The Molecules of Life Chapter 3 The Simplest




- Slides: 21

The Molecules of Life Chapter 3


The Simplest Hydrocarbon • Methane = Carbon + Hydrogen

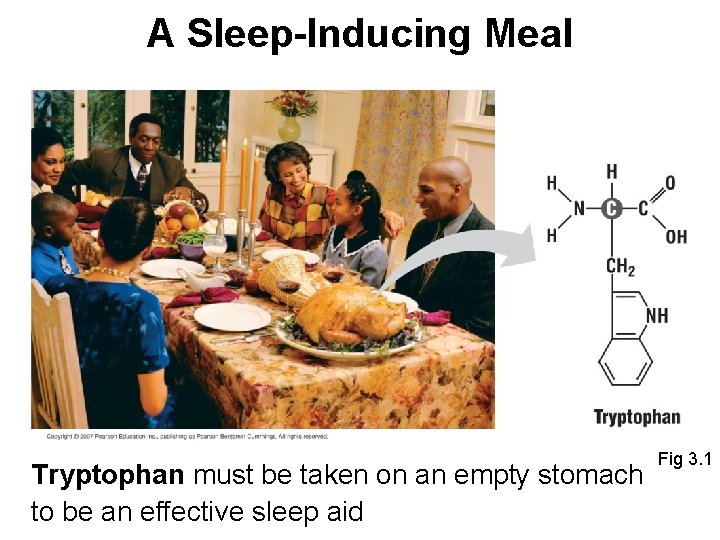
Biology and Society • Thanksgiving dinner: carbohydrates (mashed potatoes); fats (butter/gravy); proteins (meat) • After finishing a huge Thanksgiving dinner many people feel especially lethargic - many think that turkey makes you sleepy - is there a biological basis to this claim? • Turkey meat is high in trytophan - it is converted to serotonin, a chemical that can act on the brain to promote sleep

A Sleep-Inducing Meal Tryptophan must be taken on an empty stomach to be an effective sleep aid Fig 3. 1

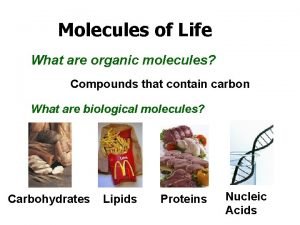
Organic Molecules • A cell is mostly water but the rest consists mainly of carbon based molecules • Compounds that contain carbon are called organic compounds • Carbon has the ability to form the large, complex diverse, molecules necessary for life functions • Why are carbon atoms so versatile as molecular ingredients?

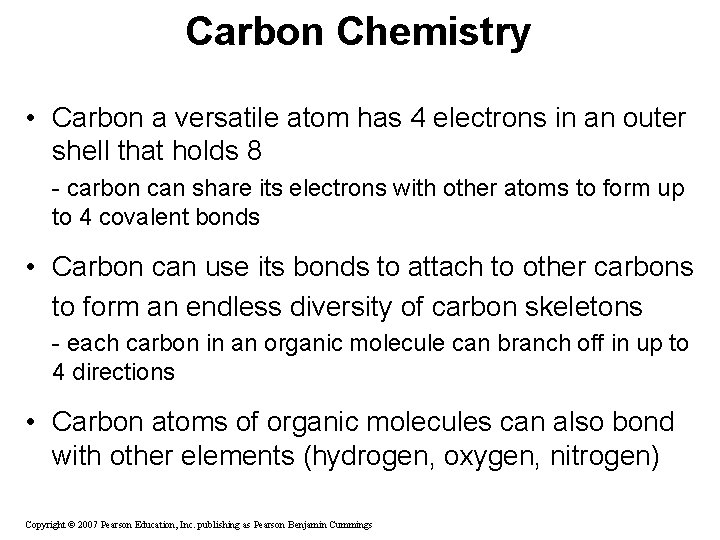
Carbon Chemistry • Carbon a versatile atom has 4 electrons in an outer shell that holds 8 - carbon can share its electrons with other atoms to form up to 4 covalent bonds • Carbon can use its bonds to attach to other carbons to form an endless diversity of carbon skeletons - each carbon in an organic molecule can branch off in up to 4 directions • Carbon atoms of organic molecules can also bond with other elements (hydrogen, oxygen, nitrogen) Copyright © 2007 Pearson Education, Inc. publishing as Pearson Benjamin Cummings

