Optical Isomers Constitutional Isomers Have same molecular form


- Slides: 10

Optical Isomers

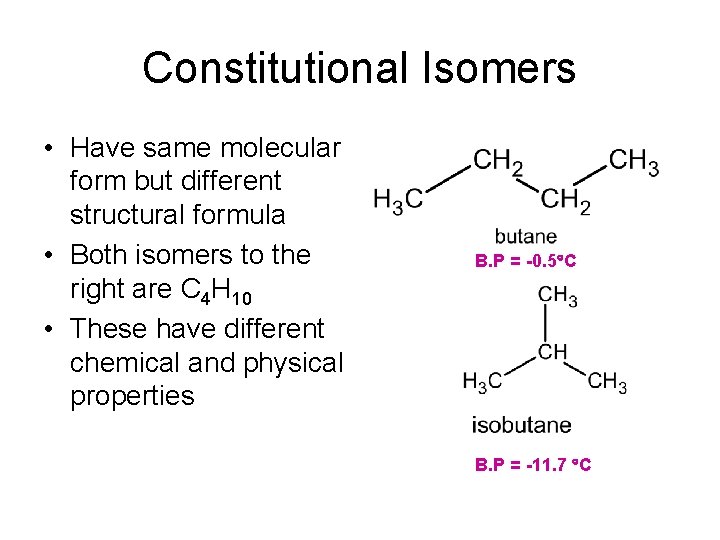
Constitutional Isomers • Have same molecular form but different structural formula • Both isomers to the right are C 4 H 10 • These have different chemical and physical properties B. P = -0. 5 C B. P = -11. 7 C

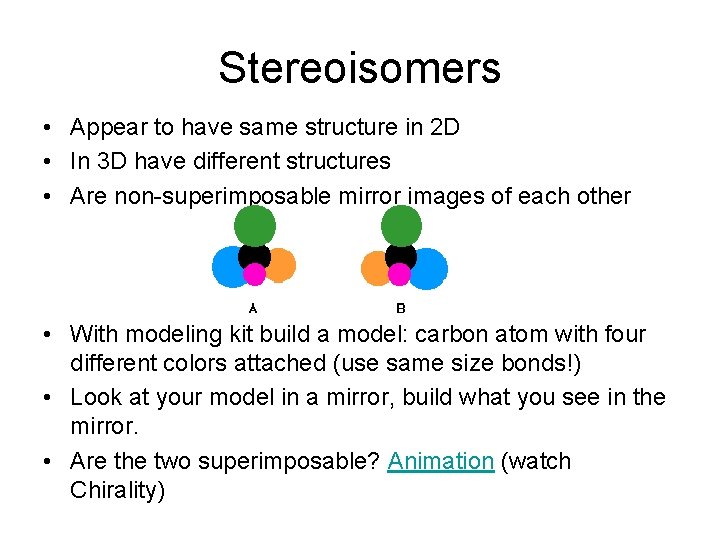
Stereoisomers • Appear to have same structure in 2 D • In 3 D have different structures • Are non-superimposable mirror images of each other • With modeling kit build a model: carbon atom with four different colors attached (use same size bonds!) • Look at your model in a mirror, build what you see in the mirror. • Are the two superimposable? Animation (watch Chirality)

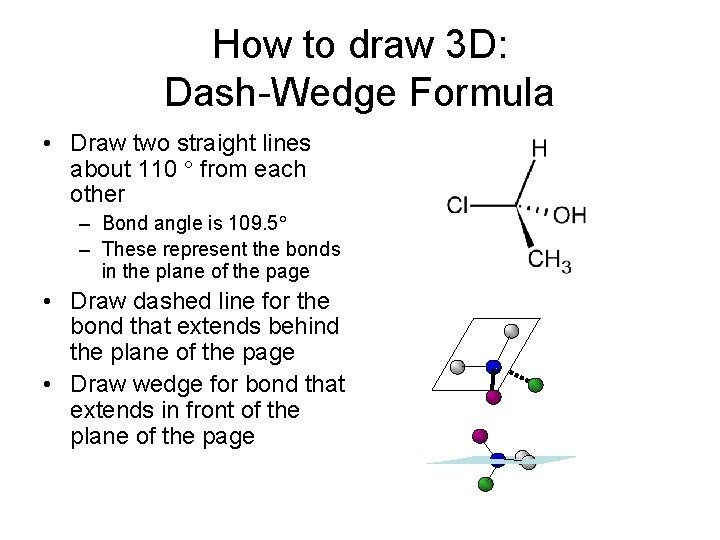
How to draw 3 D: Dash-Wedge Formula • Draw two straight lines about 110 from each other – Bond angle is 109. 5 – These represent the bonds in the plane of the page • Draw dashed line for the bond that extends behind the plane of the page • Draw wedge for bond that extends in front of the plane of the page

