CHEMICAL ISOMERS What are isomers Isomers are molecules






- Slides: 19

CHEMICAL ISOMERS

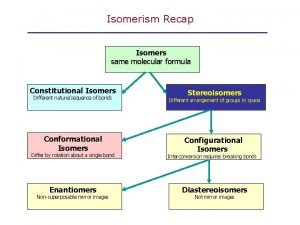
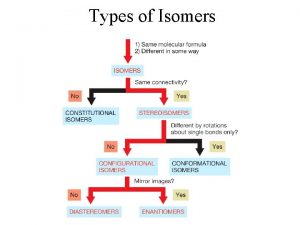
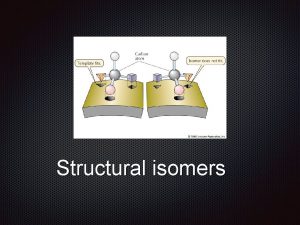
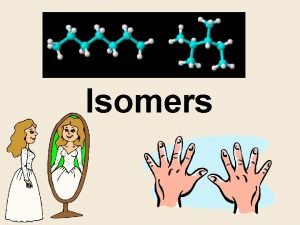
What are isomers Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space.

What are stereoisomers In stereoisomerism, the atoms making up the isomers are joined up in the same order, but still manage to have a different spatial arrangement. Optical isomerism is one form of stereoisomerism.

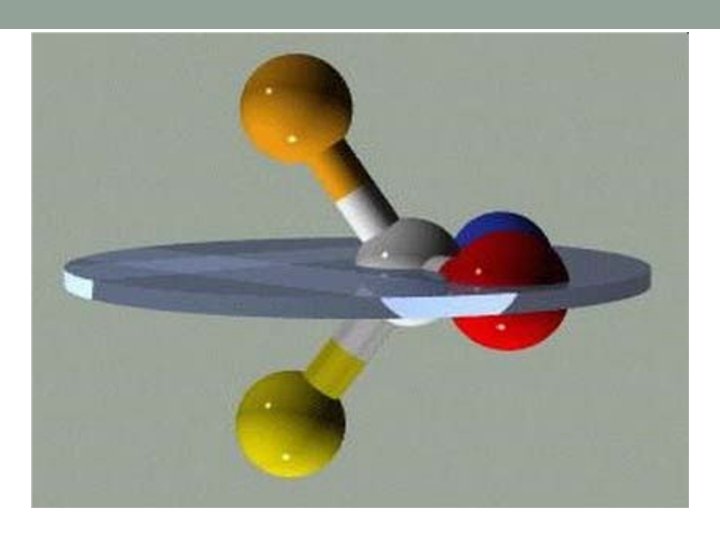
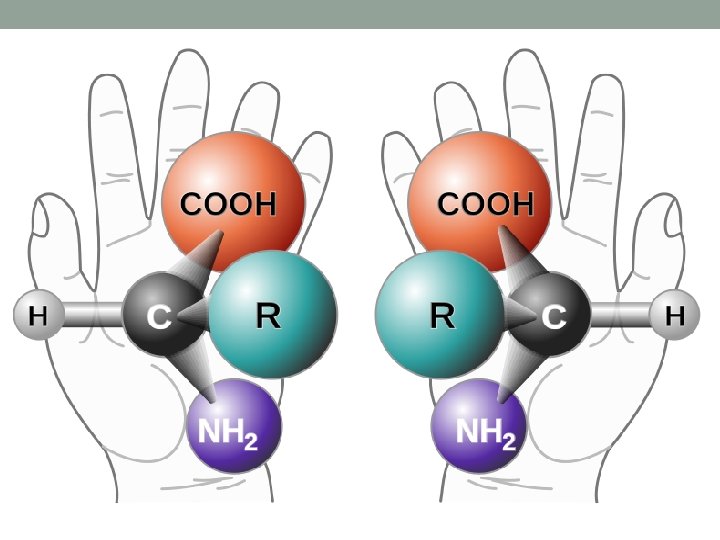
Optical isomers Chiral molecule is a type of molecule that has a non-superposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom. The term chiral in general is used to describe an object that is not superposable on its mirror image.

A chiral (not chiral) objects are objects that are identical to their mirror image. Human hands are perhaps the most universally recognized example of chirality: The left hand is a nonsuperposable mirror image of the right hand; no matter how the two hands are oriented, it is impossible for all the major features of both hands to coincide.

This difference in symmetry becomes obvious if someone attempts to shake the right hand of a person using his left hand, or if a left-handed glove is placed on a right hand.

