Isomers 1 Structural 2 Stereo isomers geometric optical






- Slides: 28

Isomers 1. Structural 2. Stereo isomers - geometric - optical Author: J R Reid

Starter: Draw the 2 possible products formed when HCl is added to 2 -methylbut-2 -ene. Name the products and identify which is the major product. (Markovnikov’s rule) Which type of reaction is this? addition, elimination or substitution

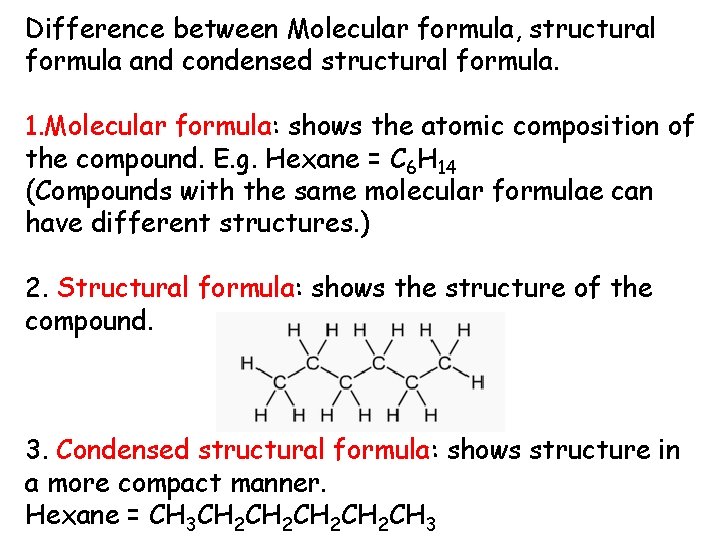
Difference between Molecular formula, structural formula and condensed structural formula. 1. Molecular formula: shows the atomic composition of the compound. E. g. Hexane = C 6 H 14 (Compounds with the same molecular formulae can have different structures. ) 2. Structural formula: shows the structure of the compound. 3. Condensed structural formula: shows structure in a more compact manner. Hexane = CH 3 CH 2 CH 2 CH 3

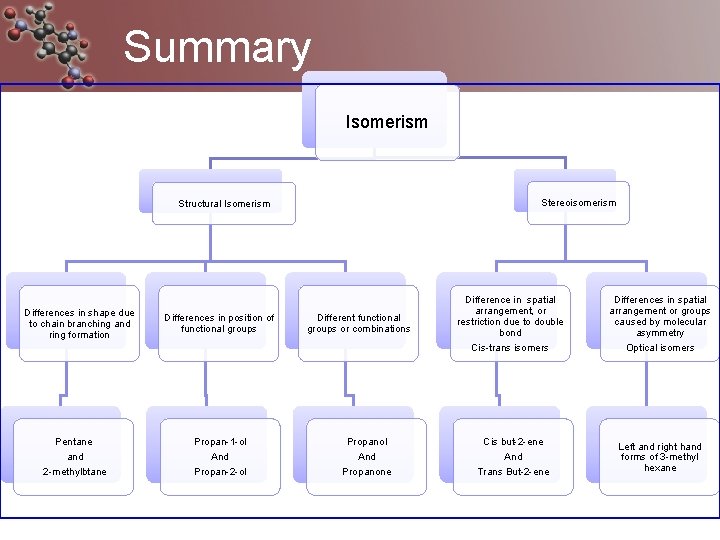
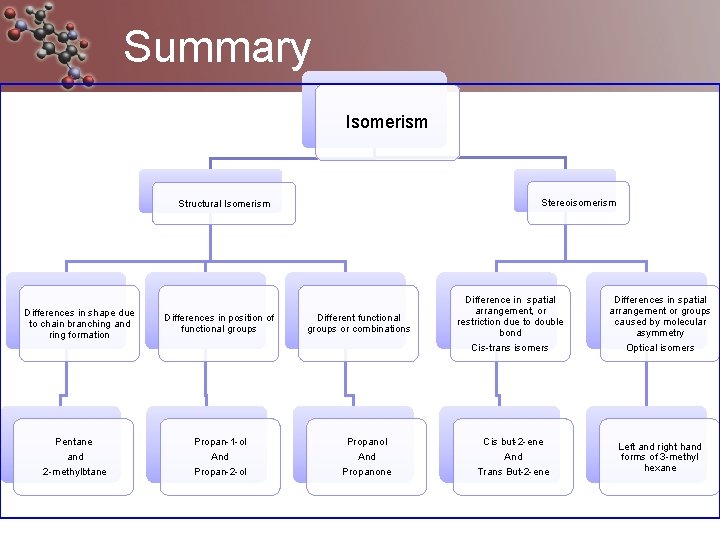
Summary Isomerism Stereoisomerism Structural Isomerism Differences in shape due to chain branching and ring formation Pentane and 2 -methylbtane Differences in position of functional groups Propan-1 -ol And Propan-2 -ol Different functional groups or combinations Propanol And Propanone Difference in spatial arrangement, or restriction due to double bond Cis-trans isomers Differences in spatial arrangement or groups caused by molecular asymmetry Optical isomers Cis but-2 -ene And Trans But-2 -ene Left and right hand forms of 3 -methyl hexane

