Alcohols Contain a hydroxyl OH group Intermolecular forces

- Slides: 32

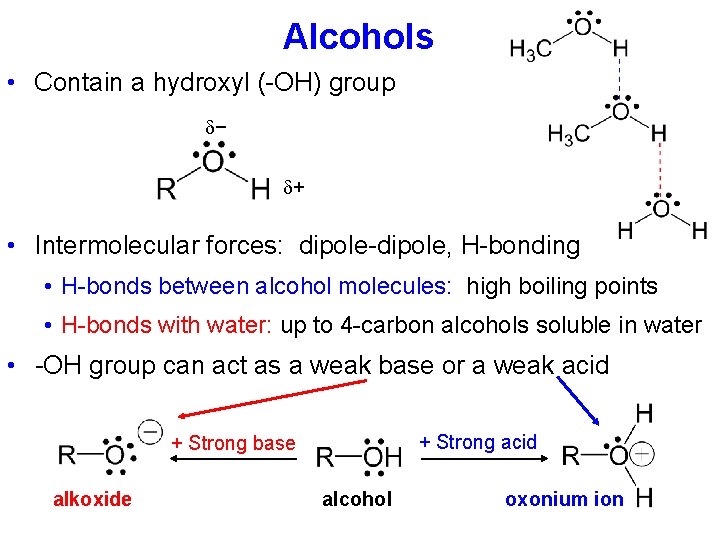
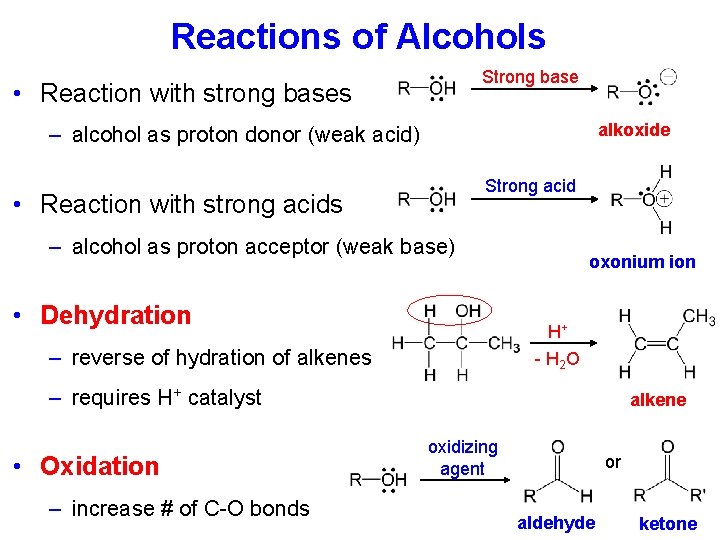
Alcohols • Contain a hydroxyl (-OH) group − + • Intermolecular forces: dipole-dipole, H-bonding • H-bonds between alcohol molecules: high boiling points • H-bonds with water: up to 4 -carbon alcohols soluble in water • -OH group can act as a weak base or a weak acid + Strong base alkoxide alcohol oxonium ion

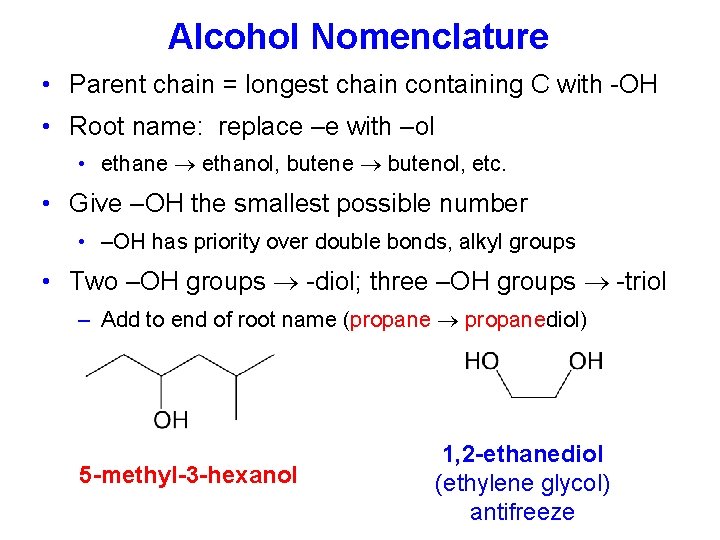
Alcohol Nomenclature • Parent chain = longest chain containing C with -OH • Root name: replace –e with –ol • ethane ethanol, butene butenol, etc. • Give –OH the smallest possible number • –OH has priority over double bonds, alkyl groups • Two –OH groups -diol; three –OH groups -triol – Add to end of root name (propane propanediol) 5 -methyl-3 -hexanol 1, 2 -ethanediol (ethylene glycol) antifreeze

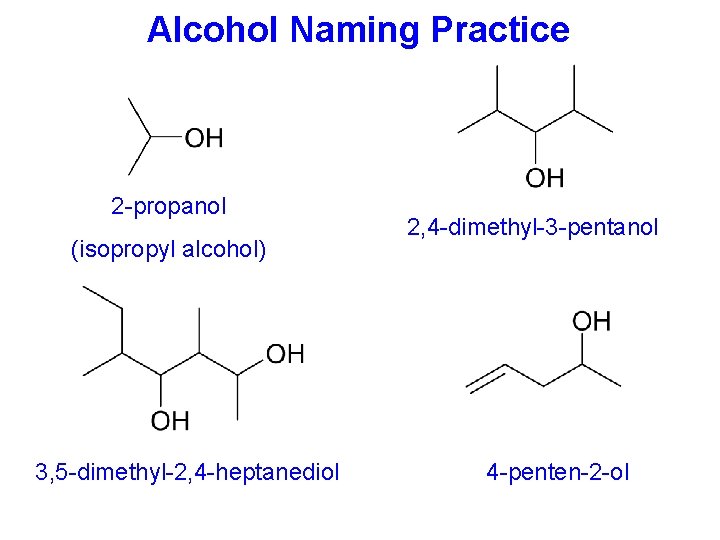
Alcohol Naming Practice 2 -propanol (isopropyl alcohol) 3, 5 -dimethyl-2, 4 -heptanediol 2, 4 -dimethyl-3 -pentanol 4 -penten-2 -ol