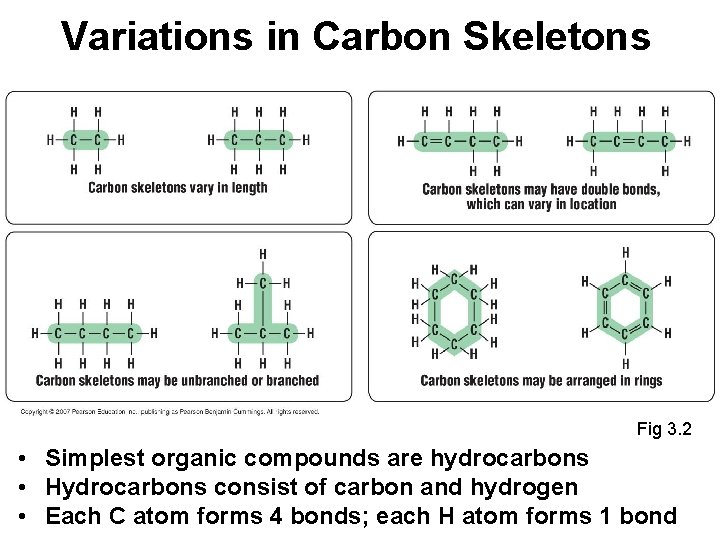
Variations in Carbon Skeletons Fig 3. 2 • Simplest organic compounds are hydrocarbons • Hydrocarbons consist of carbon and hydrogen • Each C atom forms 4 bonds; each H atom forms 1 bond

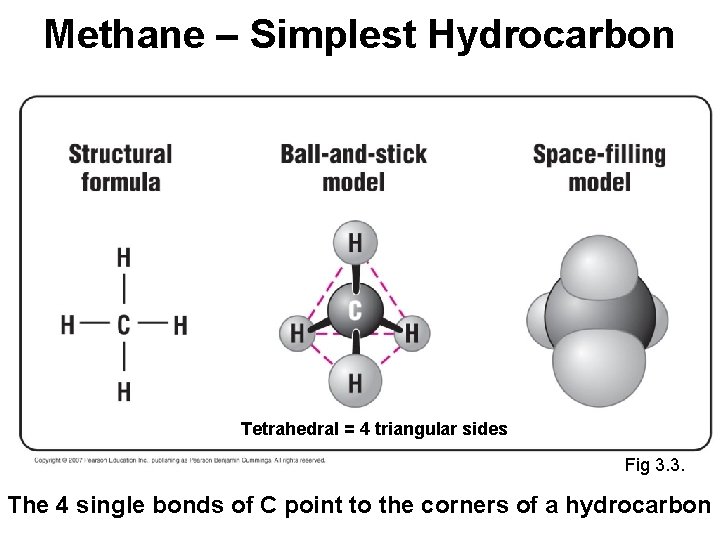
Methane – Simplest Hydrocarbon Tetrahedral = 4 triangular sides Fig 3. 3. The 4 single bonds of C point to the corners of a hydrocarbon

Larger Hydrocarbons Fig 3. 4 • Main molecules in the gasoline we burn in our cars • Hydrocarbons of fat molecules provide energy for our bodies

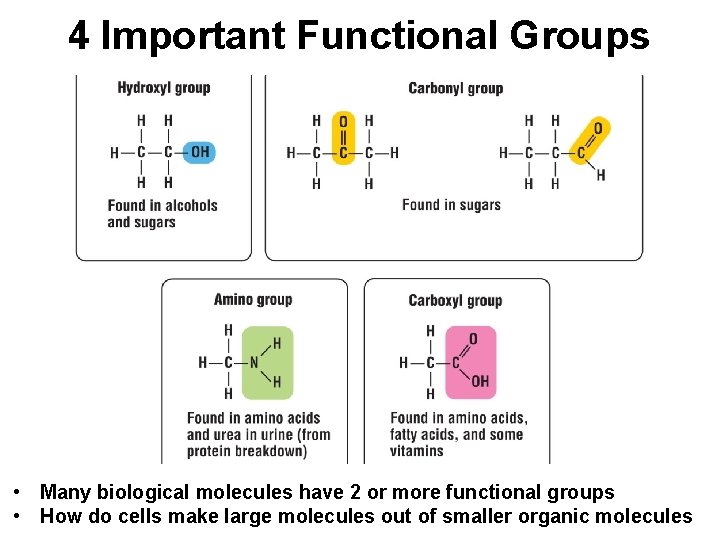
Functional Groups • Each type of organic molecule has a unique 3 dimensional shape that defines its function in an organism - the molecules of your body recognize one another based on their shapes • The unique properties of an organic compound depend not only on its carbon skeleton but also on the atoms attached to the skeleton - these atoms are called functional groups • Functional groups behave consistently from one organic molecule to another

