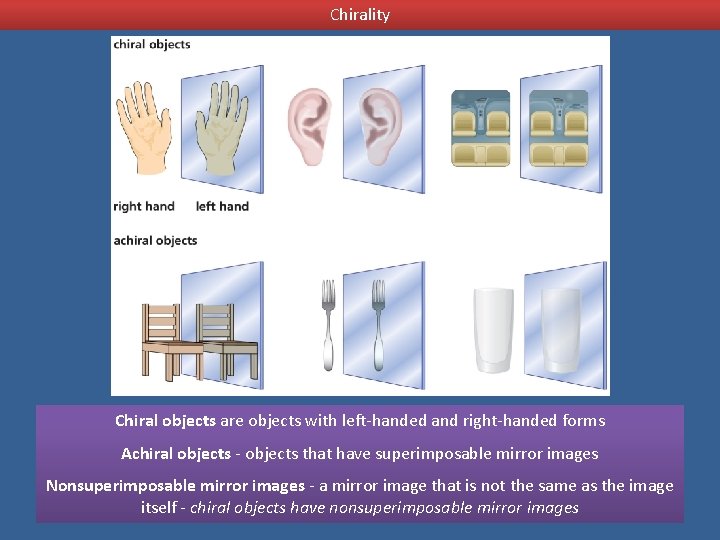
Chirality Chiral objects are objects with left-handed and right-handed forms Achiral objects - objects that have superimposable mirror images Nonsuperimposable mirror images - a mirror image that is not the same as the image itself - chiral objects have nonsuperimposable mirror images

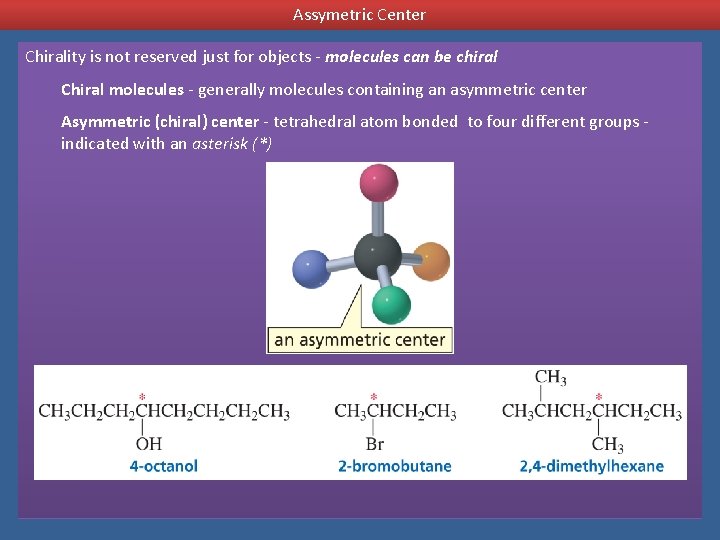
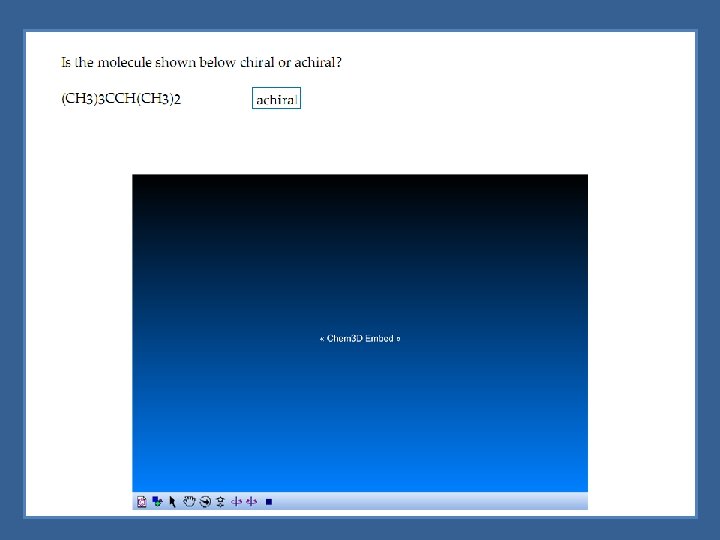
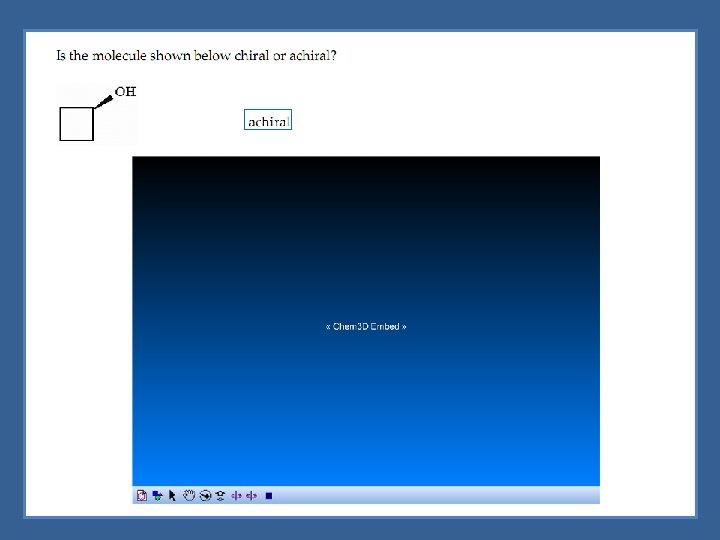