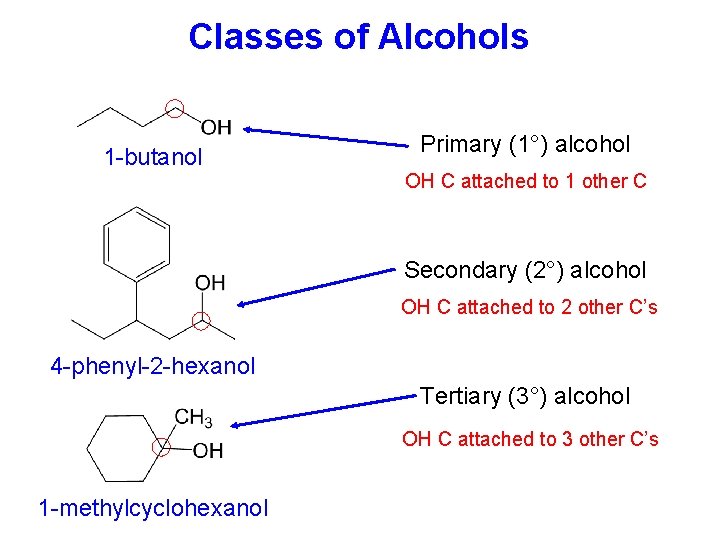
Classes of Alcohols 1 -butanol Primary (1°) alcohol OH C attached to 1 other C Secondary (2°) alcohol OH C attached to 2 other C’s 4 -phenyl-2 -hexanol Tertiary (3°) alcohol OH C attached to 3 other C’s 1 -methylcyclohexanol

Reactions of Alcohols Strong base • Reaction with strong bases alkoxide – alcohol as proton donor (weak acid) Strong acid • Reaction with strong acids – alcohol as proton acceptor (weak base) • Dehydration oxonium ion H+ - H 2 O – reverse of hydration of alkenes – requires H+ catalyst • Oxidation – increase # of C-O bonds alkene oxidizing agent or aldehyde ketone

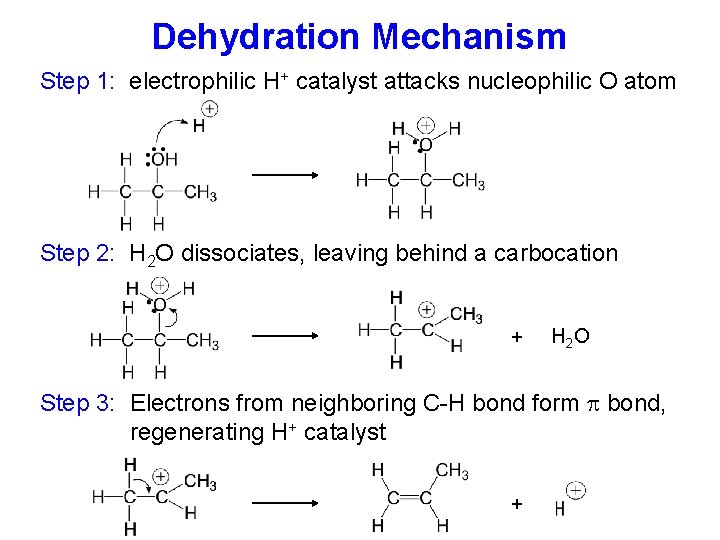
Dehydration Mechanism Step 1: electrophilic H+ catalyst attacks nucleophilic O atom Step 2: H 2 O dissociates, leaving behind a carbocation + H 2 O Step 3: Electrons from neighboring C-H bond form bond, regenerating H+ catalyst +

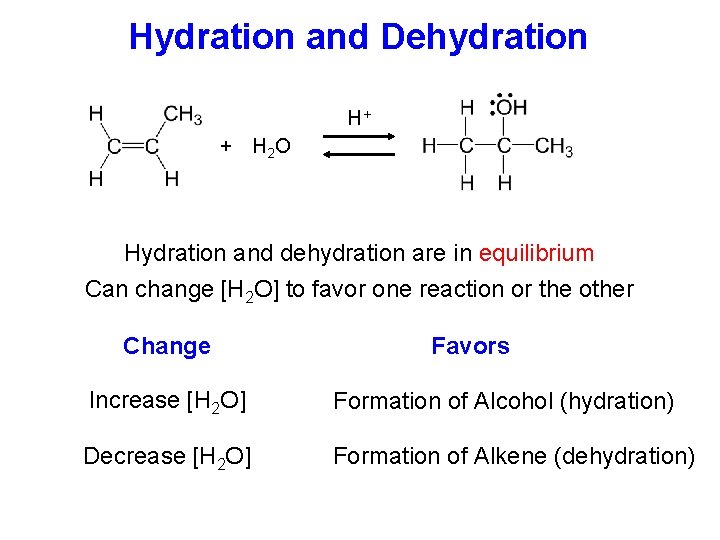
Hydration and Dehydration H+ + H 2 O Hydration and dehydration are in equilibrium Can change [H 2 O] to favor one reaction or the other Change Favors Increase [H 2 O] Formation of Alcohol (hydration) Decrease [H 2 O] Formation of Alkene (dehydration)

