Chapter 6 Chirality the Handedness of Molecules Isomers




- Slides: 33

Chapter 6 Chirality - the Handedness of Molecules

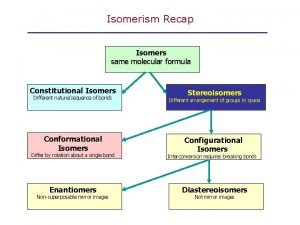
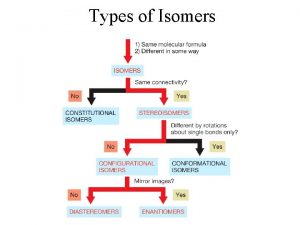
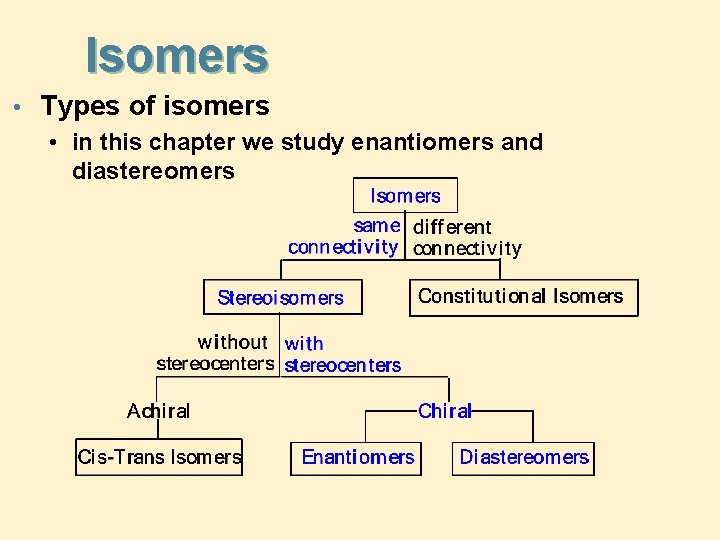
Isomers • Types of isomers • in this chapter we study enantiomers and diastereomers

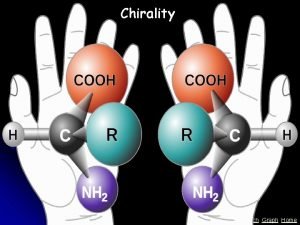
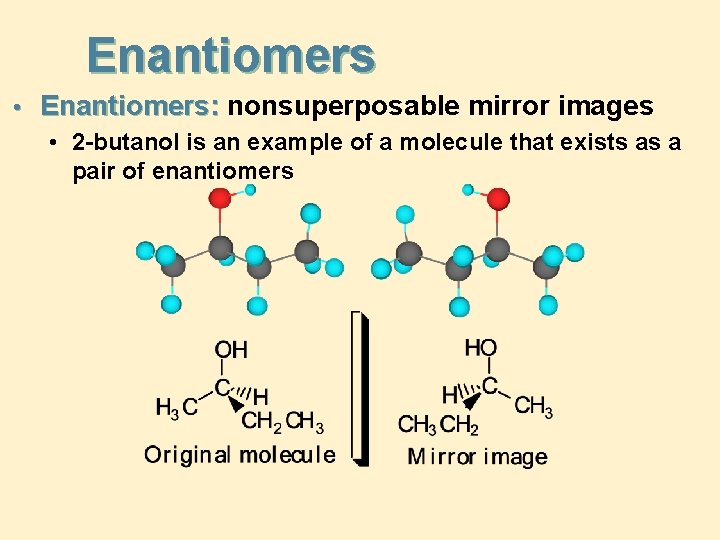
Enantiomers • Enantiomers: nonsuperposable mirror images • 2 -butanol is an example of a molecule that exists as a pair of enantiomers

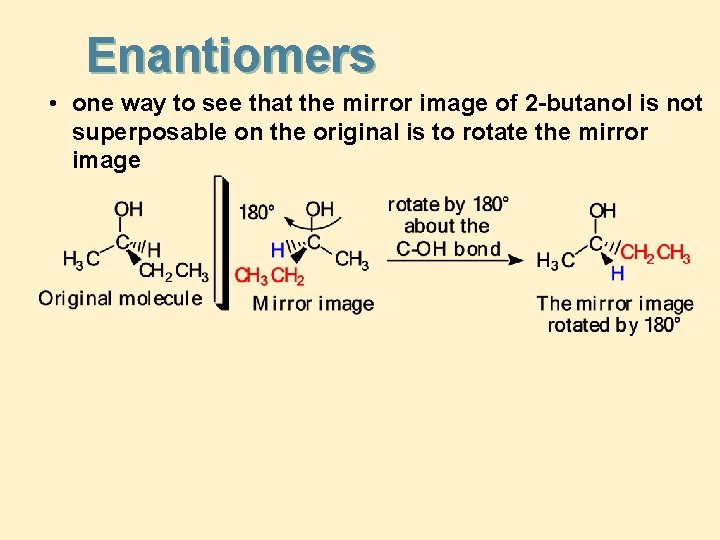
Enantiomers • one way to see that the mirror image of 2 -butanol is not superposable on the original is to rotate the mirror image