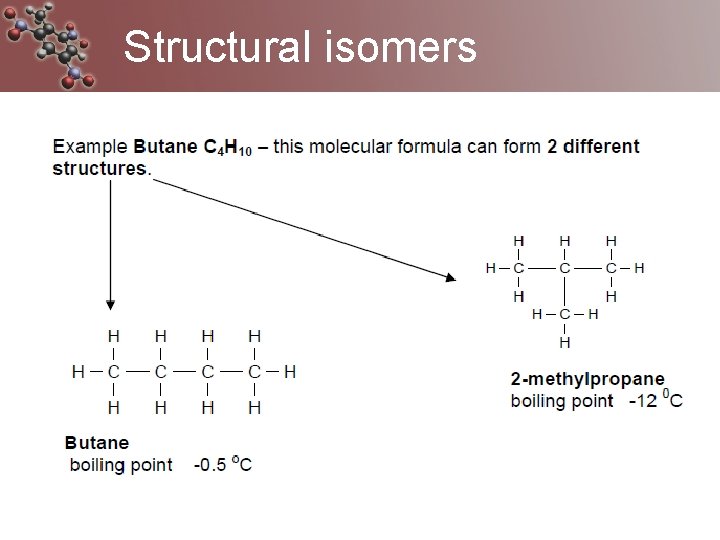
Structural isomers Sometimes called constitutional isomers Same molecular formula but a different arrangement of atoms (structure) Can have different physical and chemical properties.

Structural isomers

Stereoisomers Atoms arranged in same order but different position in space There are two types 1. Geometric 2. Optical

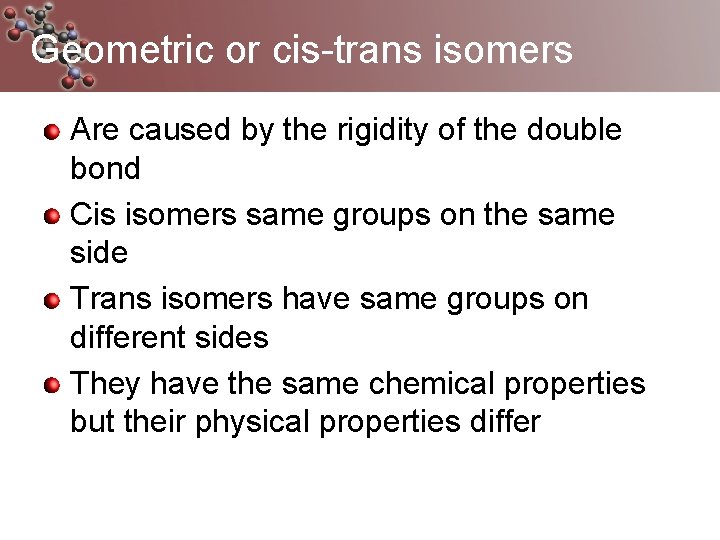
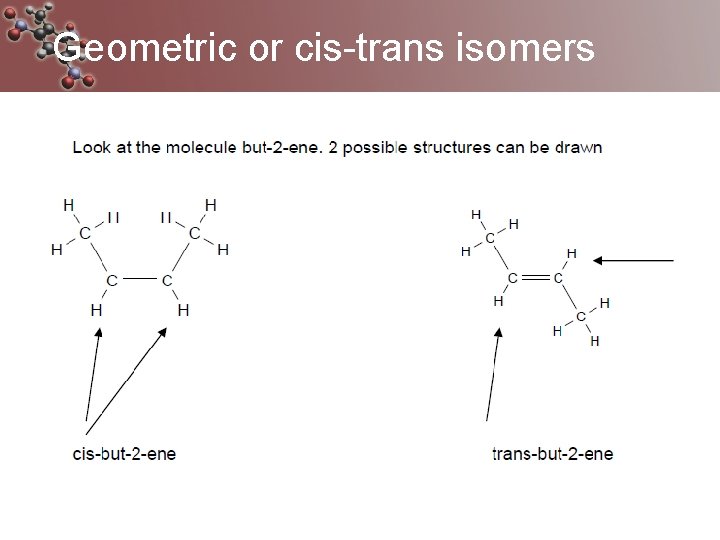
Geometric or cis-trans isomers Are caused by the rigidity of the double bond Cis isomers same groups on the same side Trans isomers have same groups on different sides They have the same chemical properties but their physical properties differ