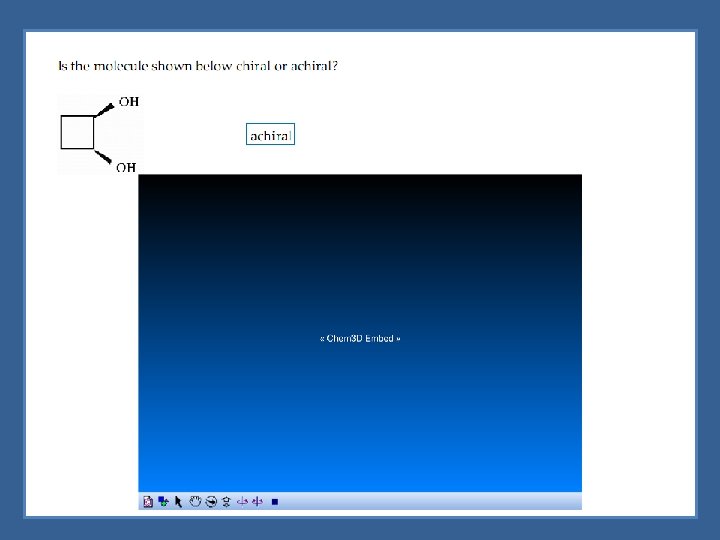
Assymetric Center Chirality is not reserved just for objects - molecules can be chiral Chiral molecules - generally molecules containing an asymmetric center Asymmetric (chiral) center - tetrahedral atom bonded to four different groups indicated with an asterisk (*)

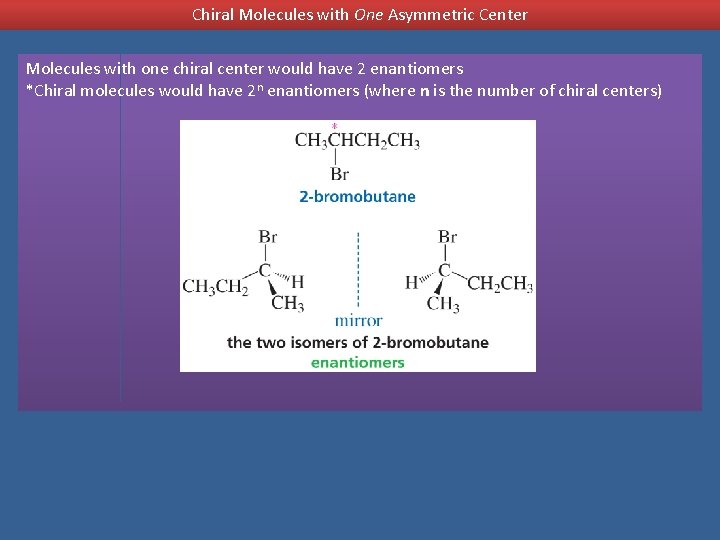
Chiral Molecules with One Asymmetric Center Molecules with one chiral center would have 2 enantiomers *Chiral molecules would have 2 n enantiomers (where n is the number of chiral centers)

