Carbon and Organic Molecules Organic Molecules Organic molecules
Carbon and Organic Molecules

Organic Molecules Organic molecules —compounds mostly found in living things and containing the element carbon. Ex. Carbohydrates, lipids. Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 4 -1

Organic molecule origins • Vitalism – organic molecules can only be made by living organisms. • Mechanism -is the view that organic molecules can be created through physical and chemical means. • Miller proved mechanism in an experiment. Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 4 -2 EXPERIMENT “Atmosphere” CH 4 Water vapor Electrode NH 3 H 2 Condenser Cooled water containing organic molecules H 2 O “sea” Sample for chemical analysis Cold water

4 reasons: Why use carbon -4 valence electrons, can form 4 bonds -can link together in chains, branches, or rings -can form single, double, or triple bonds -bonds with a variety of elements Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 4 -4 Hydrogen (valence = 1) Oxygen (valence = 6) Nitrogen (valence = 5) Carbon (valence = 4) H O N C

Fig. 4 -3 Name (a) Methane (b) Ethane (c) Ethene (ethylene) Molecular Formula Structural Formula Ball-and-Stick Model Space-Filling Model

Hydrocarbons – only carbon and hydrogen Ethane Propane (a) Length Butane (b) Branching 1 -Butene 2 -Butene (c) Double bonds 2 -Methylpropane (commonly called isobutane) Cyclohexane (d) Rings Benzene

Isomers • Isomers are compounds with the same molecular formula but different shapes: – Structural isomers have different covalent arrangements of their atoms Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
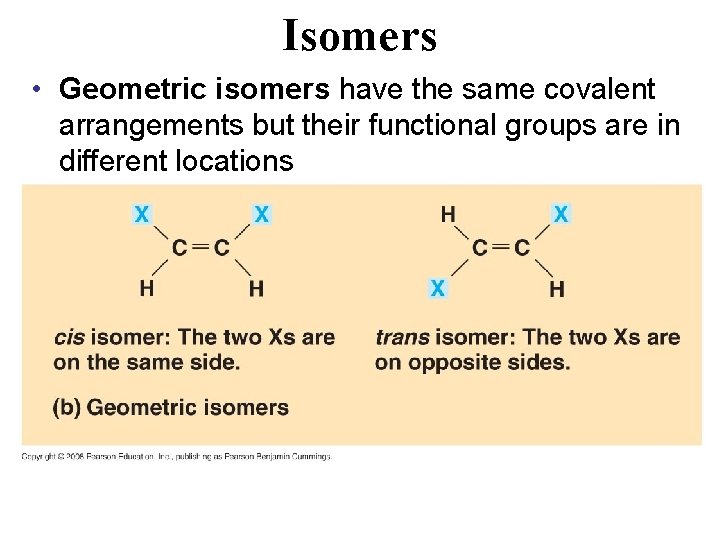
Isomers • Geometric isomers have the same covalent arrangements but their functional groups are in different locations
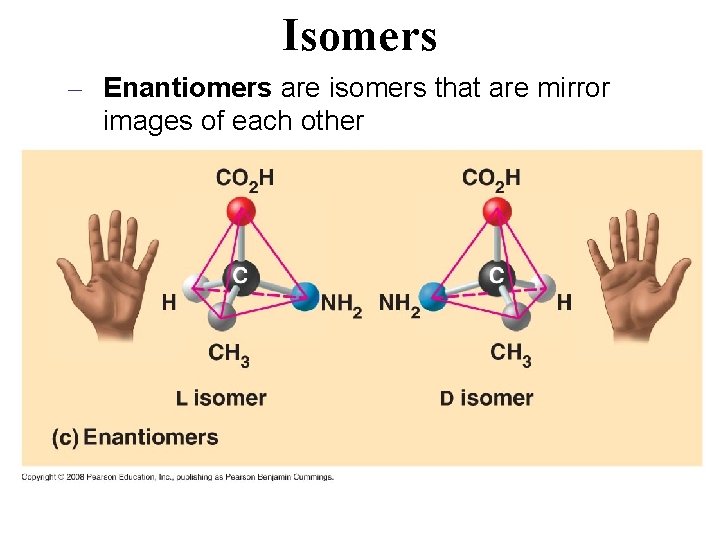
Isomers – Enantiomers are isomers that are mirror images of each other
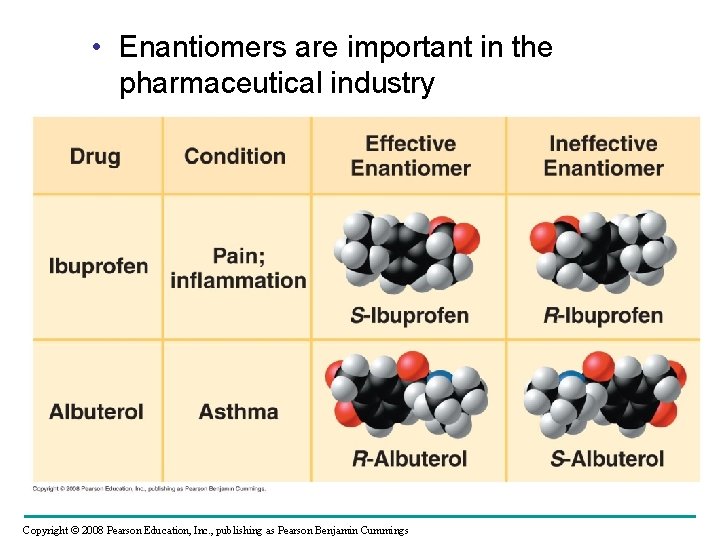
• Enantiomers are important in the pharmaceutical industry Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Functional Groups • Functional groups – They are the components of organic molecules that are most commonly involved in chemical reactions. They give the molecules specific properties. Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