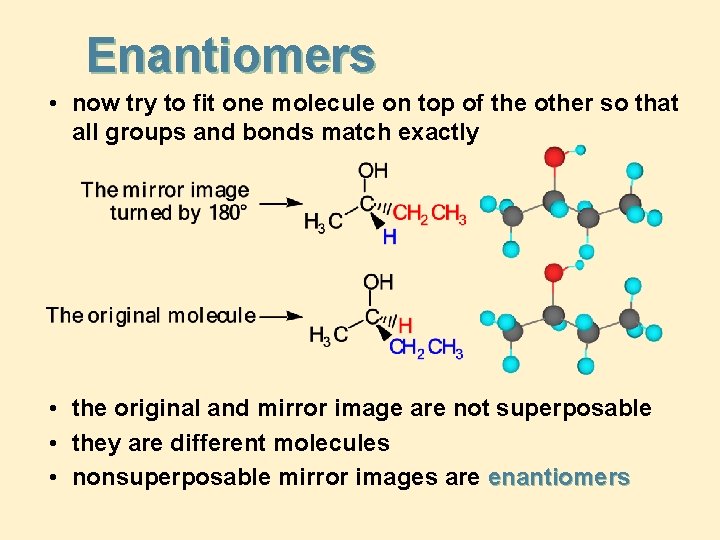
Enantiomers • now try to fit one molecule on top of the other so that all groups and bonds match exactly • the original and mirror image are not superposable • they are different molecules • nonsuperposable mirror images are enantiomers

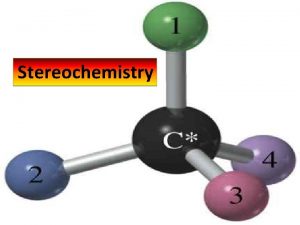
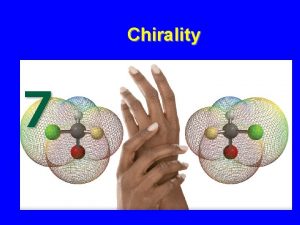
Enantiomers • Objects that are not superposable on their mirror images are chiral (from the Greek: cheir, hand) • they show handedness • The most common cause of enantiomerism in organic molecules is the presence of a carbon with four different groups bonded to it • a carbon with four different groups bonded to it is called a stereocenter

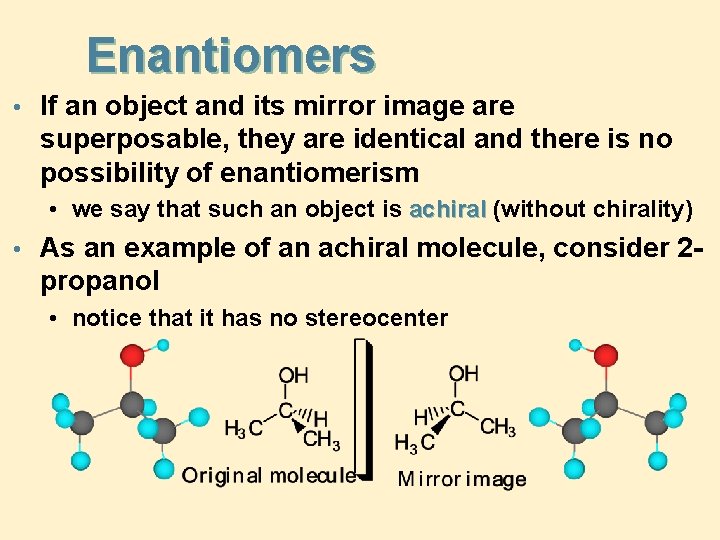
Enantiomers • If an object and its mirror image are superposable, they are identical and there is no possibility of enantiomerism • we say that such an object is achiral (without chirality) • As an example of an achiral molecule, consider 2 - propanol • notice that it has no stereocenter

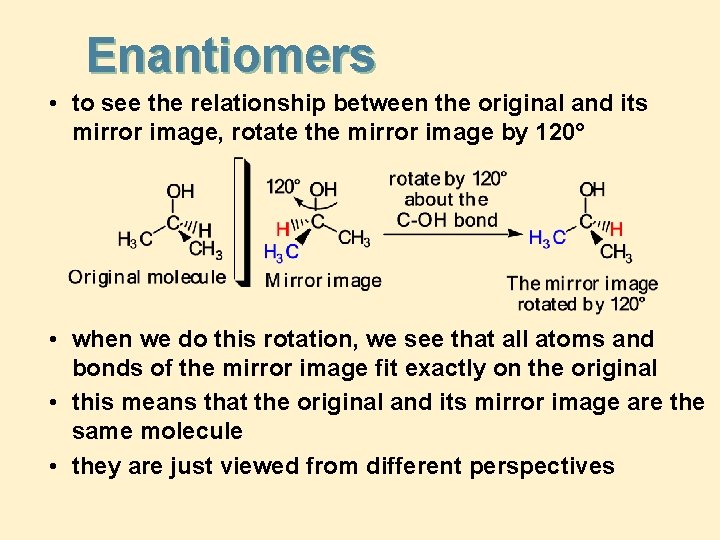
Enantiomers • to see the relationship between the original and its mirror image, rotate the mirror image by 120° • when we do this rotation, we see that all atoms and bonds of the mirror image fit exactly on the original • this means that the original and its mirror image are the same molecule • they are just viewed from different perspectives