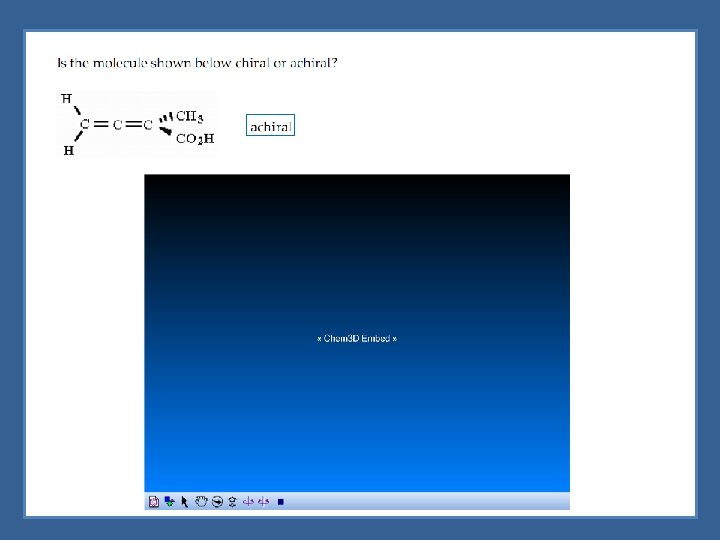
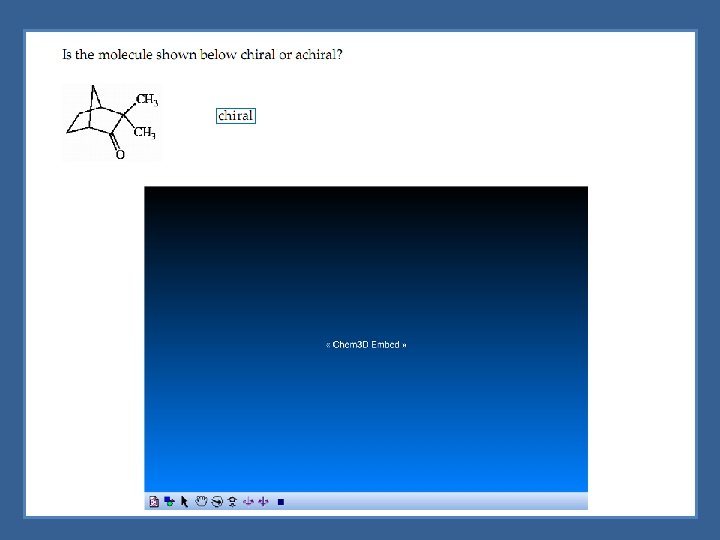
Chiral vs Achiral






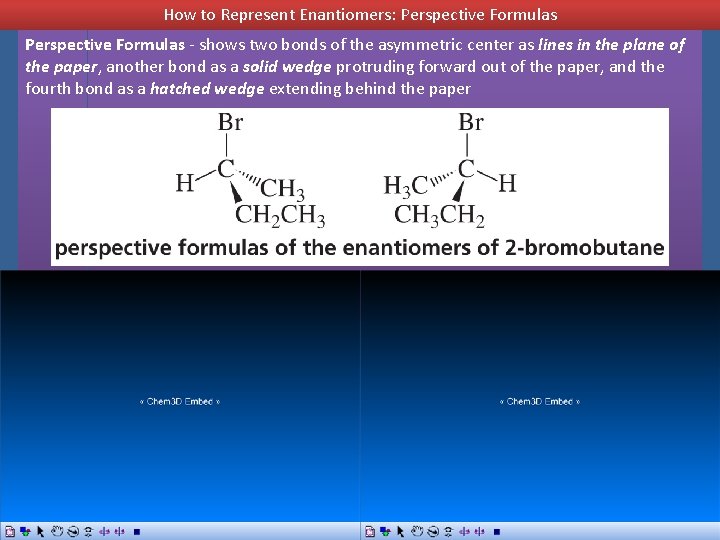
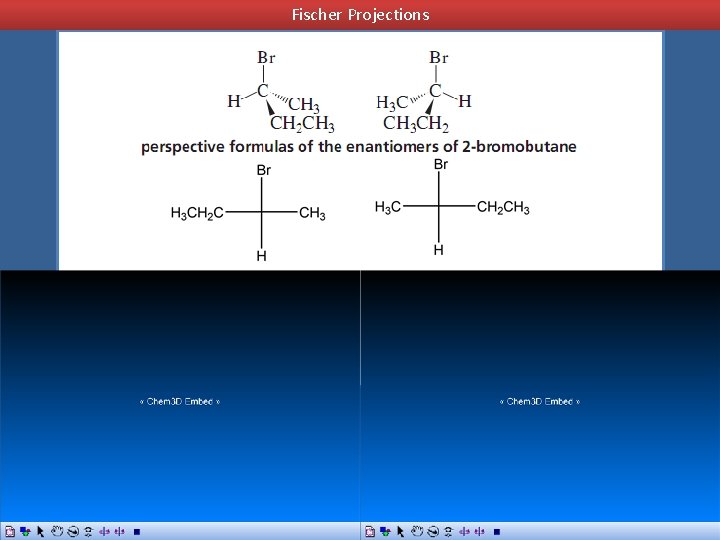
How to Represent Enantiomers: Perspective Formulas - shows two bonds of the asymmetric center as lines in the plane of the paper, another bond as a solid wedge protruding forward out of the paper, and the fourth bond as a hatched wedge extending behind the paper