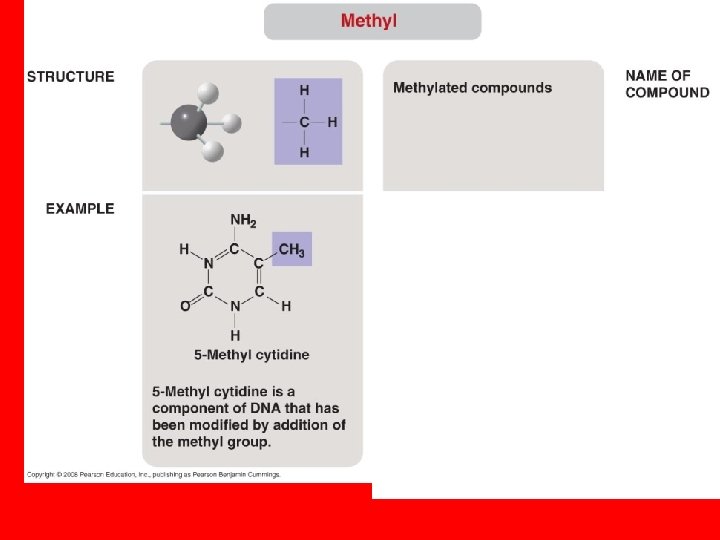
• The seven functional groups that are most important in the chemistry of life: – Hydroxyl group – Carbonyl group – Carboxyl group – Amino group – Sulfhydryl group – Phosphate group – Methyl group Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
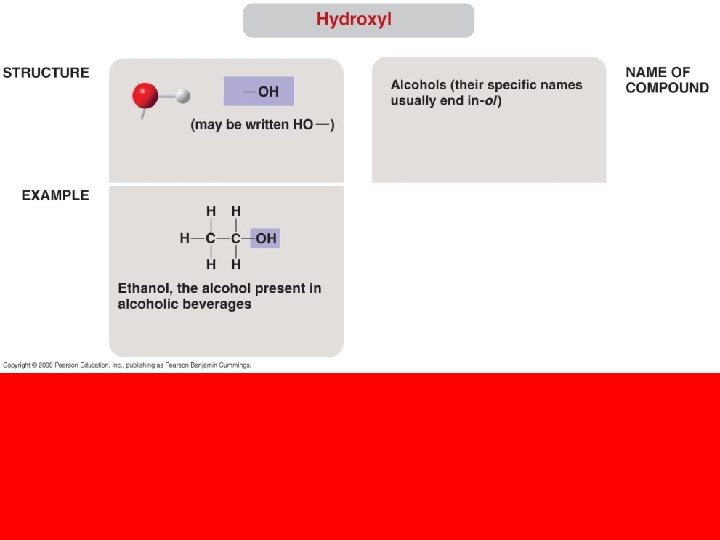