Drawing Enantiomers • Draw the molecule you built, using the dash-wedge formula and colored pens • Use a mirror to see the enantiomer • Sketch the mirror image, using the dashwedge formula • Draw the two stereoisomers of 1 -chloro-1 bromo-ethane. – There is no convention for which atom is attached to a wedge.

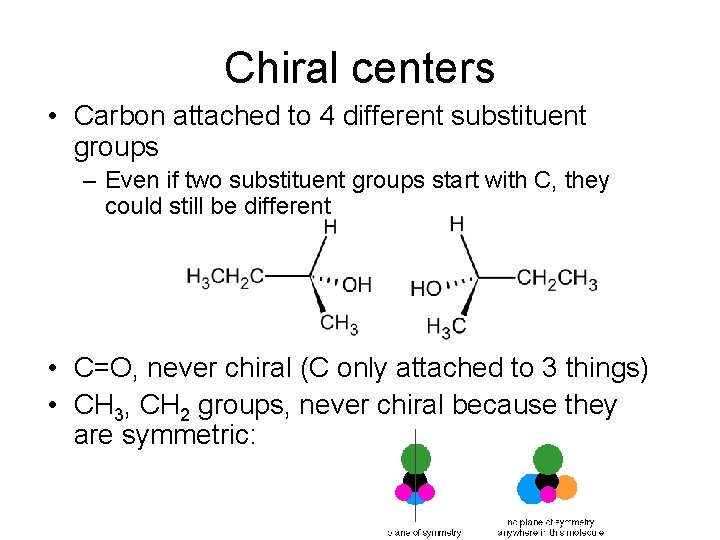
Chiral centers • Carbon attached to 4 different substituent groups – Even if two substituent groups start with C, they could still be different • C=O, never chiral (C only attached to 3 things) • CH 3, CH 2 groups, never chiral because they are symmetric:

Vocabulary • Stereoisomers have chiral centers. • Carbon atoms can be – chiral or achiral – Asymmetric or symmetric • If two molecules are stereoisomers, they are also called enantiomers • Chiral molecules are optically active • Enantiomers are optical isomers

Properties of Optical Isomers • same physical and chemical properties • rotate plane polarized light – Each isomer rotates it in a different direction – What is plane polarized light? • Animation (watch Optical Activity) – Try polarizers

Optical Activity • Stereoisomers are said to be optically active if the rotate plane polarized light • Each type of enantiomer rotates light the same amount, but in different directions. • Amount and direction of rotation must be experimentally determined using a polarimeter

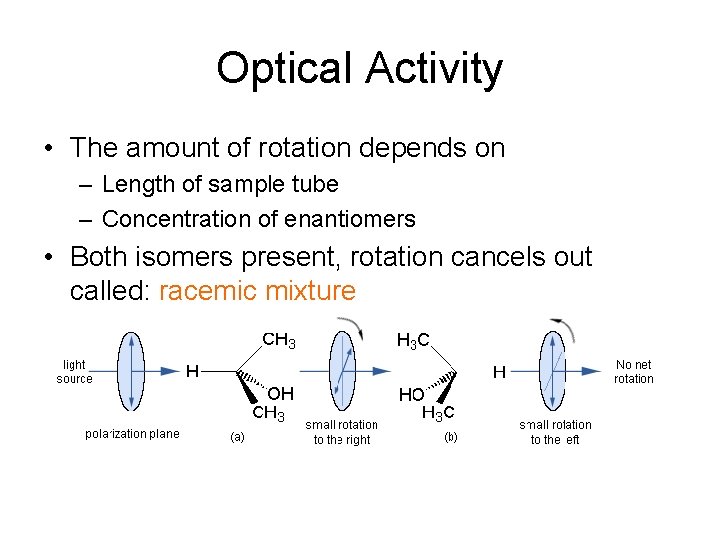
Optical Activity • The amount of rotation depends on – Length of sample tube – Concentration of enantiomers • Both isomers present, rotation cancels out called: racemic mixture