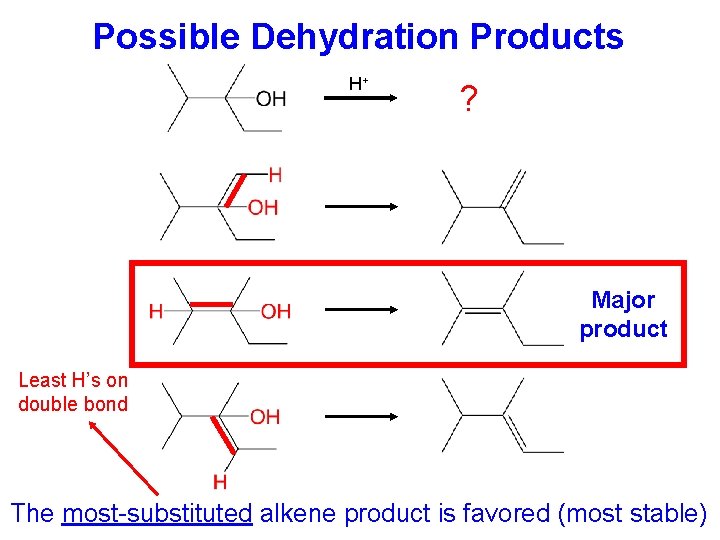
Possible Dehydration Products H+ ? Major product Least H’s on double bond The most-substituted alkene product is favored (most stable)

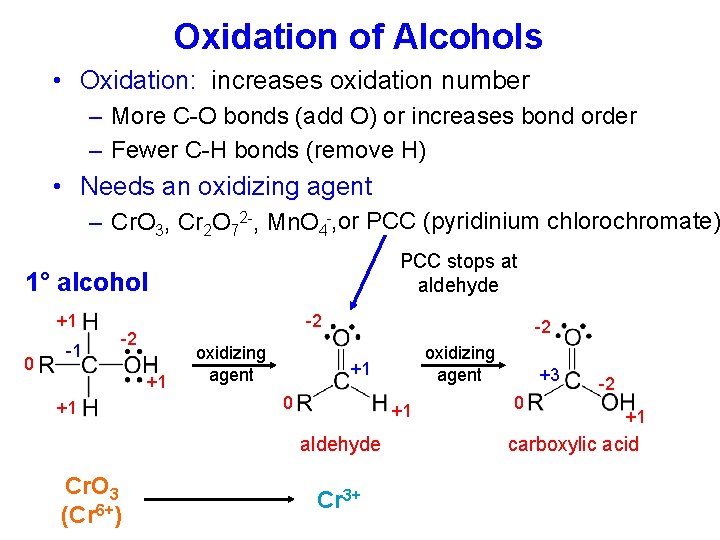
Oxidation of Alcohols • Oxidation: increases oxidation number – More C-O bonds (add O) or increases bond order – Fewer C-H bonds (remove H) • Needs an oxidizing agent – Cr. O 3, Cr 2 O 72 -, Mn. O 4 -, or PCC (pyridinium chlorochromate) PCC stops at aldehyde 1° alcohol +1 0 -1 -2 -2 +1 +1 oxidizing agent -2 +1 0 +1 aldehyde Cr. O 3 (Cr 6+) oxidizing agent Cr 3+ +3 0 -2 +1 carboxylic acid

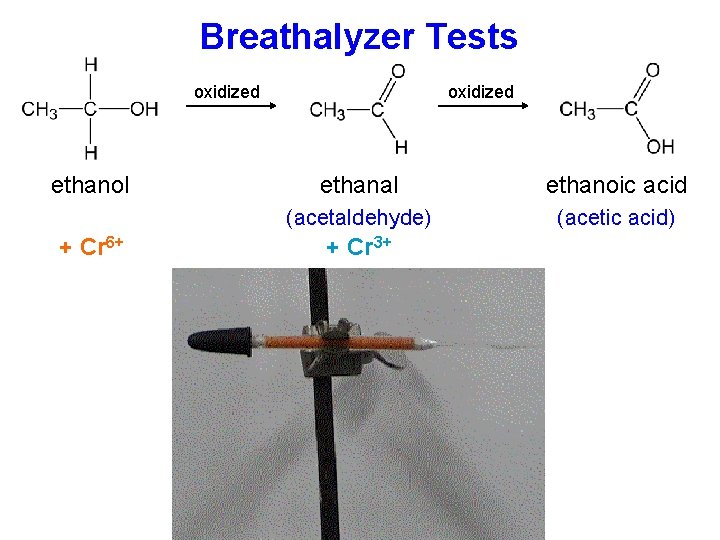
Breathalyzer Tests oxidized ethanol + Cr 6+ oxidized ethanal ethanoic acid (acetaldehyde) (acetic acid) + Cr 3+

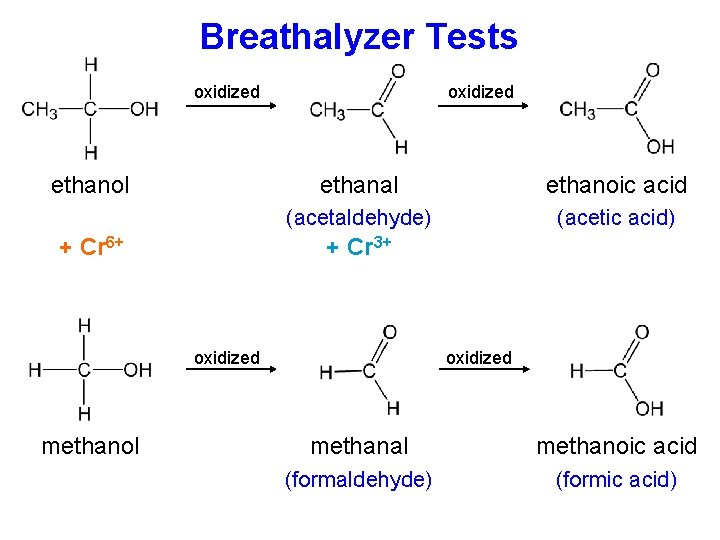
Breathalyzer Tests oxidized ethanol + Cr 6+ oxidized ethanal ethanoic acid (acetaldehyde) (acetic acid) + Cr 3+ oxidized methanol oxidized methanal methanoic acid (formaldehyde) (formic acid)