Enantiomers • To summarize • objects that are nonsuperposable on their mirror images are chiral (they show handedness) • the most common cause of chirality among organic molecules is the presence of a carbon with four different groups bonded to it • we call a carbon with four different groups bonded to it a stereocenter • objects that are superposable on their mirror images are achiral (without chirality) • nonsuperposable mirror images are called enantiomers • enantiomers always come in pairs

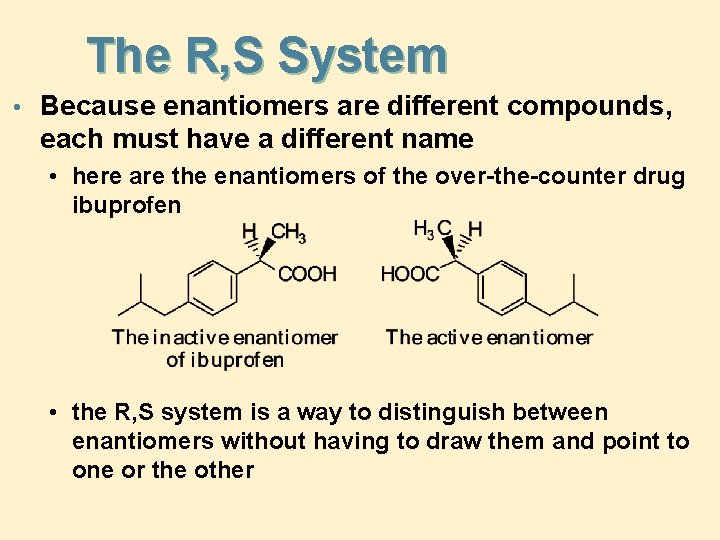
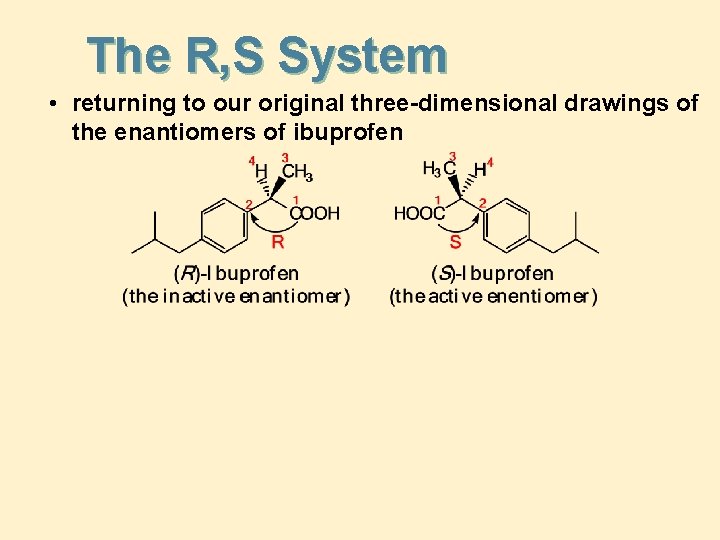
The R, S System • Because enantiomers are different compounds, each must have a different name • here are the enantiomers of the over-the-counter drug ibuprofen • the R, S system is a way to distinguish between enantiomers without having to draw them and point to one or the other

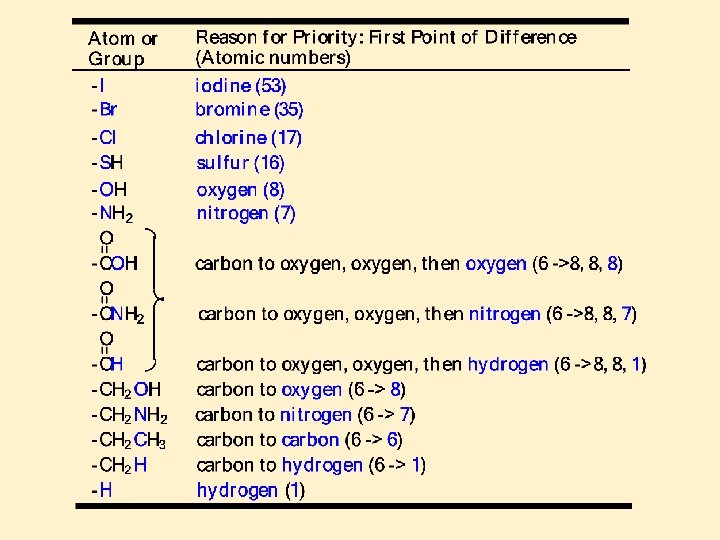
The R, S System • The first step in assigning an R or S configuration to a stereocenter is to arrange the groups on the stereocenter in order of priority • priority is based on atomic number • the higher the atomic number, the higher the priority If priority cannot be assigned on the basis of the atoms directly attached to the stereo center look at the next atom and continue to the first point of difference.