Geometric or cis-trans isomers

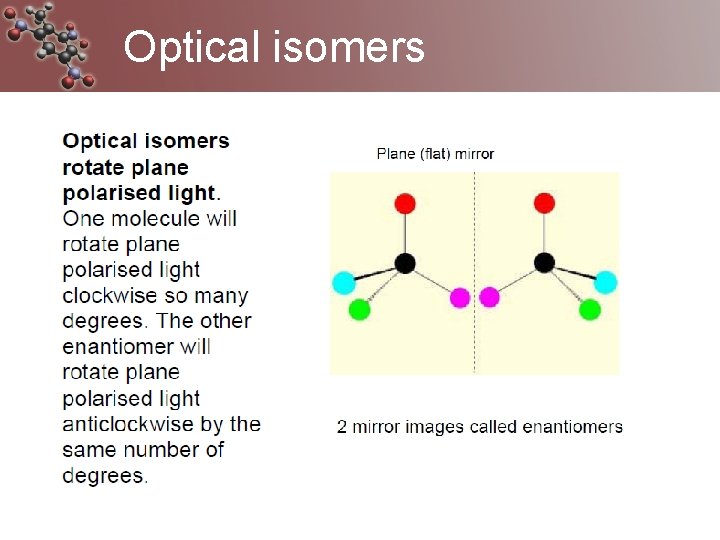
Optical Isomers A form of stereoisomerism • In stereoisomerism, the atoms making up the isomers are joined up in the same order, but still manage to have a different spatial arrangement. Why optical isomers? • Optical isomers are named like this because of their effect on plane polarised light. • Simple substances which show optical isomerism exist as two isomers known as enantiomers.