In medical chemistry, chirality usually refers to molecules. Two mirror images of a chiral molecule are called enantiomers or optical isomers. Pairs of enantiomers are often designated as "right-" and "lefthanded". Molecular chirality is of interest because of its application to stereochemistry in inorganic chemistry, physical chemistry, biochemistry, and supramolecular chemistry.



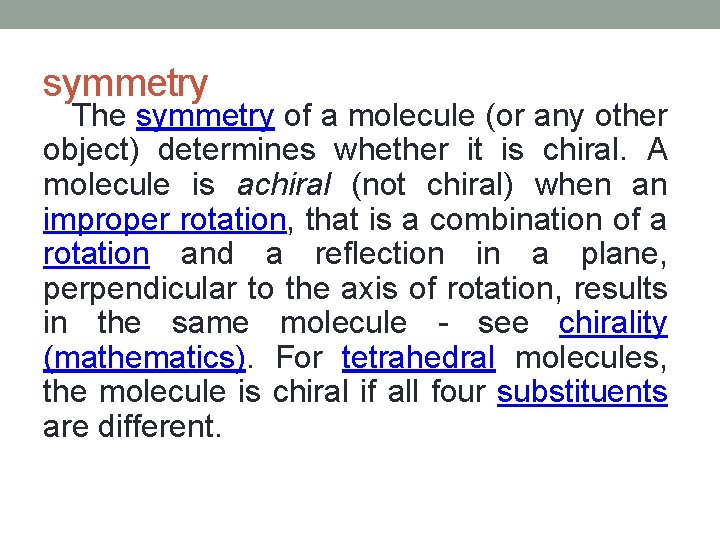
symmetry The symmetry of a molecule (or any other object) determines whether it is chiral. A molecule is achiral (not chiral) when an improper rotation, that is a combination of a rotation and a reflection in a plane, perpendicular to the axis of rotation, results in the same molecule - see chirality (mathematics). For tetrahedral molecules, the molecule is chiral if all four substituents are different.

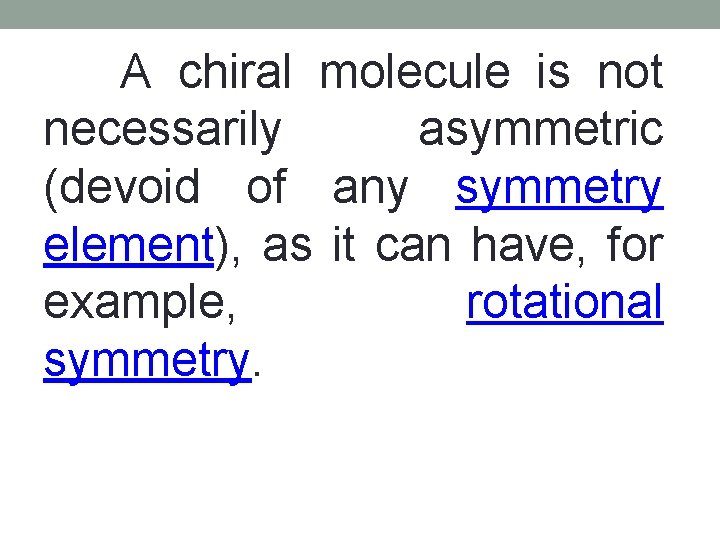
A chiral molecule is not necessarily asymmetric (devoid of any symmetry element), as it can have, for example, rotational symmetry.

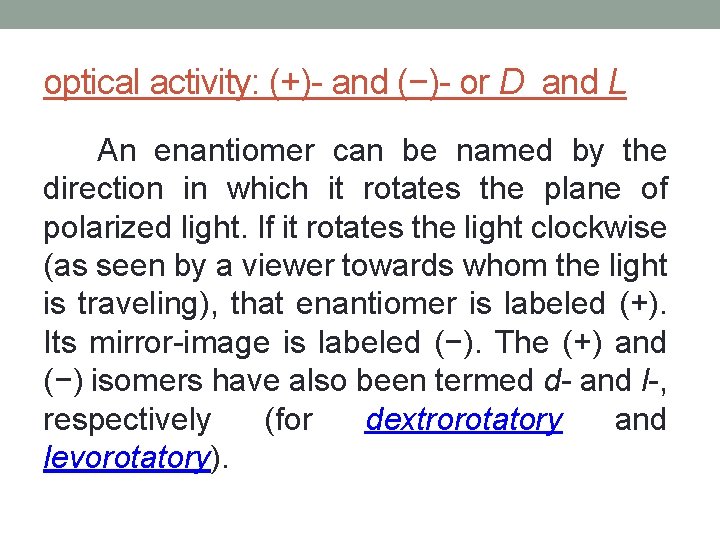
optical activity: (+)- and (−)- or D and L An enantiomer can be named by the direction in which it rotates the plane of polarized light. If it rotates the light clockwise (as seen by a viewer towards whom the light is traveling), that enantiomer is labeled (+). Its mirror-image is labeled (−). The (+) and (−) isomers have also been termed d- and l-, respectively (for dextrorotatory and levorotatory).