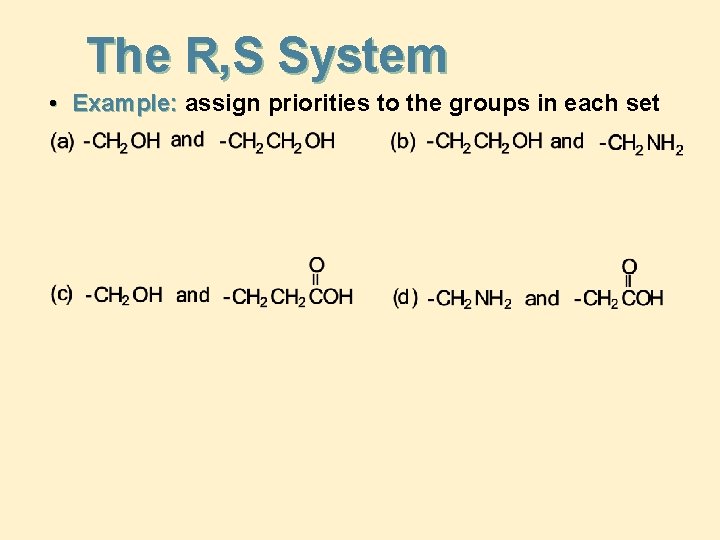
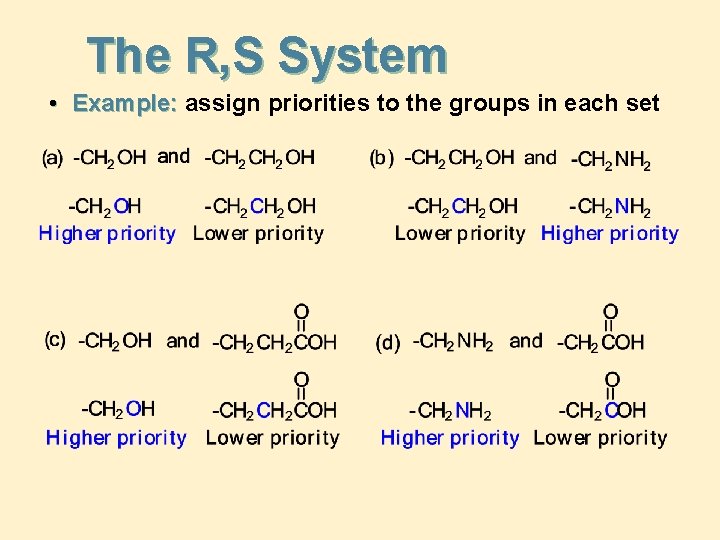
The R, S System • Example: assign priorities to the groups in each set

The R, S System • Example: assign priorities to the groups in each set

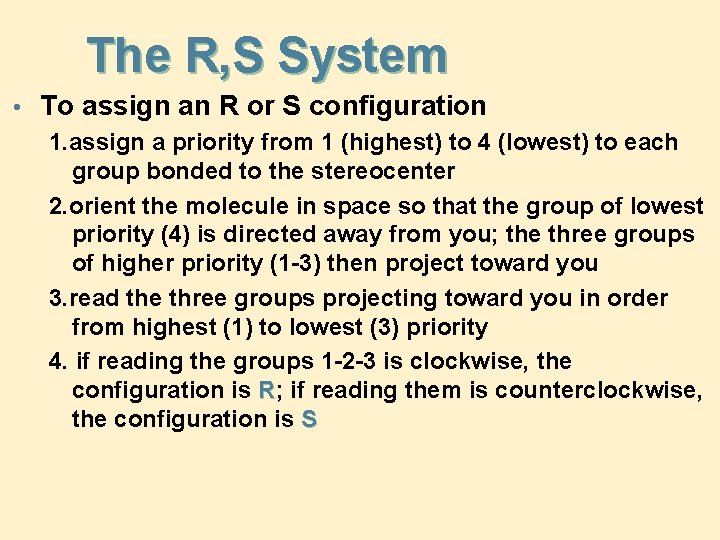
The R, S System • To assign an R or S configuration 1. assign a priority from 1 (highest) to 4 (lowest) to each group bonded to the stereocenter 2. orient the molecule in space so that the group of lowest priority (4) is directed away from you; the three groups of higher priority (1 -3) then project toward you 3. read the three groups projecting toward you in order from highest (1) to lowest (3) priority 4. if reading the groups 1 -2 -3 is clockwise, the configuration is R; if reading them is counterclockwise, the configuration is S