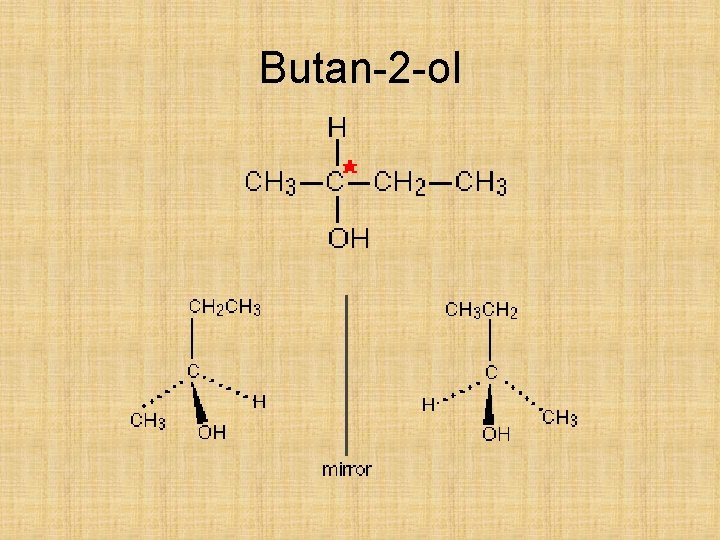
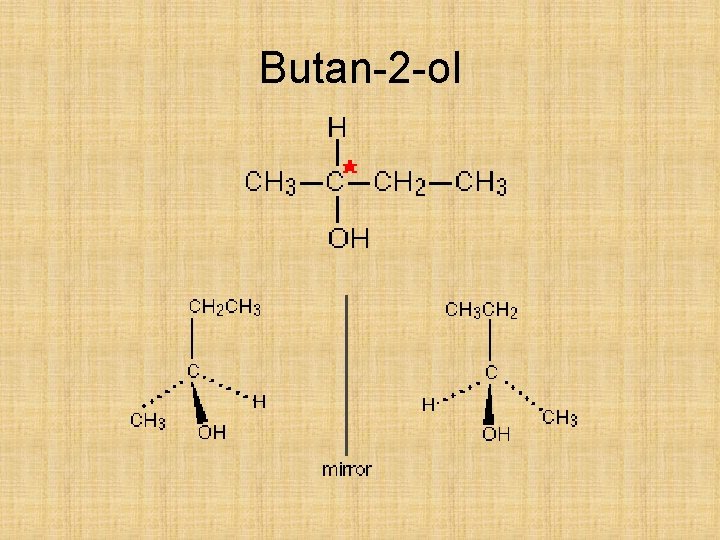
Butan-2 -ol

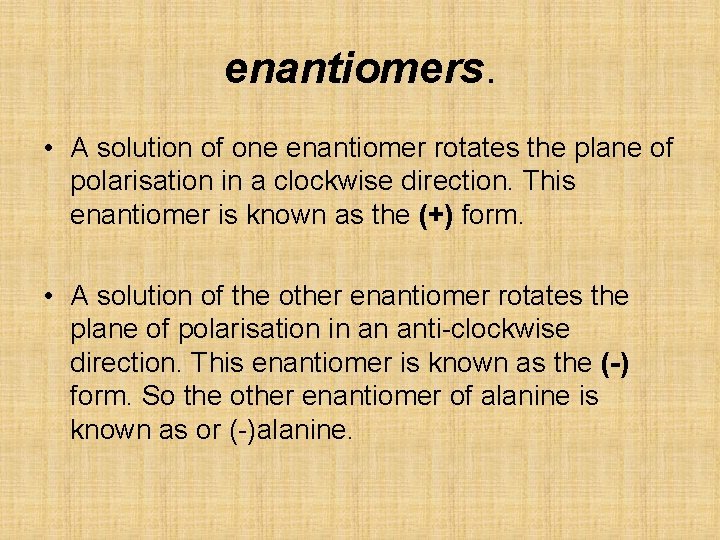
enantiomers. • A solution of one enantiomer rotates the plane of polarisation in a clockwise direction. This enantiomer is known as the (+) form. • A solution of the other enantiomer rotates the plane of polarisation in an anti-clockwise direction. This enantiomer is known as the (-) form. So the other enantiomer of alanine is known as or (-)alanine.

racemic mixture • When optically active substances are made in the lab, they often occur as a 50/50 mixture of the two enantiomers. This is known as a racemic mixture or racemate. • It has no effect on plane polarised light.