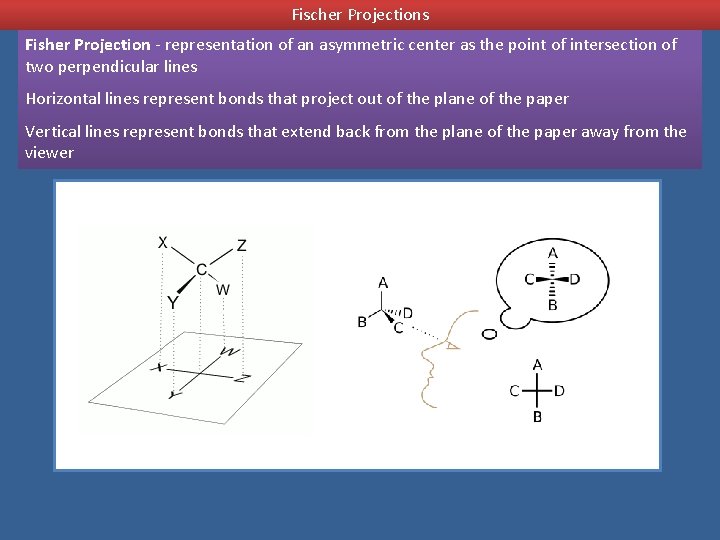
Fischer Projections Fisher Projection - representation of an asymmetric center as the point of intersection of two perpendicular lines Horizontal lines represent bonds that project out of the plane of the paper Vertical lines represent bonds that extend back from the plane of the paper away from the viewer

Fischer Projections



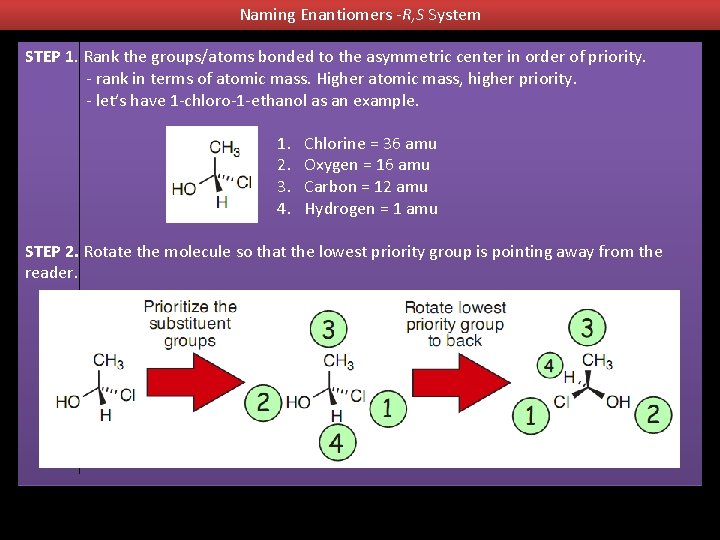