sugars
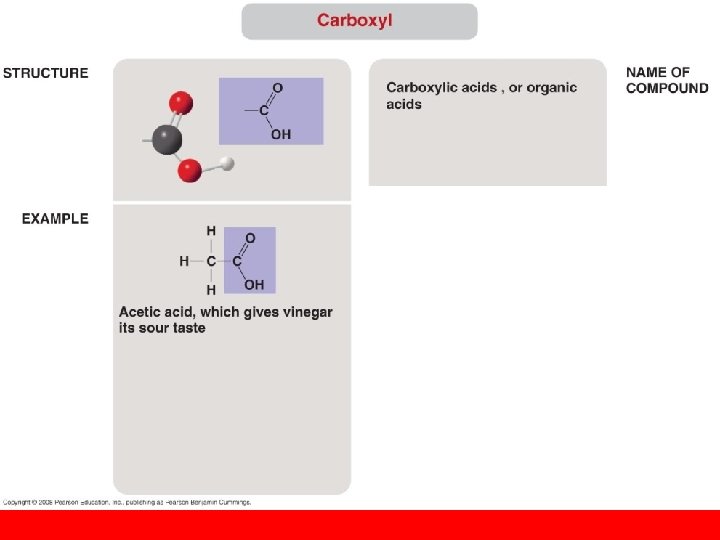

(Builds proteins)

Found in proteins

(fats)
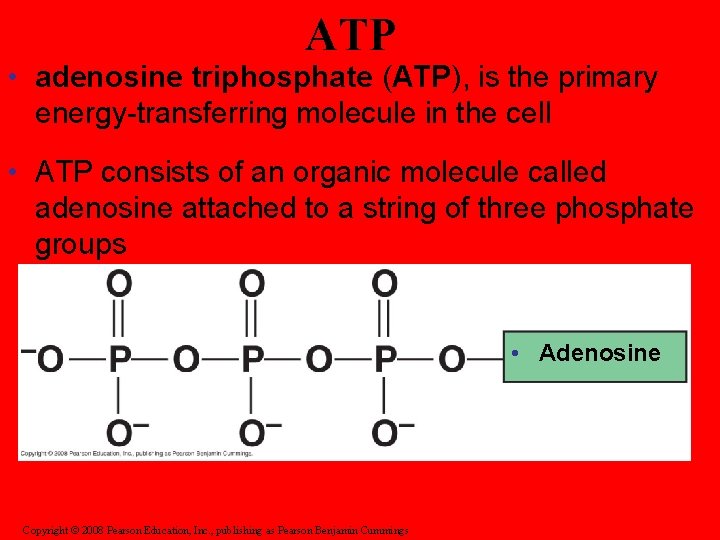
ATP • adenosine triphosphate (ATP), is the primary energy-transferring molecule in the cell • ATP consists of an organic molecule called adenosine attached to a string of three phosphate groups • Adenosine Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
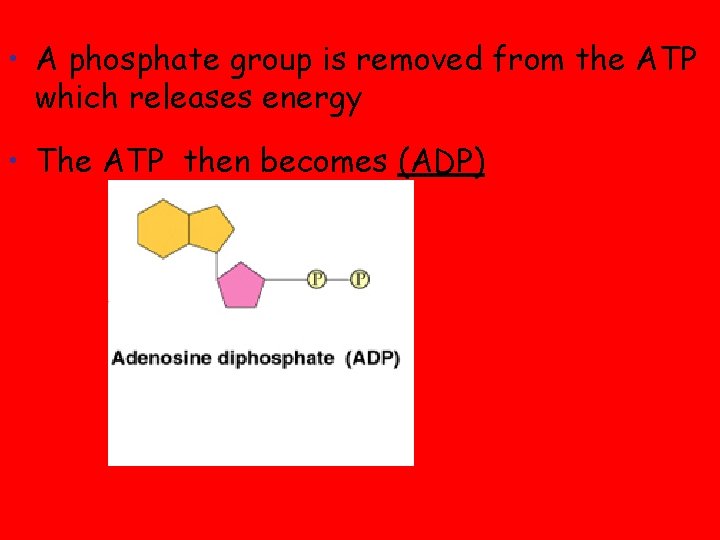
• A phosphate group is removed from the ATP which releases energy • The ATP then becomes (ADP)

• A third phosphate group can be added back on to ADP so it become ATP again (recycling)

You should now be able to: 1. Explain how carbon’s electron configuration explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isomers: structural, geometric, and enantiomer Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
4. Name the major functional groups found in organic molecules; describe the basic structure of each functional group and outline the chemical properties of the organic molecules in which they occur 5. Explain how ATP functions as the primary energy transfer molecule in living cells Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
- Slides: 27