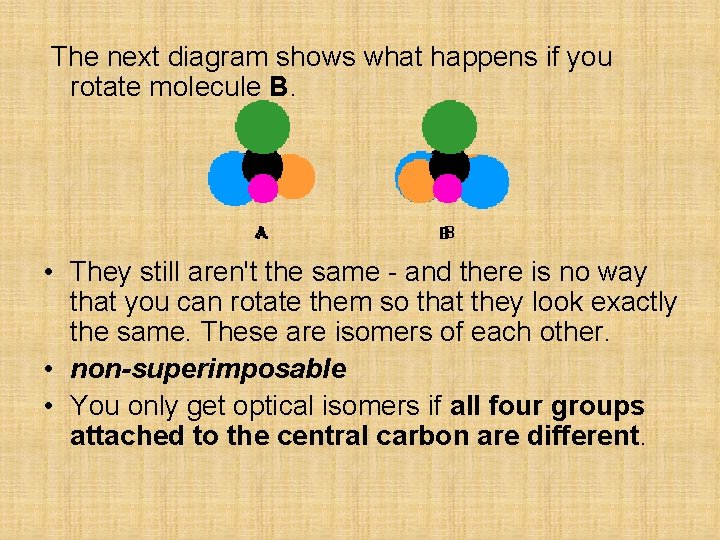
The examples of organic optical isomers required at this level all contain a carbon atom joined to four different groups. These two models each have the same groups joined to the central carbon atom, but still manage to be different: Obviously as they are drawn, the orange and blue groups aren't aligned the same way. Could you get them to align by rotating one of the molecules?

The next diagram shows what happens if you rotate molecule B. • They still aren't the same - and there is no way that you can rotate them so that they look exactly the same. These are isomers of each other. • non-superimposable • You only get optical isomers if all four groups attached to the central carbon are different.

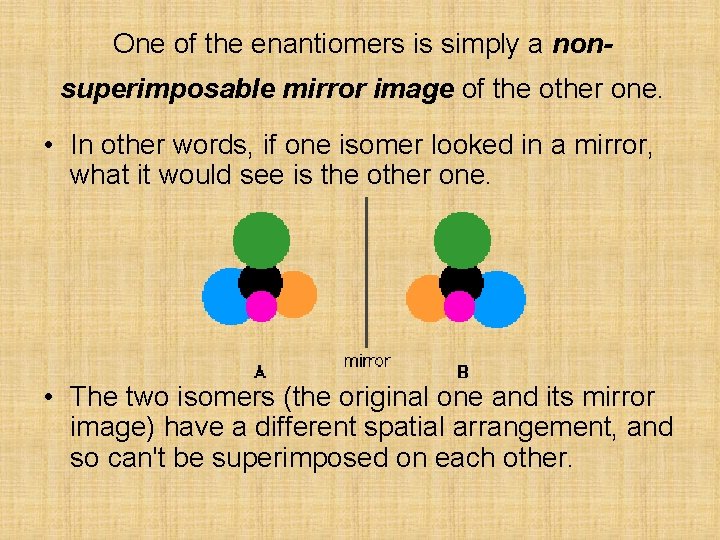
One of the enantiomers is simply a nonsuperimposable mirror image of the other one. • In other words, if one isomer looked in a mirror, what it would see is the other one. • The two isomers (the original one and its mirror image) have a different spatial arrangement, and so can't be superimposed on each other.