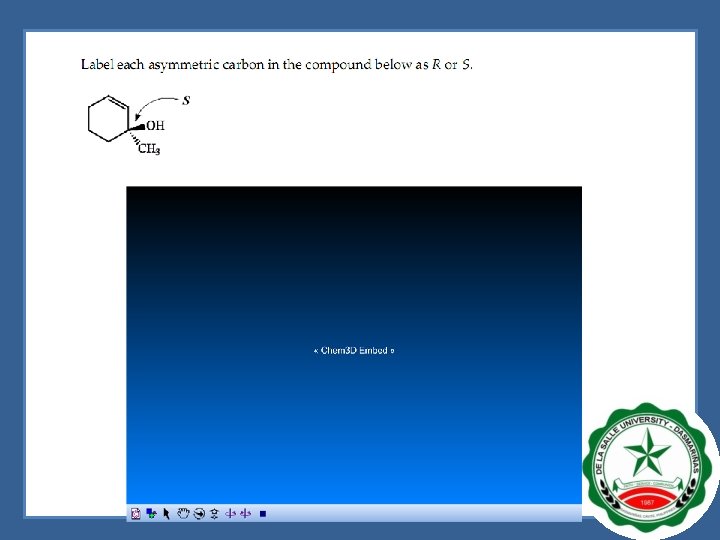
Naming Enantiomers -R, S System STEP 1. Rank the groups/atoms bonded to the asymmetric center in order of priority. - rank in terms of atomic mass. Higher atomic mass, higher priority. - let’s have 1 -chloro-1 -ethanol as an example. 1. 2. 3. 4. Chlorine = 36 amu Oxygen = 16 amu Carbon = 12 amu Hydrogen = 1 amu STEP 2. Rotate the molecule so that the lowest priority group is pointing away from the reader.