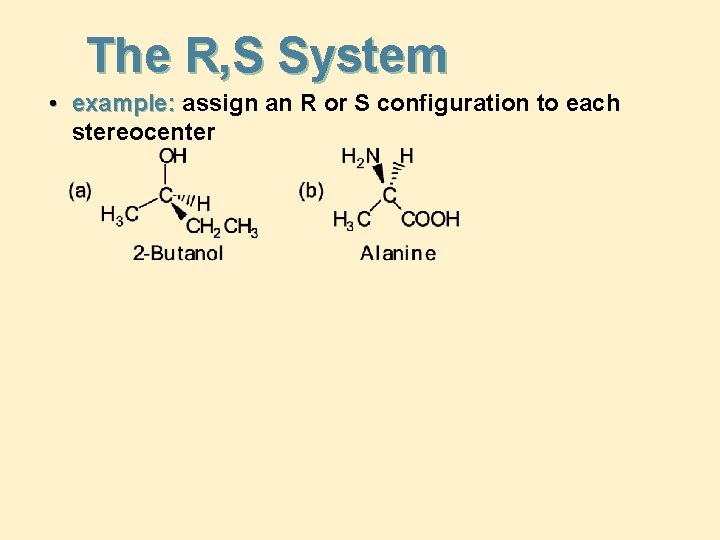
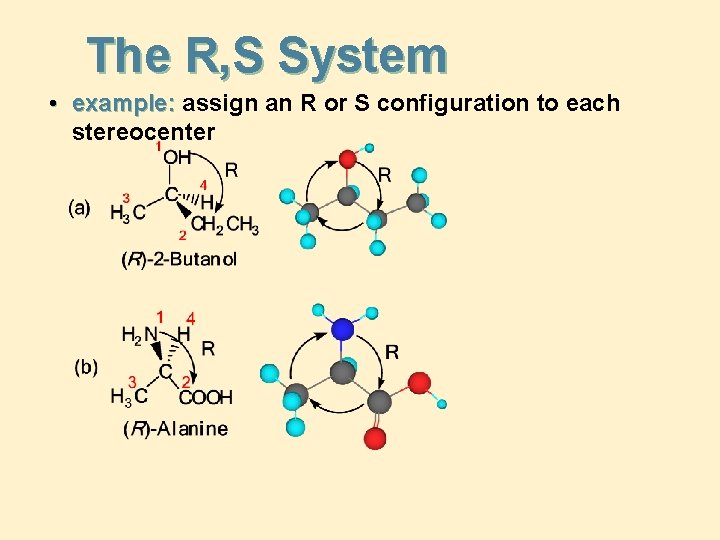
The R, S System • example: assign an R or S configuration to each stereocenter

The R, S System • example: assign an R or S configuration to each stereocenter

The R, S System • returning to our original three-dimensional drawings of the enantiomers of ibuprofen

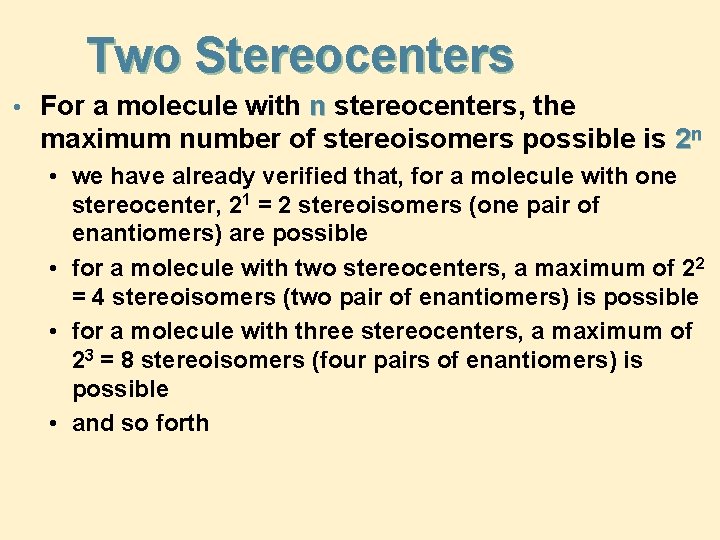
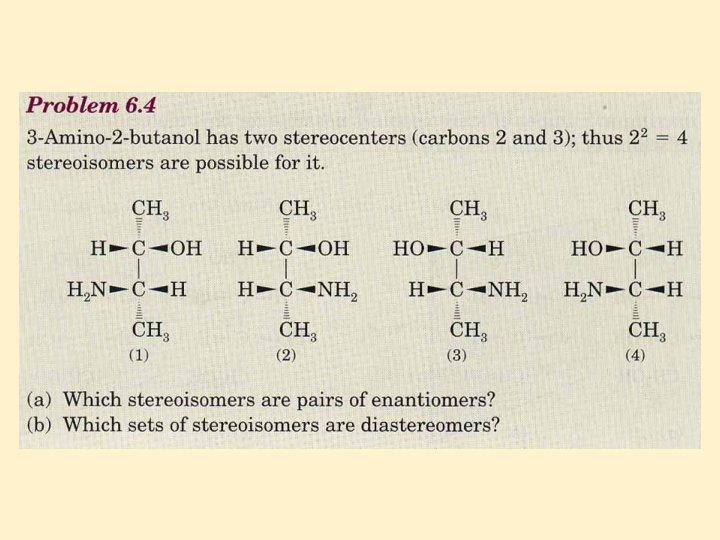
Two Stereocenters • For a molecule with n stereocenters, the maximum number of stereoisomers possible is 2 n • we have already verified that, for a molecule with one stereocenter, 21 = 2 stereoisomers (one pair of enantiomers) are possible • for a molecule with two stereocenters, a maximum of 22 = 4 stereoisomers (two pair of enantiomers) is possible • for a molecule with three stereocenters, a maximum of 23 = 8 stereoisomers (four pairs of enantiomers) is possible • and so forth