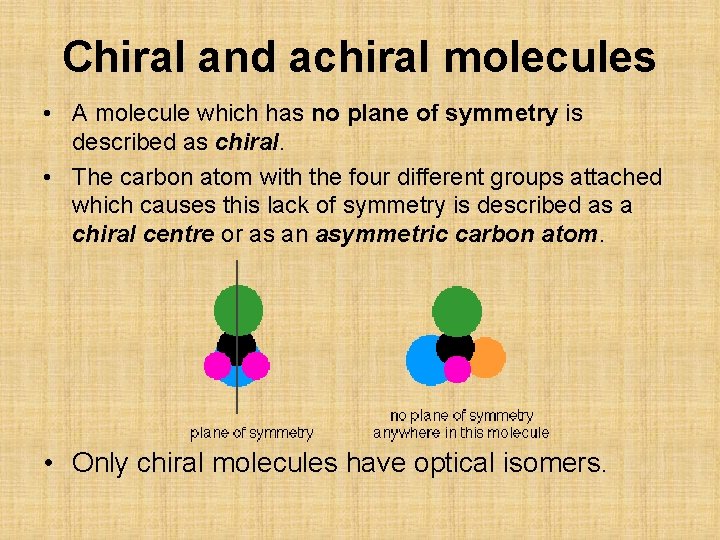
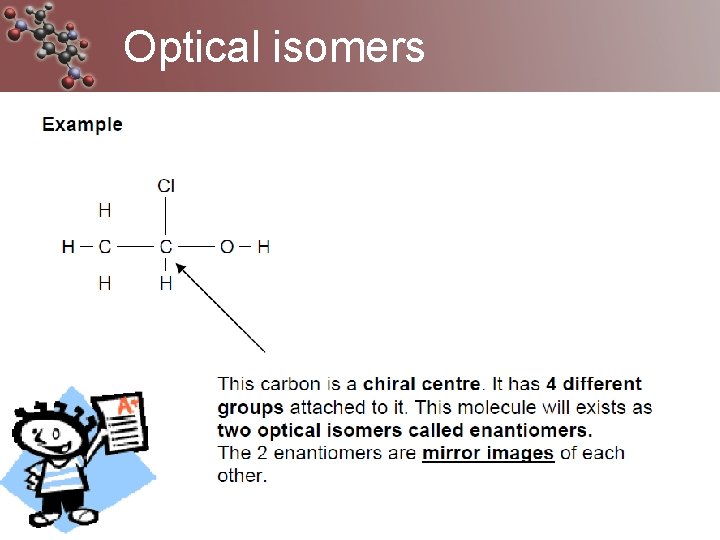
Chiral and achiral molecules • A molecule which has no plane of symmetry is described as chiral. • The carbon atom with the four different groups attached which causes this lack of symmetry is described as a chiral centre or as an asymmetric carbon atom. • Only chiral molecules have optical isomers.

Butan-2 -ol

Summary Isomerism Stereoisomerism Structural Isomerism Differences in shape due to chain branching and ring formation Pentane and 2 -methylbtane Differences in position of functional groups Propan-1 -ol And Propan-2 -ol Different functional groups or combinations Propanol And Propanone Difference in spatial arrangement, or restriction due to double bond Cis-trans isomers Differences in spatial arrangement or groups caused by molecular asymmetry Optical isomers Cis but-2 -ene And Trans But-2 -ene Left and right hand forms of 3 -methyl hexane

Key words Structural isomers Stereo isomers Cis and trans isomers Optical isomers or enantiomers Asymmetric carbon Chiral racemic

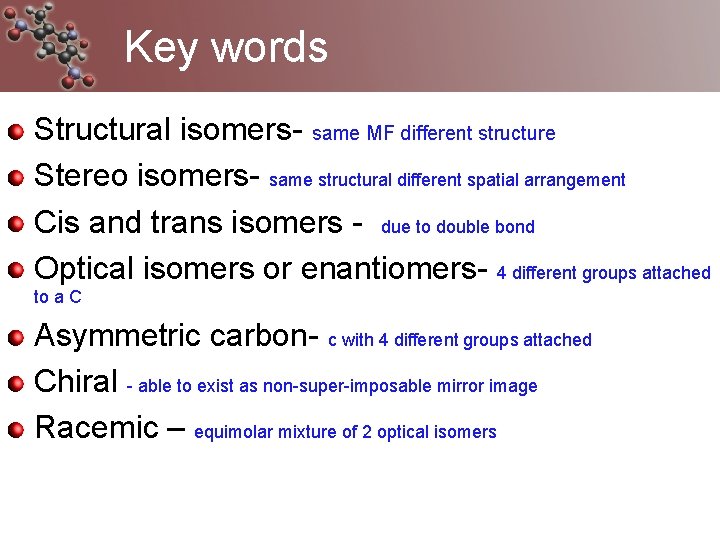
Key words Structural isomers- same MF different structure Stereo isomers- same structural different spatial arrangement Cis and trans isomers - due to double bond Optical isomers or enantiomers- 4 different groups attached to a C Asymmetric carbon- c with 4 different groups attached Chiral - able to exist as non-super-imposable mirror image Racemic – equimolar mixture of 2 optical isomers