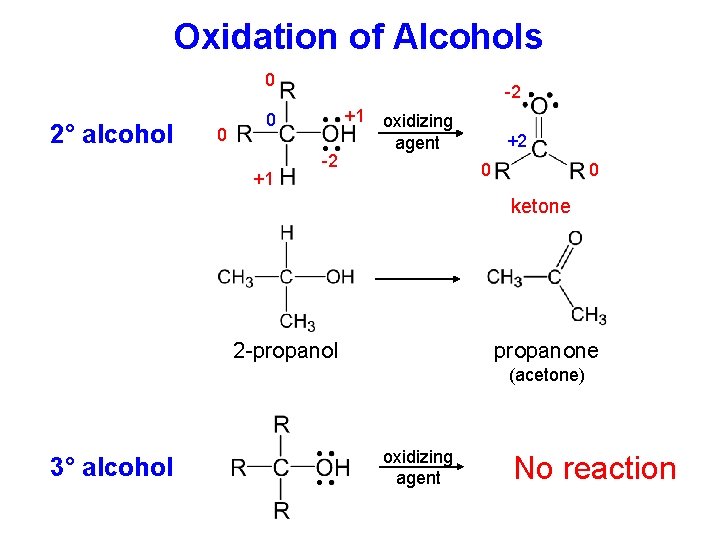
Oxidation of Alcohols 0 2° alcohol 0 -2 +1 0 +1 -2 oxidizing agent +2 0 0 ketone propanone 2 -propanol (acetone) 3° alcohol oxidizing agent No reaction

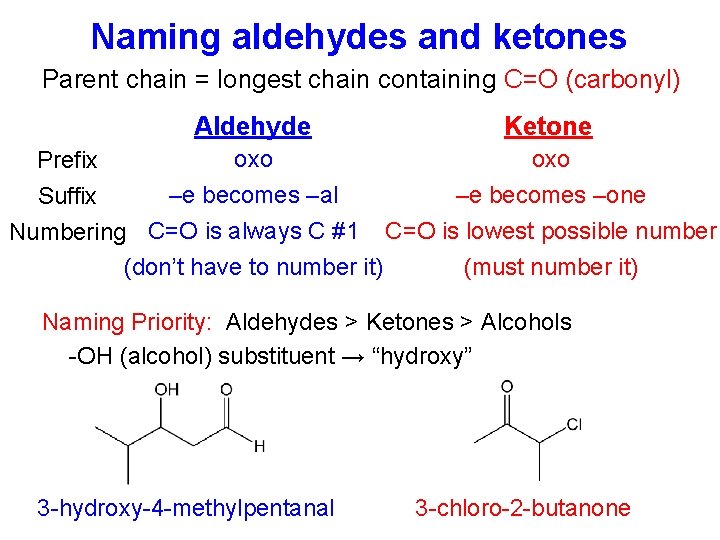
Naming aldehydes and ketones Parent chain = longest chain containing C=O (carbonyl) Aldehyde Ketone oxo Prefix –e becomes –al –e becomes –one Suffix Numbering C=O is always C #1 C=O is lowest possible number (don’t have to number it) (must number it) Naming Priority: Aldehydes > Ketones > Alcohols -OH (alcohol) substituent → “hydroxy” 3 -hydroxy-4 -methylpentanal 3 -chloro-2 -butanone

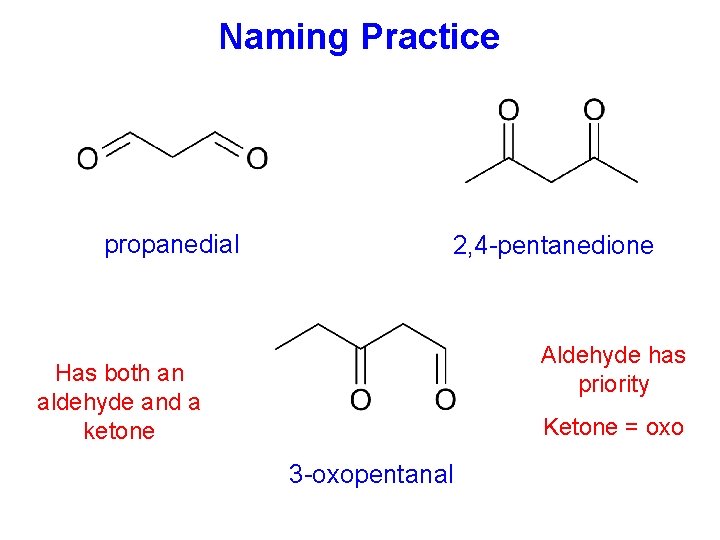
Naming Practice propanedial 2, 4 -pentanedione Aldehyde has priority Has both an aldehyde and a ketone Ketone = oxo 3 -oxopentanal

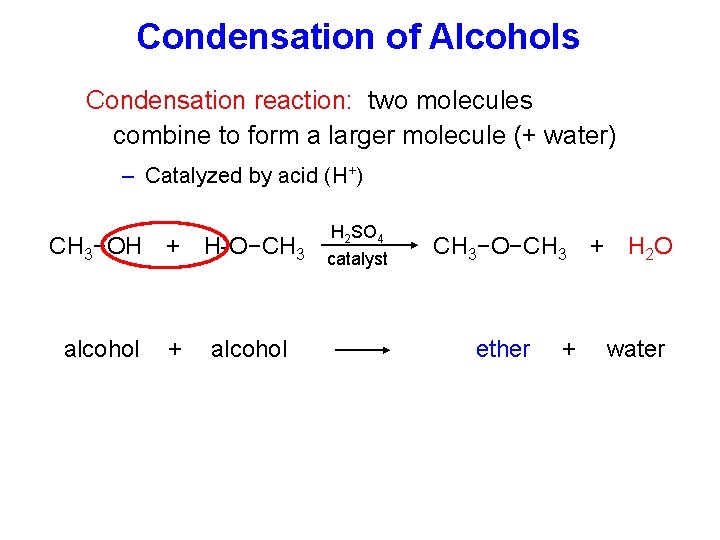
Condensation of Alcohols Condensation reaction: two molecules combine to form a larger molecule (+ water) – Catalyzed by acid (H+) CH 3−OH + H-O−CH 3 alcohol + alcohol H 2 SO 4 catalyst CH 3−O−CH 3 + H 2 O ether + water