4 Important Functional Groups • Many biological molecules have 2 or more functional groups • How do cells make large molecules out of smaller organic molecules

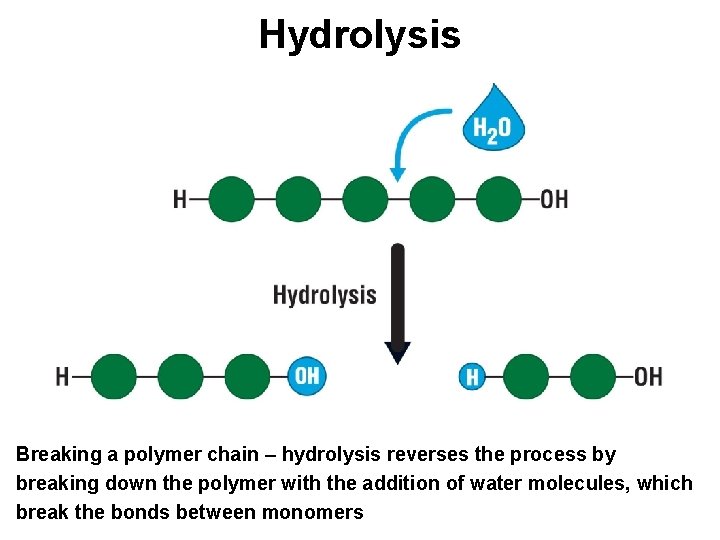
Building Blocks • On a molecular scale, many of life’s molecules are gigantic - biologists call them macromolecules (macro = ‘big’) such as DNA, carbohydrates, proteins • Most macromolecules are polymers - polymers are made by stringing together many smaller molecules called monomers - cells link monomers together through a dehydration reaction (removes a molecule of water) • Organisms break down macromolecules (digestion) - cells do this by a process called hydrolysis (hydro = ‘water’ lyse = ‘break’; to break with water)

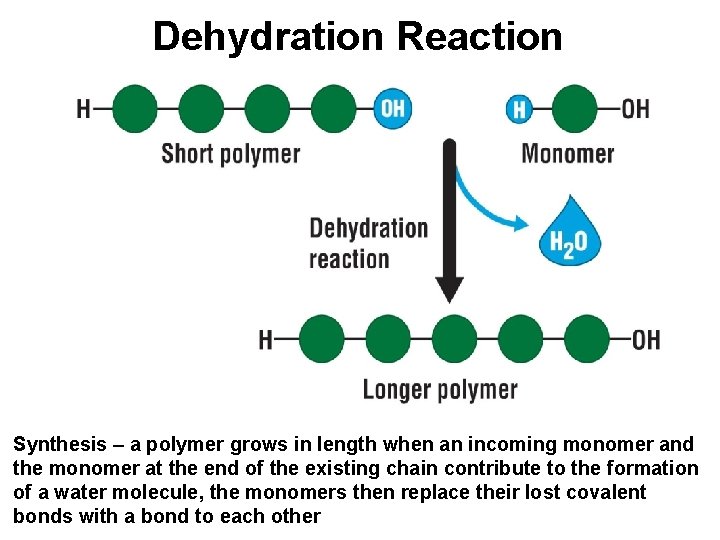
Dehydration Reaction Synthesis – a polymer grows in length when an incoming monomer and the monomer at the end of the existing chain contribute to the formation of a water molecule, the monomers then replace their lost covalent bonds with a bond to each other

Hydrolysis Breaking a polymer chain – hydrolysis reverses the process by breaking down the polymer with the addition of water molecules, which break the bonds between monomers

Biological Molecules There are 4 categories of large molecules in cells: • Carbohydrates • Lipids • Proteins • Nucleic Acids

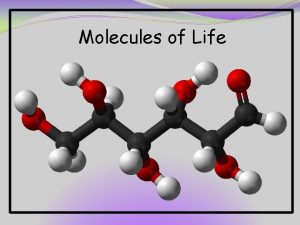
Carbohydrates • ‘Carbs’ - from small sugar molecules in soft drinks to long starch molecules in pasta and potatoes - serve as a primary source of dietary energy - used as building material to form the body of a plant • Monosaccharides (mono = ‘one’, and sacchar = ‘sugar’) are simple sugars: - glucose found in sports drinks - fructose found in fruits • Monosaccharides glucose and fructose are isomers - they have the same molecular formula, but their atoms are arranged differently