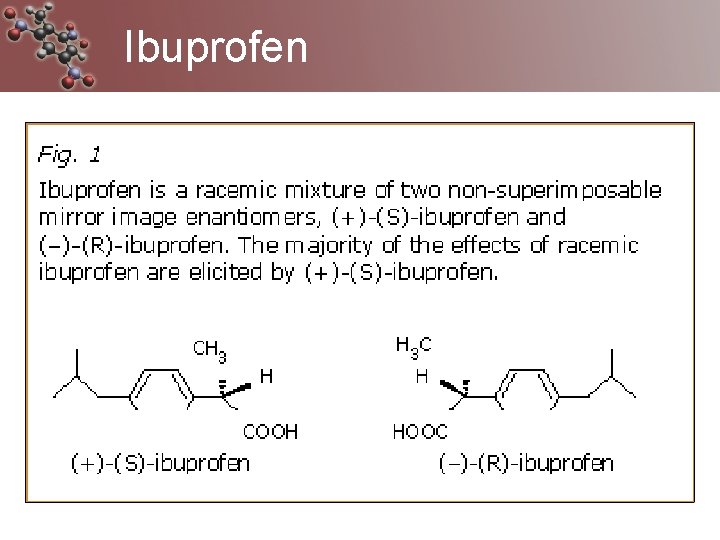
Ibuprofen


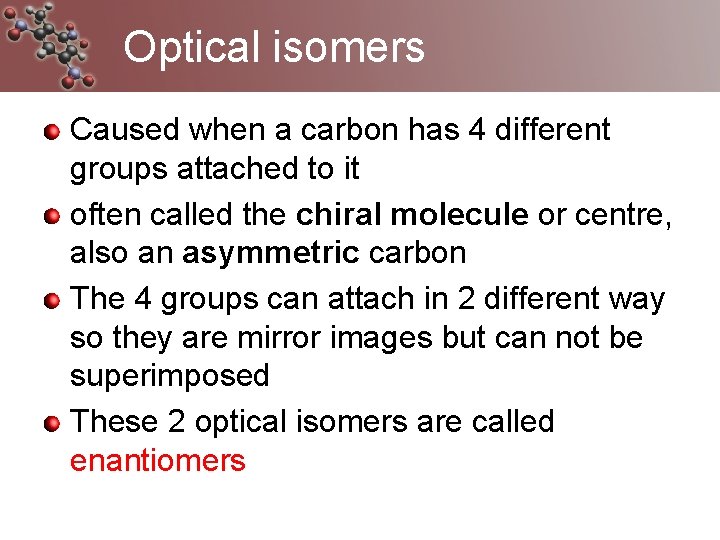
Optical isomers Caused when a carbon has 4 different groups attached to it often called the chiral molecule or centre, also an asymmetric carbon The 4 groups can attach in 2 different way so they are mirror images but can not be superimposed These 2 optical isomers are called enantiomers

Optical isomers

Optical isomers

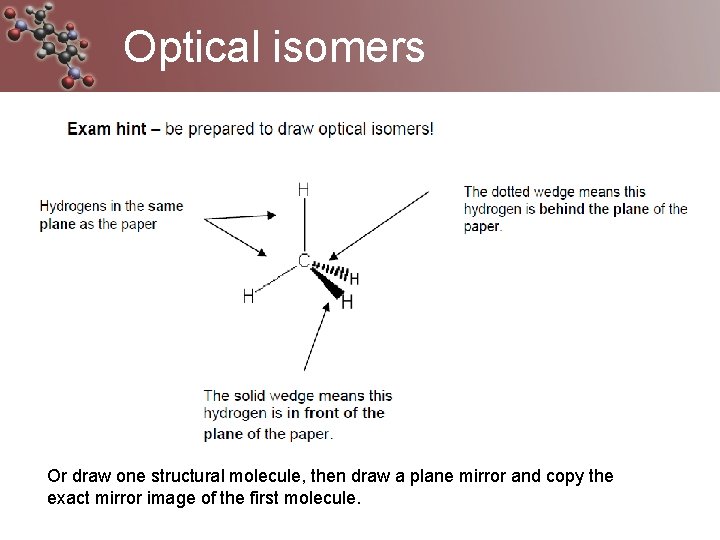
Optical isomers Or draw one structural molecule, then draw a plane mirror and copy the exact mirror image of the first molecule.

Optical isomers Try to draw the two optical isomers of butan-2 -ol