Chemistry Do Now 101617 Directions Take out your



















- Slides: 40

Chemistry Do Now 10/16/17 • Directions: Take out your Do Now sheet and answer each question based on the notes. • • Compare and contrast solids, liquids, and gases in terms of IMFs and thermal energy. • Explain how ions are formed. • What is the difference between intermolecular forces and intramolecular forces?

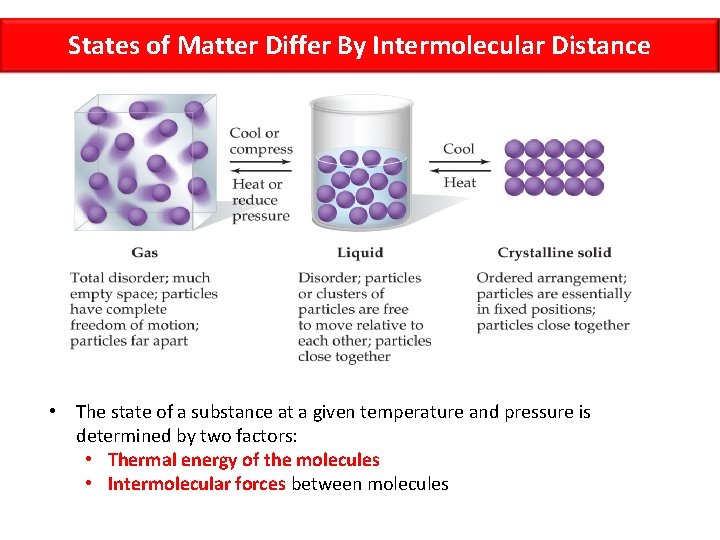
Chemistry Do Now 10/16/17 Key • Compare and contrast solids, liquids, and gases in terms of IMFs and thermal energy. Solid IMF Strong Thermal Low Energy Liquid Gas Moderate Weak Moderate High

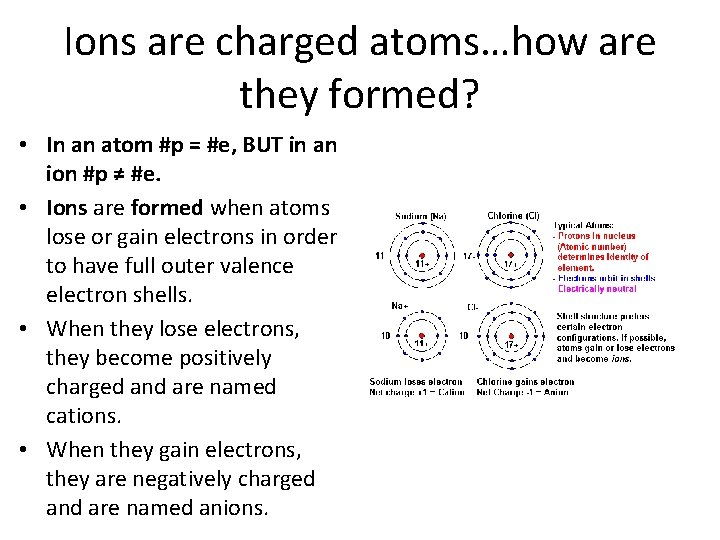
Chemistry Do Now 10/16/17 Key • Explain how ions are formed. Ions are formed when there is a transfer of electrons between two elements. The element that loses an electron from its outer shell is called a cation and will carry a positive charge since it now has more protons than electrons. The element that gains an electron to its outer shell is called an anion and will carry a negative charge since it now has more electrons than protons. On the periodic table of elements, metals typically will lose 1, 2, or 3 electrons from their outer electron shell; whereas, nonmetals typically will gain 1, 2, or 3 electrons to its outer electron shell. Metals have a poor affinity for attracting electrons; whereas, nonmetals have a high affinity for attracting electrons. As a result of the transfer of electrons, each element becomes stable.

Chemistry Do Now 10/16/17 Key • What is the difference between intermolecular forces and intramolecular forces? Intermolecular forces are the attractive forces that exist between molecules. These are not the same as intramolecular forces (i. e. chemical bonds) which exist within a molecule. Intermolecular forces are much weaker than intramolecular forces.

Homework - Copy • Complete lab questions for ice cream lab

Objective • Students will know how the intermolecular forces (IMFs) between atoms determine the state of matter and properties of that atom by performing a close read, taking notes, and demonstrating the freezing depression point (making ice cream). • Mastery Level: 70 pts. on close read annotations, 4/6 on TDQs, and 2/2 on exit ticket questions

Engage • Show the students the You. Tube video “Freezing Depression” • Source: https: //www. youtube. com/watch? v=Vehpw. RVTl UI • Show the students the You. Tube video “Freezing Point Depression” • Source: https: //www. youtube. com/watch? v=Ygl. P 2 El_cq U

Explore • Students will perform a close read on the article titled “Freezing Physics”. As the students read the text, they will annotate using the symbols posted on charts in the classroom. Following their annotations, the students will answer 6 scaffolded textdependent questions. • Source: https: //www. eduplace. com/kids/hmxs/g 6/we atherwater/cricket/sect 2 cc. shtml

Which word(s) stand out?