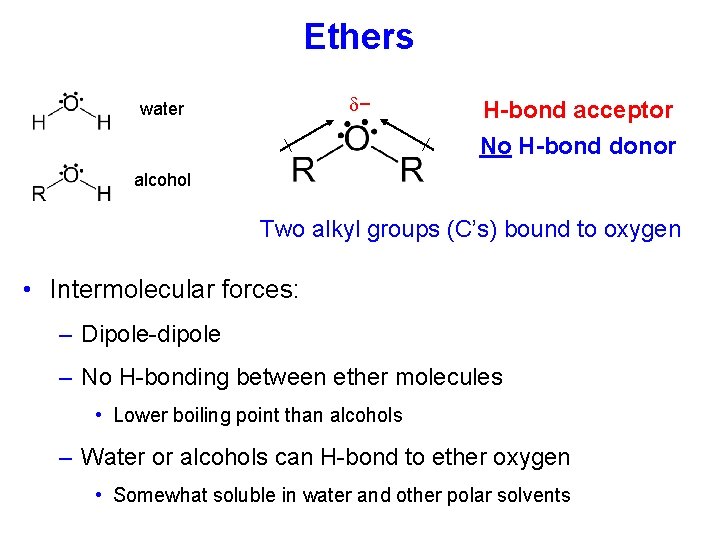
Ethers − water H-bond acceptor No H-bond donor alcohol Two alkyl groups (C’s) bound to oxygen • Intermolecular forces: – Dipole-dipole – No H-bonding between ether molecules • Lower boiling point than alcohols – Water or alcohols can H-bond to ether oxygen • Somewhat soluble in water and other polar solvents

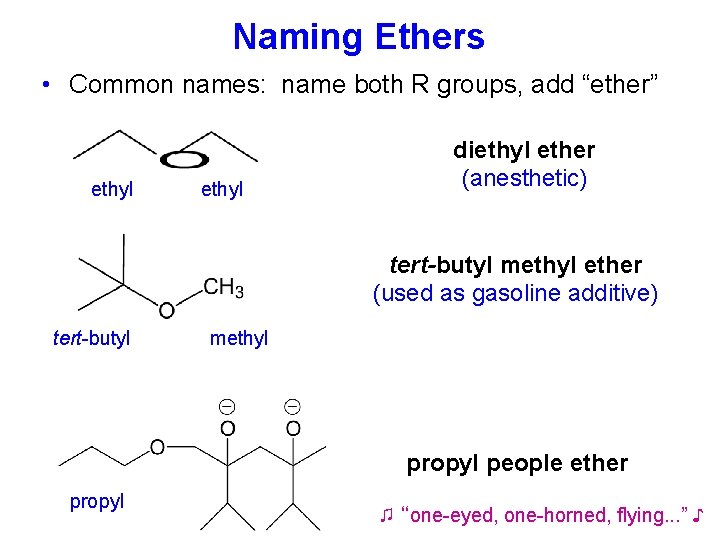
Naming Ethers • Common names: name both R groups, add “ether” ethyl diethyl ether (anesthetic)

Naming Ethers • Common names: name both R groups, add “ether” ethyl diethyl ether (anesthetic) tert-butyl methyl ether (used as gasoline additive) tert-butyl methyl propyl people ether propyl ♫ “one-eyed, one-horned, flying. . . ” ♪

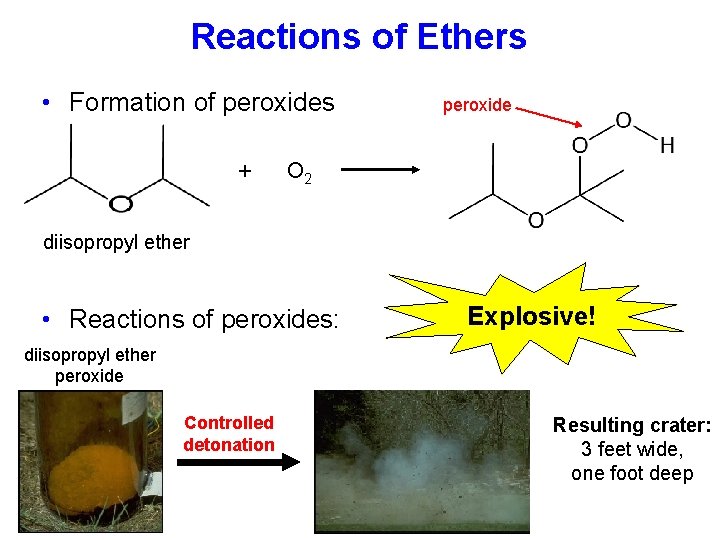
Reactions of Ethers • Formation of peroxides + peroxide O 2 diisopropyl ether • Reactions of peroxides: Explosive! diisopropyl ether peroxide Controlled detonation Resulting crater: 3 feet wide, one foot deep

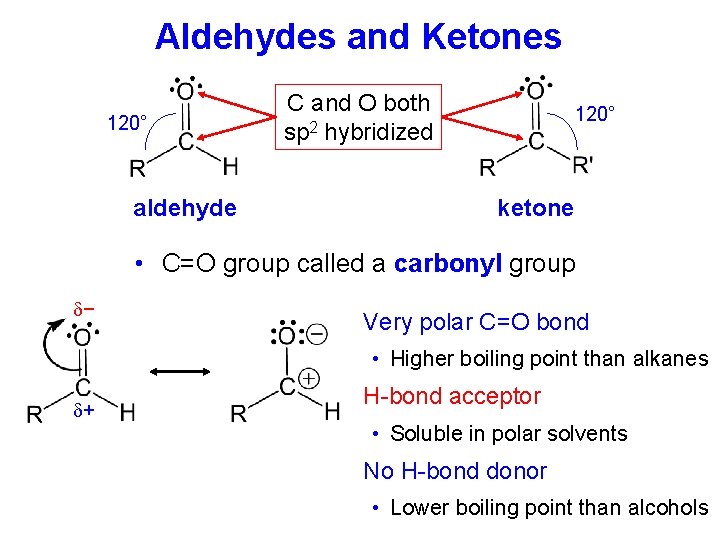
Aldehydes and Ketones 120° aldehyde C and O both sp 2 hybridized 120° ketone • C=O group called a carbonyl group − Very polar C=O bond • Higher boiling point than alkanes + H-bond acceptor • Soluble in polar solvents No H-bond donor • Lower boiling point than alcohols