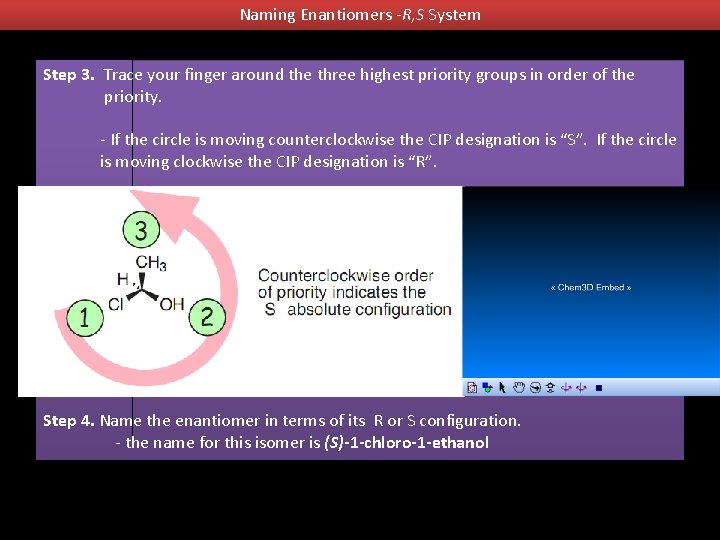
Naming Enantiomers -R, S System Step 3. Trace your finger around the three highest priority groups in order of the priority. - If the circle is moving counterclockwise the CIP designation is “S”. If the circle is moving clockwise the CIP designation is “R”. Step 4. Name the enantiomer in terms of its R or S configuration. - the name for this isomer is (S)-1 -chloro-1 -ethanol

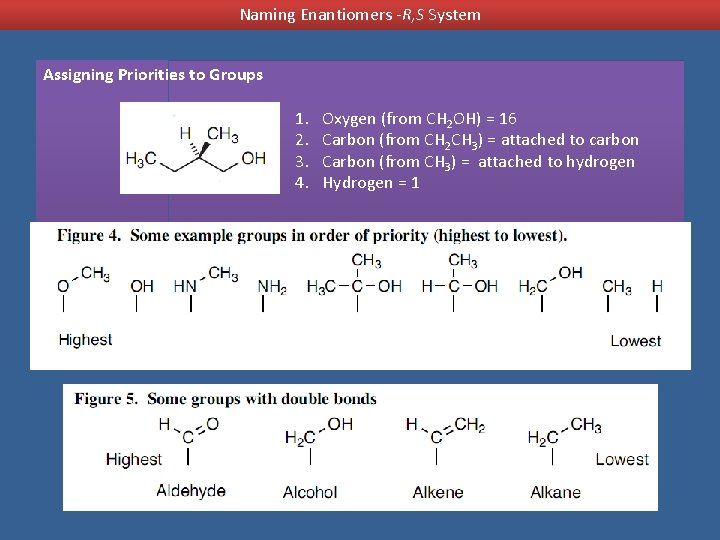
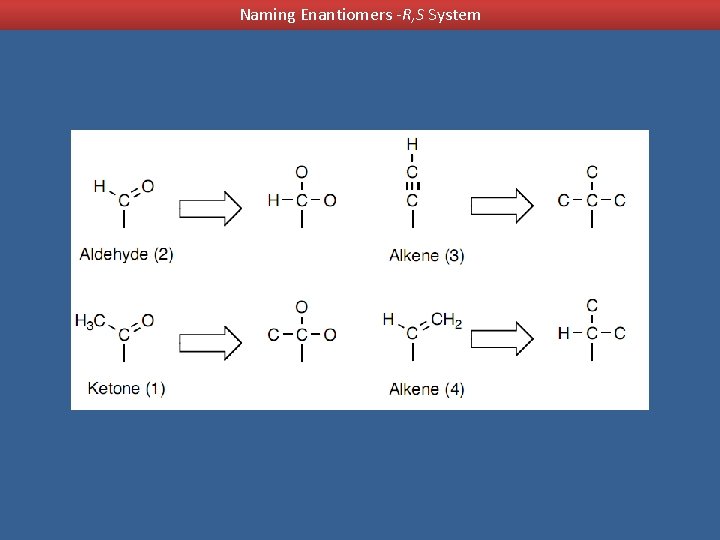
Naming Enantiomers -R, S System Assigning Priorities to Groups 1. 2. 3. 4. Oxygen (from CH 2 OH) = 16 Carbon (from CH 2 CH 3) = attached to carbon Carbon (from CH 3) = attached to hydrogen Hydrogen = 1

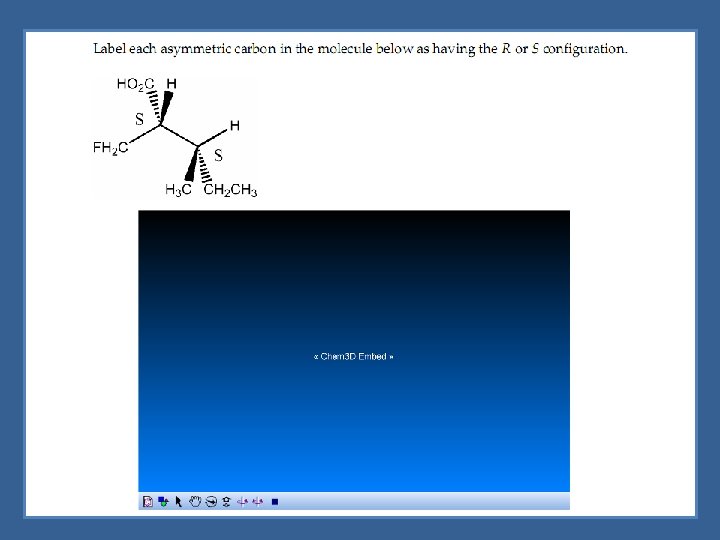
Naming Enantiomers -R, S System


 Insidan region jh
Insidan region jh Diastereomers vs constitutional isomers
Diastereomers vs constitutional isomers Introduction to stereochemistry
Introduction to stereochemistry Introduction to stereochemistry
Introduction to stereochemistry What is protien
What is protien 2 3 4-trihydroxybutanal stereoisomers
2 3 4-trihydroxybutanal stereoisomers Trans-1-chloro-3-methylcyclohexane
Trans-1-chloro-3-methylcyclohexane Indicate the relationship of the pair of molecules shown.
Indicate the relationship of the pair of molecules shown. Father of stereochemistry
Father of stereochemistry Stereochemistry
Stereochemistry Stability of chair and boat conformation
Stability of chair and boat conformation Stereochemistry of cephalosporins
Stereochemistry of cephalosporins Optical isomerism in octahedral complexes
Optical isomerism in octahedral complexes Diastereomers vs enantiomers
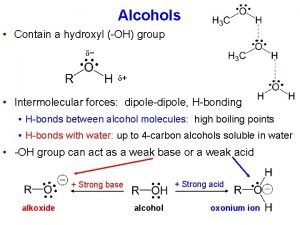
Diastereomers vs enantiomers Physical properties of amines