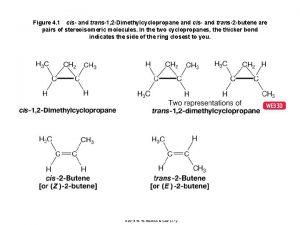
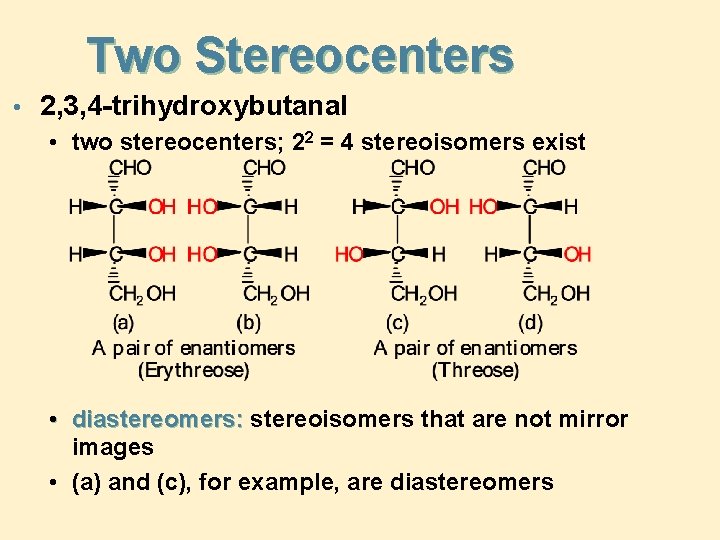
Two Stereocenters • 2, 3, 4 -trihydroxybutanal • two stereocenters; 22 = 4 stereoisomers exist • diastereomers: stereoisomers that are not mirror images • (a) and (c), for example, are diastereomers


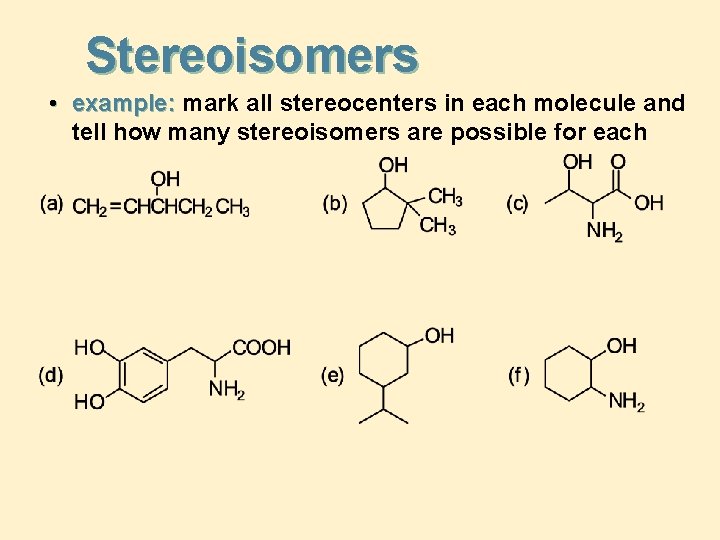
Stereoisomers • example: mark all stereocenters in each molecule and tell how many stereoisomers are possible for each

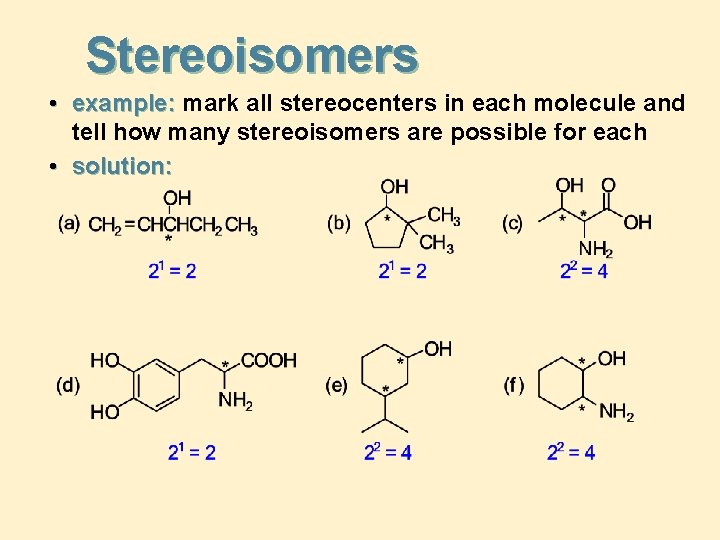
Stereoisomers • example: mark all stereocenters in each molecule and tell how many stereoisomers are possible for each • solution:

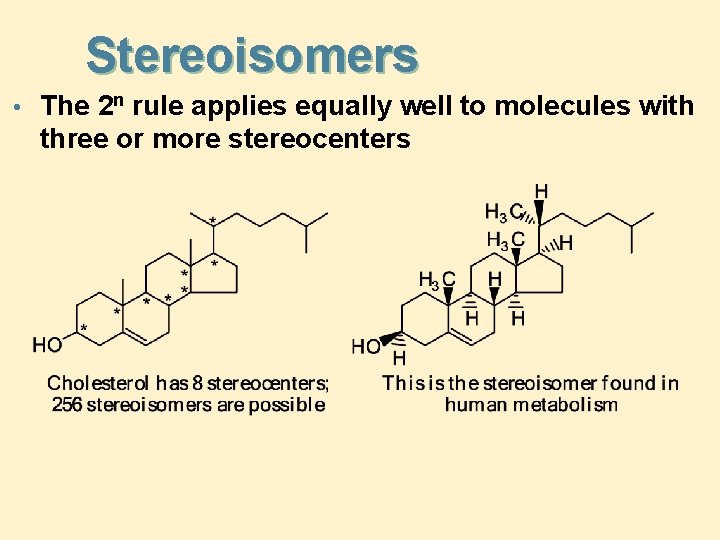
Stereoisomers • The 2 n rule applies equally well to molecules with three or more stereocenters

A pair of enantiomers have identical physical properties except for the way they rotate the plane of polarized light. A pure enantiomer is said to be optically active. Diastereomers have different physical properties.

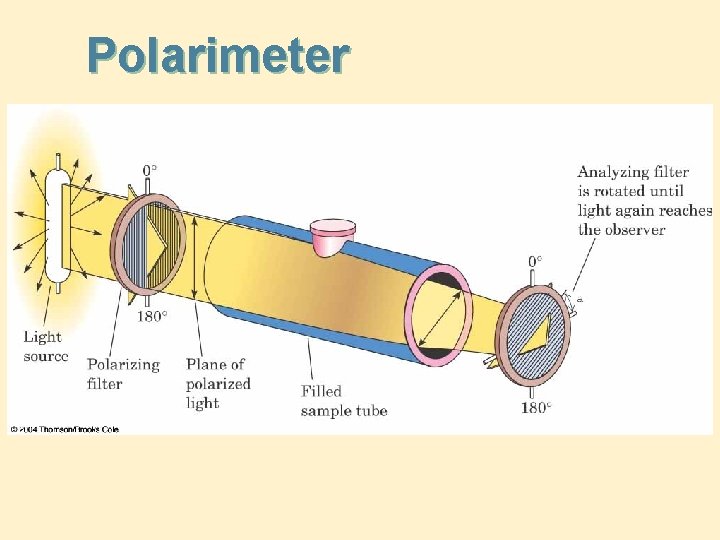
Optical Activity • Ordinary light: light waves vibrating in all planes perpendicular to its direction of propagation • Plane-polarized light: light waves vibrating only in parallel planes • Polarimeter: an instrument for measuring the ability of a compound to rotate the plane of planepolarized light • Optically active: showing that a compound rotates the plane of plane-polarized light

Polarimeter

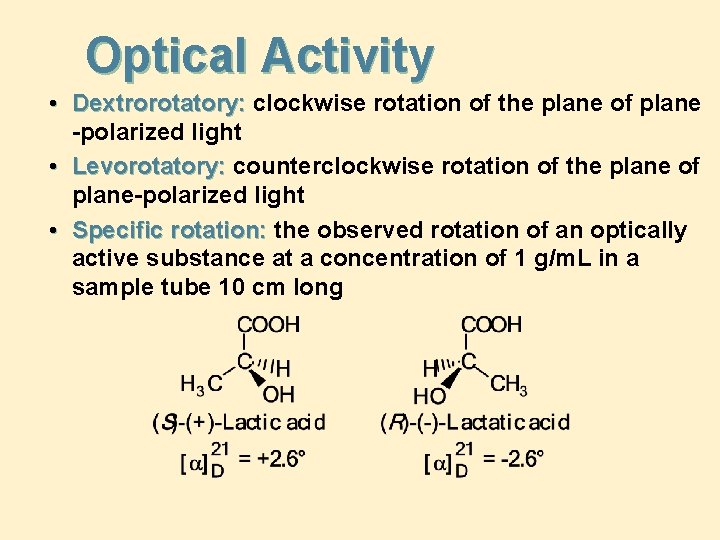
Optical Activity • Dextrorotatory: clockwise rotation of the plane of plane -polarized light • Levorotatory: counterclockwise rotation of the plane of plane-polarized light • Specific rotation: the observed rotation of an optically active substance at a concentration of 1 g/m. L in a sample tube 10 cm long

Chirality in Biomolecules • Except for inorganic salts and a few low- molecular-weight organic substances, the molecules in living systems, both plant and animal, are chiral • although these molecules can exist as a number of stereoisomers, almost invariably one stereoisomer is found in nature • instances do occur in which more than one stereoisomer is found, but these rarely exist together in the same biological system

Chirality in Biomolecules • Enzymes (protein bio-catalysts) all have many stereocenters • an example is chymotrypsin, an enzyme in the intestines of animals that catalyzes the digestion of proteins • chymotrypsin has 251 stereocenters • the maximum number of stereoisomers possible is 2251! • only one of these stereoisomers is produced and used by any given organism • because enzymes are chiral substances, most either produce or react with only substances that match their stereochemical requirements