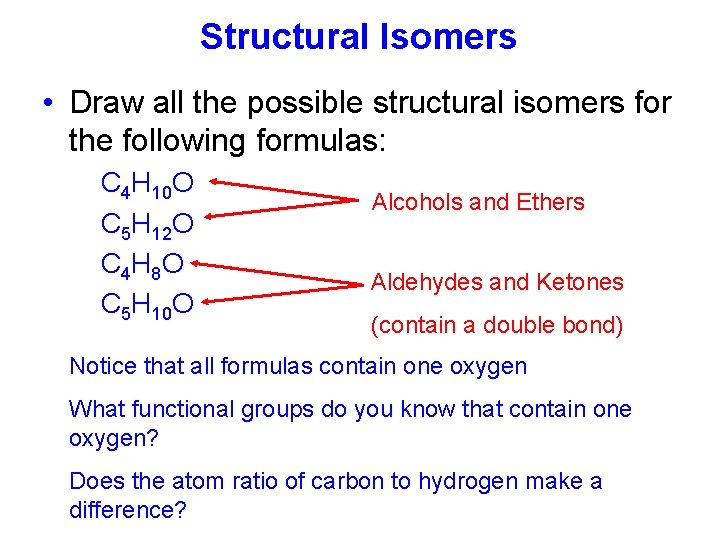
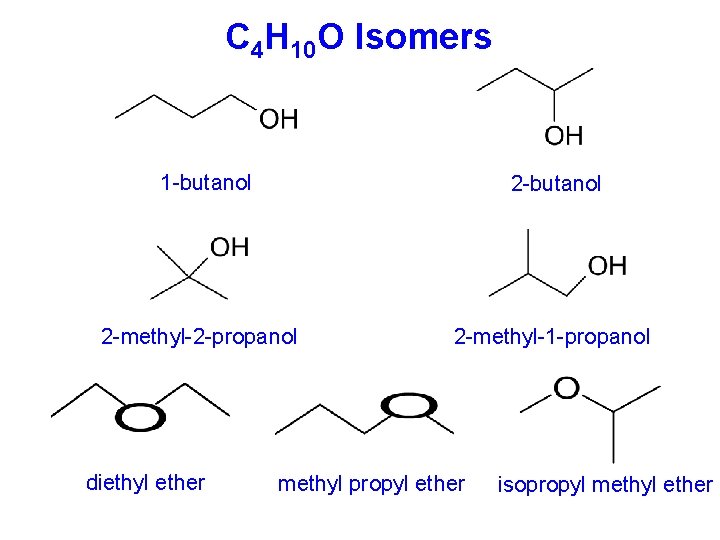
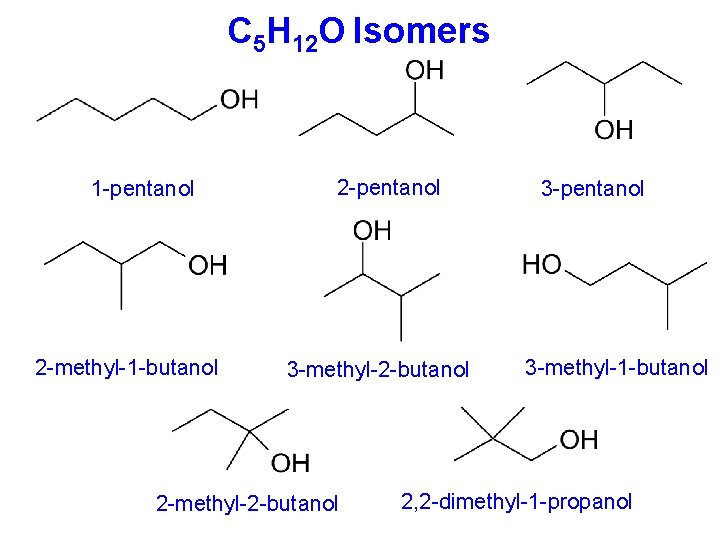
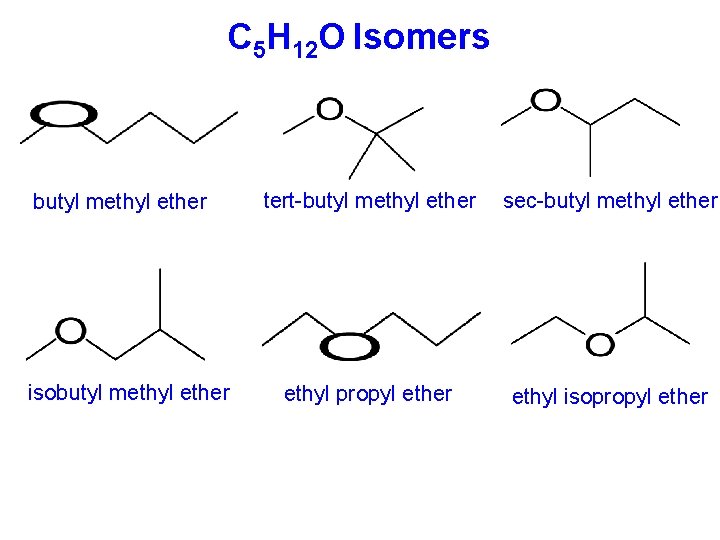
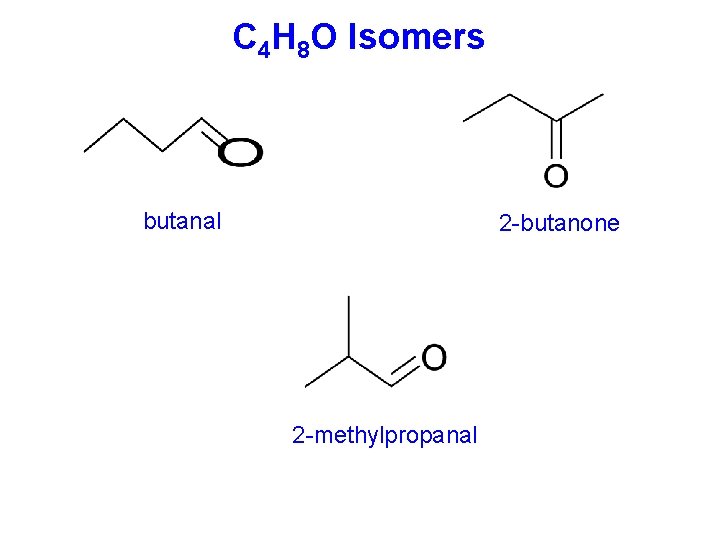
Structural Isomers • Draw all the possible structural isomers for the following formulas: C 4 H 10 O C 5 H 12 O C 4 H 8 O C 5 H 10 O Alcohols and Ethers Aldehydes and Ketones (contain a double bond) Notice that all formulas contain one oxygen What functional groups do you know that contain one oxygen? Does the atom ratio of carbon to hydrogen make a difference?