Physical properties of amines C5h12 structural isomers
C5h12 structural isomers Constitutional isomers
Constitutional isomers Is alkane saturated
Is alkane saturated Butyl isomers
Butyl isomers Spectrochemical series
Spectrochemical series Isomers of c7 h16
Isomers of c7 h16 C6h14 structural isomers
C6h14 structural isomers Alkyl substituents
Alkyl substituents R s isomers
R s isomers Tartaric acid optical isomers
Tartaric acid optical isomers Coordination isomers
Coordination isomers Geometric vs structural isomers
Geometric vs structural isomers Difference between structural and geometric isomers
Difference between structural and geometric isomers 403221
403221 Conformational isomerism
Conformational isomerism Conformational isomers are also known as
Conformational isomers are also known as E/z isomers
E/z isomers Cis trans isomers
Cis trans isomers L isomer
L isomer But-2-ene isomers
But-2-ene isomers 5 structural isomers of hexane
5 structural isomers of hexane Structural formula vs displayed formula
Structural formula vs displayed formula Structural vs geometric isomers
Structural vs geometric isomers C8h18 isomers
C8h18 isomers Functional isomers of carboxylic acids
Functional isomers of carboxylic acids Isomers of mabcd
Isomers of mabcd Consitutional isomers
Consitutional isomers 2-chlorobutane optical isomers
2-chlorobutane optical isomers 2-chlorobutane optical isomers
2-chlorobutane optical isomers Alkene structural formula
Alkene structural formula Isopropyl methyl ether
Isopropyl methyl ether E and z notation
E and z notation Diacetylferrocene isomers
Diacetylferrocene isomers What is structural isomerism
What is structural isomerism Diacetylferrocene isomers
Diacetylferrocene isomers Thalidomide optical isomers
Thalidomide optical isomers Species tree
Species tree Foragry
Foragry