Configuration: D- and L • An optical isomer can be named by the spatial configuration of its atoms. The D/L system, not to be confused with the d- and l-system, see above, does this by relating the molecule to glyceraldehyde. Glyceraldehyde is chiral itself, and its two isomers are labeled D and L

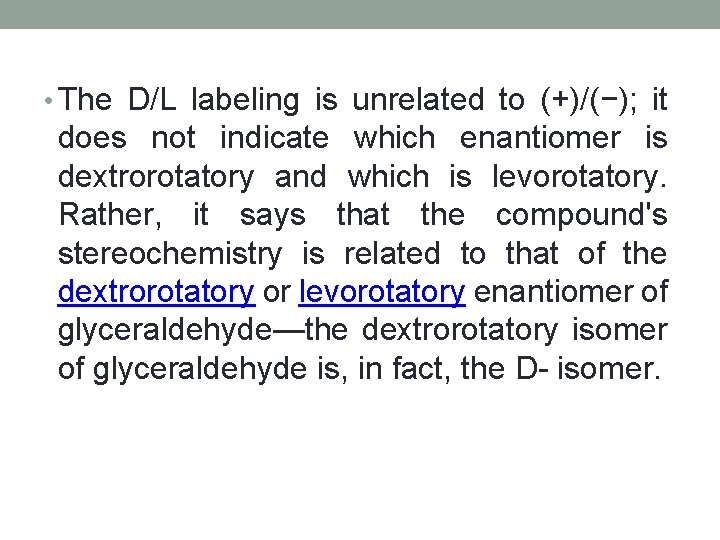
• The D/L labeling is unrelated to (+)/(−); it does not indicate which enantiomer is dextrorotatory and which is levorotatory. Rather, it says that the compound's stereochemistry is related to that of the dextrorotatory or levorotatory enantiomer of glyceraldehyde—the dextrorotatory isomer of glyceraldehyde is, in fact, the D- isomer.

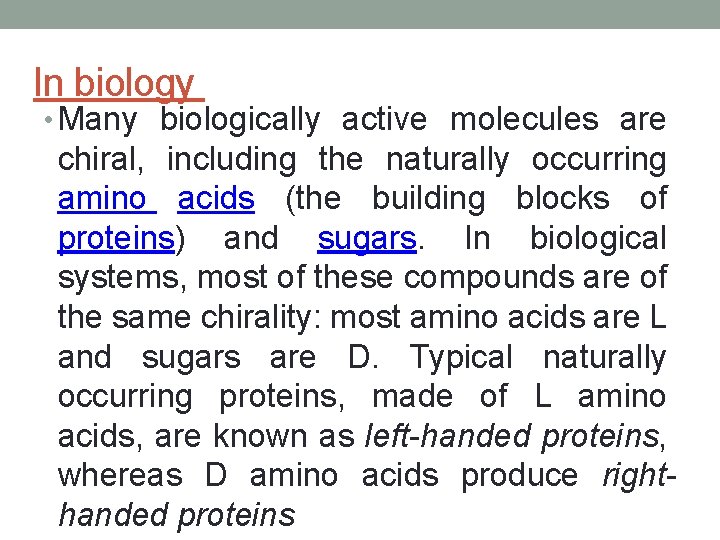
In biology • Many biologically active molecules are chiral, including the naturally occurring amino acids (the building blocks of proteins) and sugars. In biological systems, most of these compounds are of the same chirality: most amino acids are L and sugars are D. Typical naturally occurring proteins, made of L amino acids, are known as left-handed proteins, whereas D amino acids produce righthanded proteins

In drugs • Ethambutol: Whereas one enantiomer is used to treat tuberculosis, the other causes blindness. • Naproxen: One enantiomer is used to treat arthritis pain, but the other causes liver poisoning with no analgesic effect. • Steroid receptor sites also show stereoisomer specificity. • Penicillin's activity is stereoselective. The antibiotic only works on peptide links of D-alanine which occur in the cell walls of bacteria - but not in humans. The antibiotic can kill only the bacteria, and not us, because we don't have these D-amino acids. • S(-) isomer of carvedilol, a drug that interacts with adrenoceptors, is 100 times more potent as beta receptor blocker than R(+) isomer. However, both the isomers are approximately equipotent as alpha receptor blockers.