C 4 H 10 O Isomers 1 -butanol 2 -methyl-2 -propanol diethyl ether 2 -methyl-1 -propanol methyl propyl ether isopropyl methyl ether

C 5 H 12 O Isomers 1 -pentanol 2 -methyl-1 -butanol 2 -pentanol 3 -methyl-2 -butanol 2 -methyl-2 -butanol 3 -pentanol 3 -methyl-1 -butanol 2, 2 -dimethyl-1 -propanol

C 5 H 12 O Isomers butyl methyl ether isobutyl methyl ether tert-butyl methyl ether sec-butyl methyl ether ethyl propyl ether ethyl isopropyl ether

C 4 H 8 O Isomers butanal 2 -butanone 2 -methylpropanal

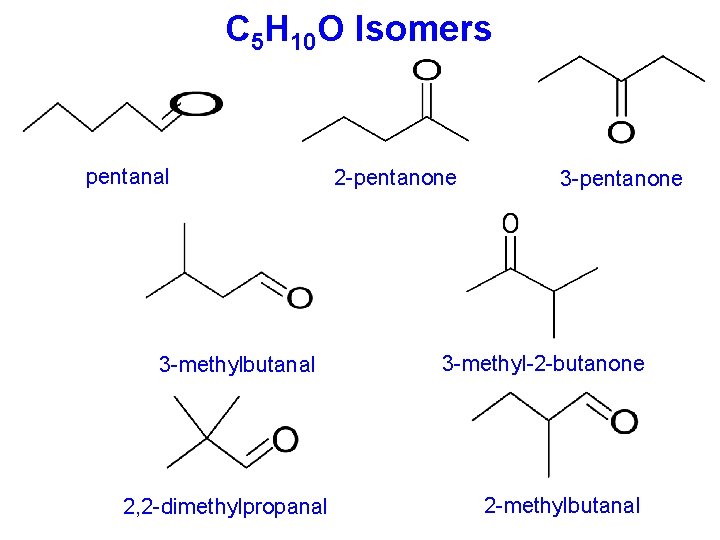
C 5 H 10 O Isomers pentanal 3 -methylbutanal 2, 2 -dimethylpropanal 2 -pentanone 3 -methyl-2 -butanone 2 -methylbutanal

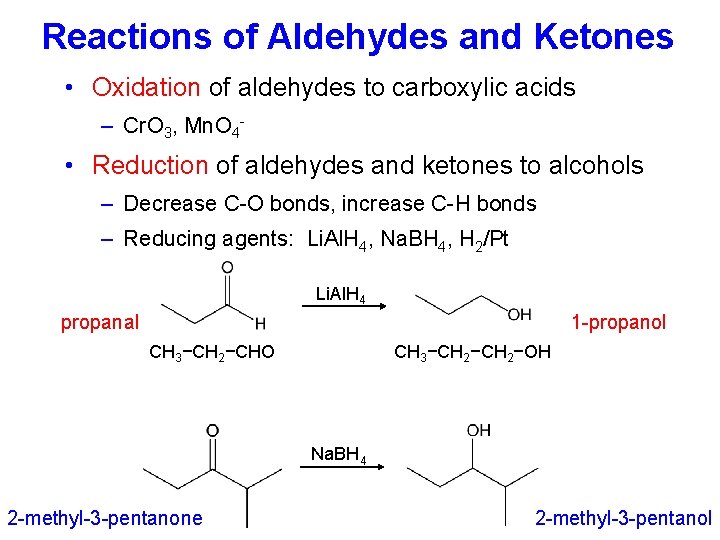
Reactions of Aldehydes and Ketones • Oxidation of aldehydes to carboxylic acids – Cr. O 3, Mn. O 4 - • Reduction of aldehydes and ketones to alcohols – Decrease C-O bonds, increase C-H bonds – Reducing agents: Li. Al. H 4, Na. BH 4, H 2/Pt Li. Al. H 4 propanal 1 -propanol CH 3−CH 2−OH CH 3−CH 2−CHO Na. BH 4 2 -methyl-3 -pentanone 2 -methyl-3 -pentanol