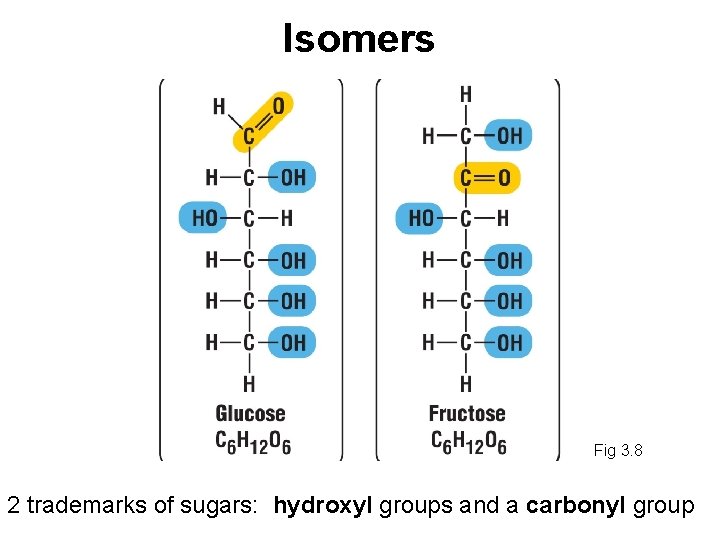
Isomers Fig 3. 8 2 trademarks of sugars: hydroxyl groups and a carbonyl group

Isomers • Molecules that have the same molecular formula but different structures - because shape is so important minor differences in the arrangement of atoms give isomers different properties - shape difference gives fructose a taste considerably sweeter than glucose • In aqueous solutions, monosaccharides form rings

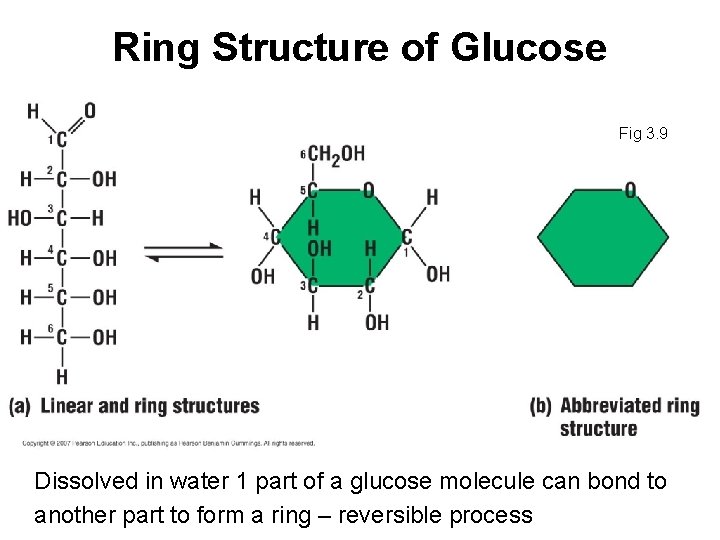
Ring Structure of Glucose Fig 3. 9 Dissolved in water 1 part of a glucose molecule can bond to another part to form a ring – reversible process

Glucose • Monosaccharides, particularly glucose, are the main fuel that cells use for cellular work • Cells break down glucose molecules and extract their stored energy - give off CO 2 as waste • Monosaccharides also provide cells with carbon skeletons that can be used as raw material
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Chapter 3 molecules of life
Chapter 3 molecules of life Chapter 8 sugar: the simplest of carbohydrates
Chapter 8 sugar: the simplest of carbohydrates The simplest form of carbohydrates.
The simplest form of carbohydrates. Biological molecules what are the building blocks of life
Biological molecules what are the building blocks of life Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Whats 45/100 in simplest form
Whats 45/100 in simplest form The simplest and most basic aerobic activity is
The simplest and most basic aerobic activity is Convenience utensils
Convenience utensils Example of sole proprietor
Example of sole proprietor Biomass pyramid
Biomass pyramid What are the principles of fingerprint
What are the principles of fingerprint Glyceraldehyde empirical formula
Glyceraldehyde empirical formula Write each fraction in simplest form
Write each fraction in simplest form Simplified format business letter
Simplified format business letter Simplest radical form of 45
Simplest radical form of 45 155 as a fraction in simplest form
155 as a fraction in simplest form 625/100 in simplest form
625/100 in simplest form Among 420 randomly selected employees
Among 420 randomly selected employees 18 1/4 in simplest radical form
18 1/4 in simplest radical form The simplest form of departmentalization is by
The simplest form of departmentalization is by The smallest unit of a textile is called a fiber
The smallest unit of a textile is called a fiber