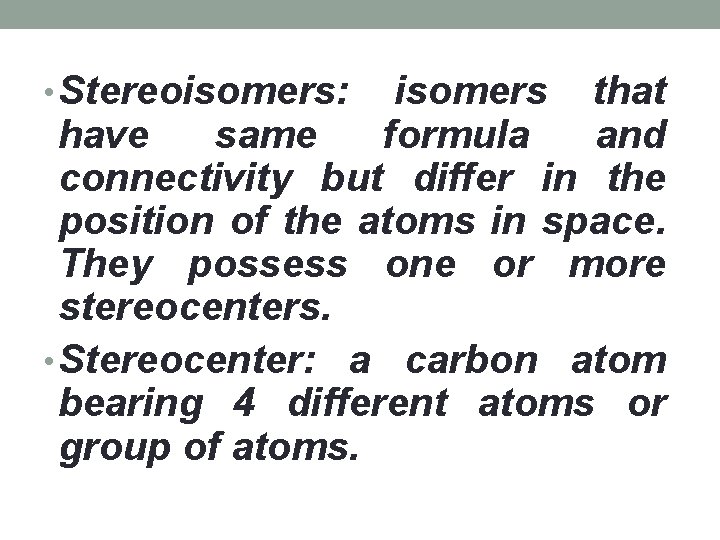
• Stereoisomers: isomers that have same formula and connectivity but differ in the position of the atoms in space. They possess one or more stereocenters. • Stereocenter: a carbon atom bearing 4 different atoms or group of atoms.

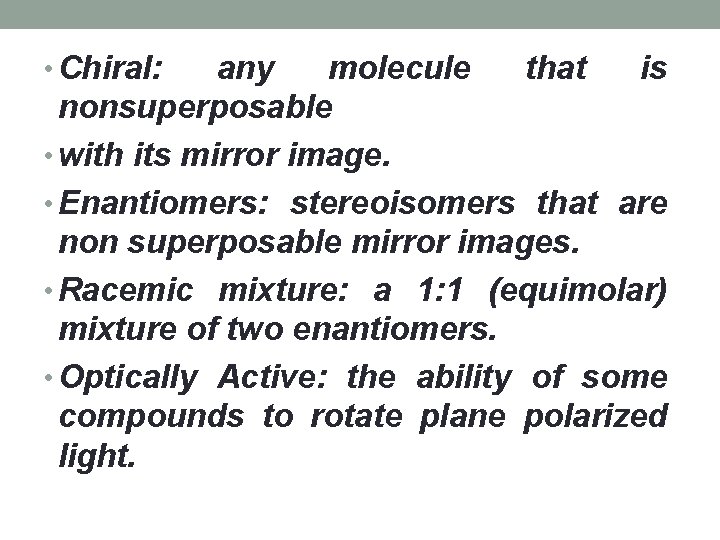
• Chiral: any molecule that is nonsuperposable • with its mirror image. • Enantiomers: stereoisomers that are non superposable mirror images. • Racemic mixture: a 1: 1 (equimolar) mixture of two enantiomers. • Optically Active: the ability of some compounds to rotate plane polarized light.
 Antigentest åre
Antigentest åre Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Constitutional isomers vs conformational isomers
Constitutional isomers vs conformational isomers Nonsuperimposable
Nonsuperimposable Section 1 chemical changes
Section 1 chemical changes Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Trinitrogen monosulfide formula
Trinitrogen monosulfide formula Empirical formula pogil
Empirical formula pogil Are kc and kp equal
Are kc and kp equal Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chapter 3 molecules of life
Chapter 3 molecules of life Name of molecules
Name of molecules When a substance absorbs heat its molecules will
When a substance absorbs heat its molecules will Target molecules
Target molecules Attraction of molecules
Attraction of molecules Chemistry molecules
Chemistry molecules Molecular geometry pogil
Molecular geometry pogil Bfcl2 molecular shape
Bfcl2 molecular shape If'n'molecules
If'n'molecules Classify the following unbalanced chemical equations
Classify the following unbalanced chemical equations