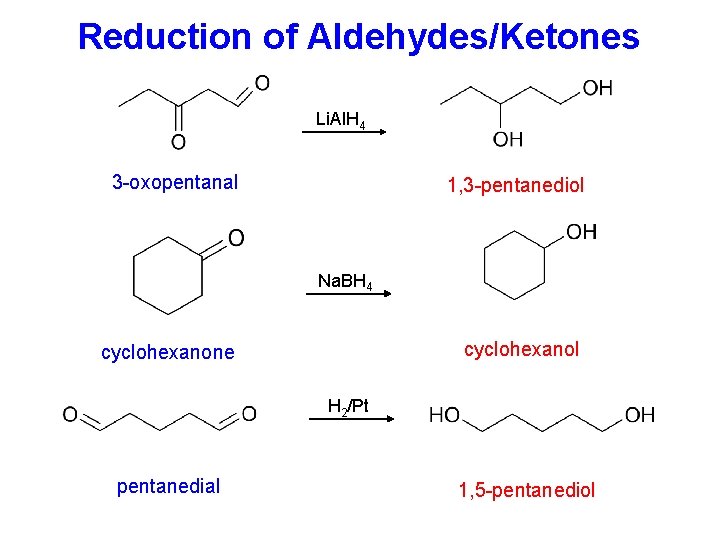
Reduction of Aldehydes/Ketones Li. Al. H 4 3 -oxopentanal 1, 3 -pentanediol Na. BH 4 cyclohexanol cyclohexanone H 2/Pt pentanedial 1, 5 -pentanediol

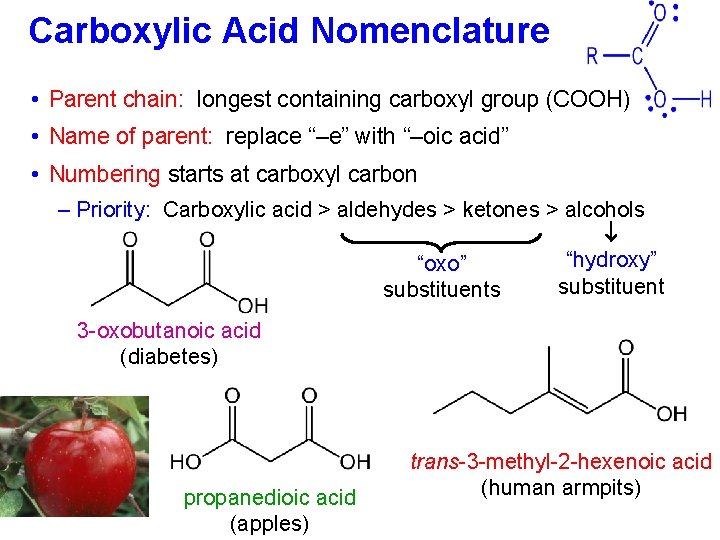
Carboxylic Acid Nomenclature • Parent chain: longest containing carboxyl group (COOH) • Name of parent: replace “–e” with “–oic acid” • Numbering starts at carboxyl carbon – Priority: Carboxylic acid > aldehydes > ketones > alcohols “hydroxy” “oxo” substituents 3 -oxobutanoic acid (diabetes) propanedioic acid (apples) trans-3 -methyl-2 -hexenoic acid (human armpits)

aspirin Tylenol Can irritate your stomach Gentle on the stomach A carboxylic acid Just an alcohol

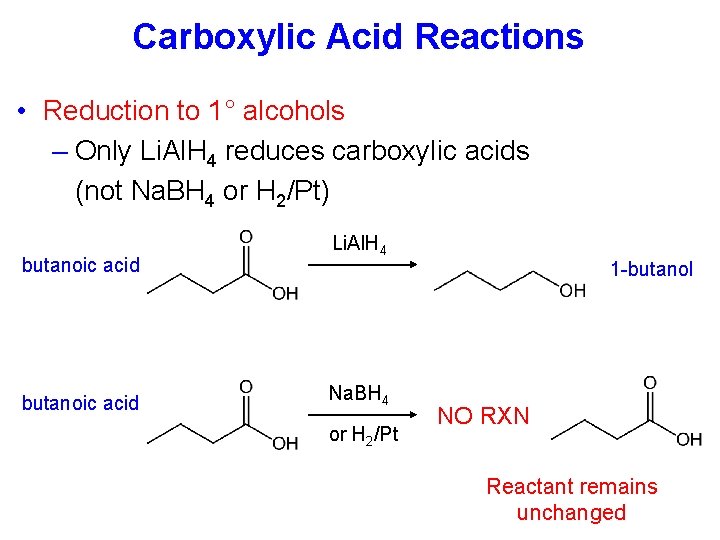
Carboxylic Acid Reactions • Reduction to 1° alcohols – Only Li. Al. H 4 reduces carboxylic acids (not Na. BH 4 or H 2/Pt) butanoic acid Li. Al. H 4 1 -butanol Na. BH 4 or H 2/Pt NO RXN Reactant remains unchanged

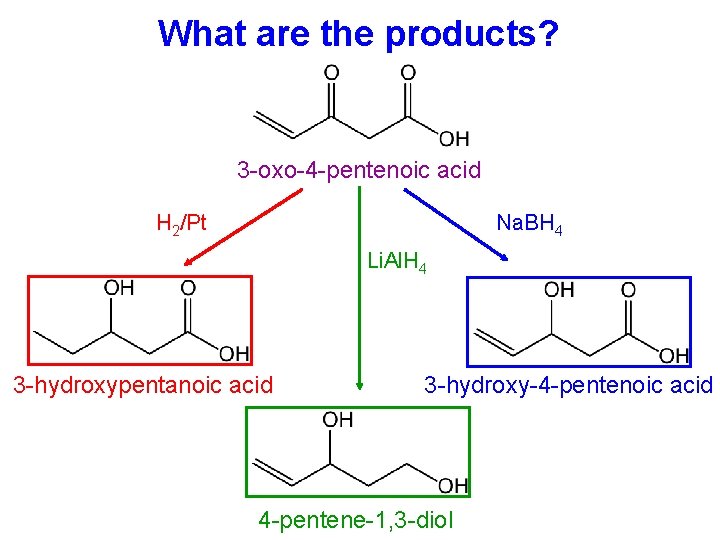
What are the products? 3 -oxo-4 -pentenoic acid H 2/Pt Na. BH 4 Li. Al. H 4 3 -hydroxypentanoic acid 3 -hydroxy-4 -pentenoic acid 4 -pentene-1, 3 -diol