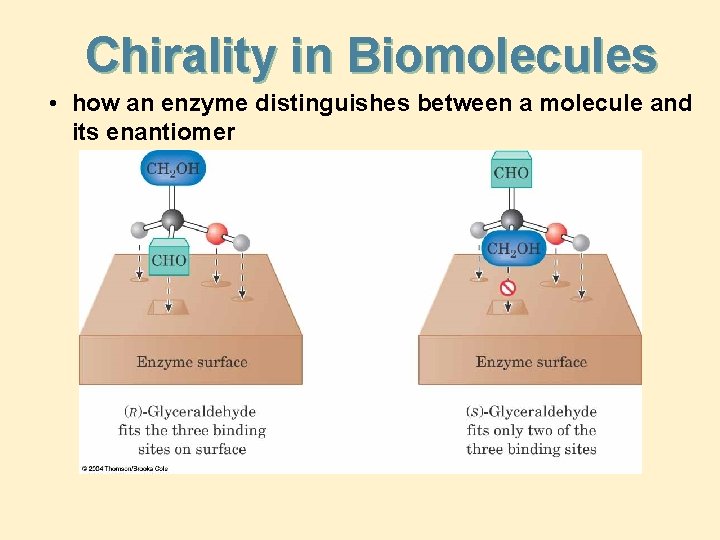
Chirality in Biomolecules • how an enzyme distinguishes between a molecule and its enantiomer

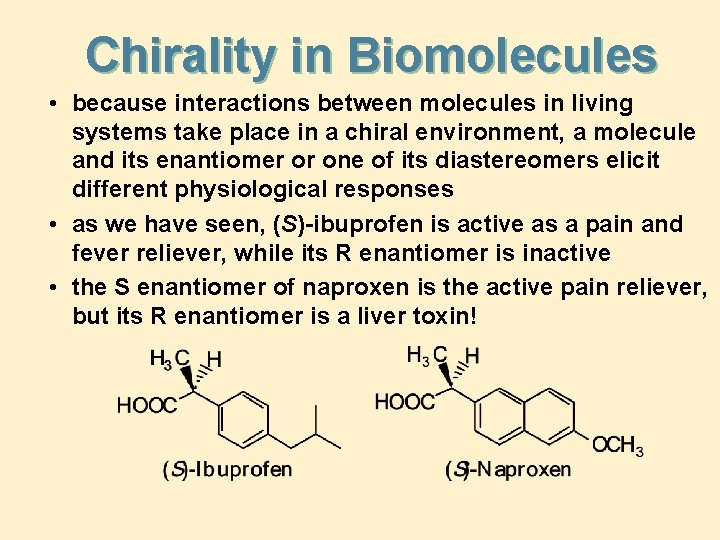
Chirality in Biomolecules • because interactions between molecules in living systems take place in a chiral environment, a molecule and its enantiomer or one of its diastereomers elicit different physiological responses • as we have seen, (S)-ibuprofen is active as a pain and fever reliever, while its R enantiomer is inactive • the S enantiomer of naproxen is the active pain reliever, but its R enantiomer is a liver toxin!

Chirality End Chapter 6
 Enantiomer vs diastereomer
Enantiomer vs diastereomer Stereoisomers always possess handedness.
Stereoisomers always possess handedness. Dextrorotatory and levorotatory
Dextrorotatory and levorotatory Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Absolute vs relative configuration
Absolute vs relative configuration Virginie simonet
Virginie simonet Cis-1 2-dichlorocyclopropane chiral
Cis-1 2-dichlorocyclopropane chiral Chirality
Chirality Chirality centers in sucralose
Chirality centers in sucralose Chirality definition
Chirality definition Chapter 3 molecules of life
Chapter 3 molecules of life Chapter 2 atoms molecules and ions
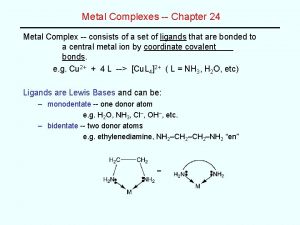
Chapter 2 atoms molecules and ions Coordination isomerism in coordination compounds
Coordination isomerism in coordination compounds Nonsuperimposable
Nonsuperimposable Physical properties of amines
Physical properties of amines C5h12 structural isomers
C5h12 structural isomers Optical purity formula
Optical purity formula Is alkane saturated
Is alkane saturated Butyl isomers
Butyl isomers Coordination outline
Coordination outline Isomers of c7 h16
Isomers of c7 h16 Structure name
Structure name Alkyl substituents
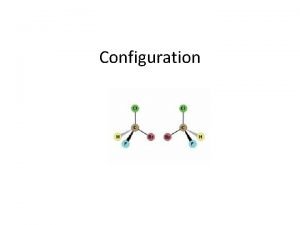
Alkyl substituents R and s isomers
R and s isomers Superimposable mirror image
Superimposable mirror image Coordination isomers
Coordination isomers Difference between structural and geometric isomers
Difference between structural and geometric isomers Difference between structural and geometric isomers
Difference between structural and geometric isomers Isomer benzena
Isomer benzena Conformations
Conformations Conformational isomers are also known as
Conformational isomers are also known as E/z isomers
E/z isomers Trans-1-chloro-3-methylcyclohexane
Trans-1-chloro-3-methylcyclohexane Cis trans isomers
Cis trans isomers