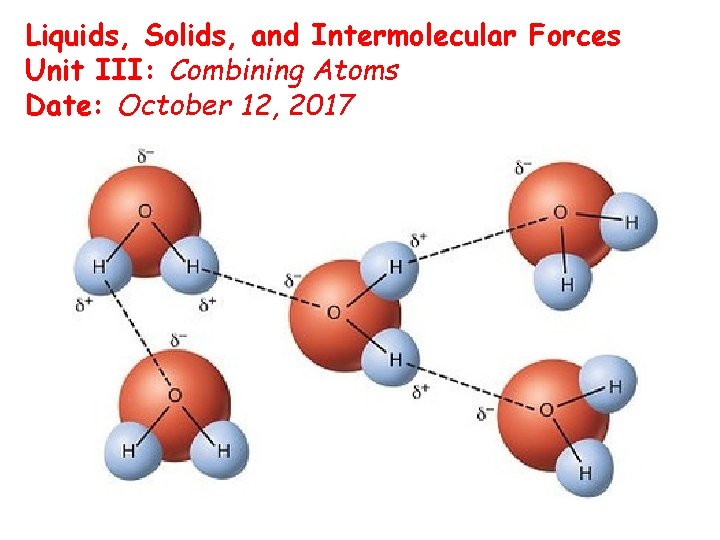
Liquids, Solids, and Intermolecular Forces Unit III: Combining Atoms Date: October 12, 2017 Suggested HW: (Ch 15) 13 -17

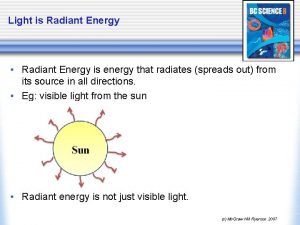
States of Matter Differ By Intermolecular Distance • The state of a substance at a given temperature and pressure is determined by two factors: • Thermal energy of the molecules • Intermolecular forces between molecules

Ions are charged atoms…how are they formed? • In an atom #p = #e, BUT in an ion #p ≠ #e. • Ions are formed when atoms lose or gain electrons in order to have full outer valence electron shells. • When they lose electrons, they become positively charged and are named cations. • When they gain electrons, they are negatively charged and are named anions.

Intermolecular Forces • Intermolecular forces are the attractive forces that exist between molecules. These are not the same as intramolecular forces (i. e. bonds) which exist within a molecule. • Intermolecular forces are much weaker than intramolecular forces. • Intermolecular forces determine many of the physical characteristics of molecules like boiling and freezing points.

Intermolecular Forces: Coulombic Attractions • As you recall, ionic compounds are solids at room temperature. There are ion-ion attractions in ionic compounds. • The force, called a Coulombic attraction, that holds ions together is very strong. Coulombic attractions are the strongest of all intermolecular forces. • Therefore, all ionic compounds have very high melting/boiling points, and are solid at room temperature.

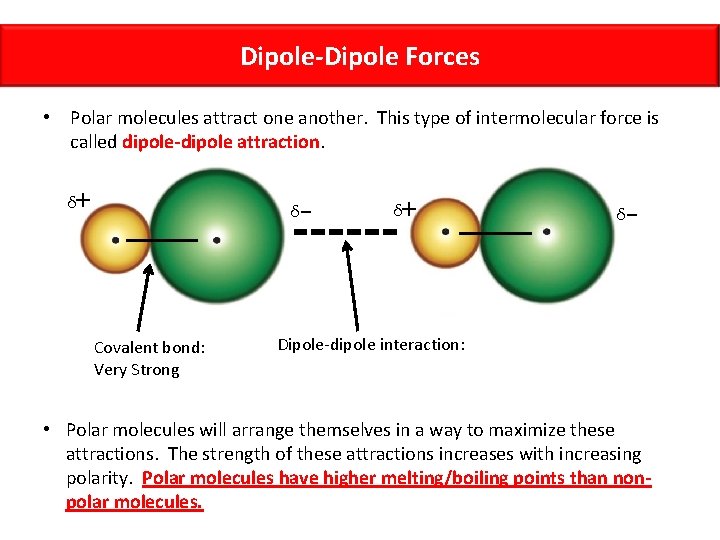
Dipole-Dipole Forces • Polar molecules attract one another. This type of intermolecular force is called dipole-dipole attraction. + δ δ Covalent bond: Very Strong - + δ δ - Dipole-dipole interaction: • Polar molecules will arrange themselves in a way to maximize these attractions. The strength of these attractions increases with increasing polarity. Polar molecules have higher melting/boiling points than nonpolar molecules.

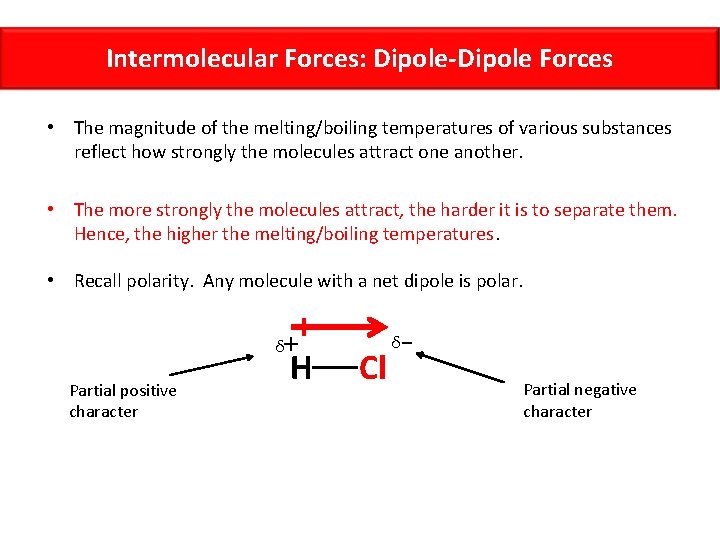
Intermolecular Forces: Dipole-Dipole Forces • The magnitude of the melting/boiling temperatures of various substances reflect how strongly the molecules attract one another. • The more strongly the molecules attract, the harder it is to separate them. Hence, the higher the melting/boiling temperatures. • Recall polarity. Any molecule with a net dipole is polar. + δ Partial positive character H Cl δ Partial negative character

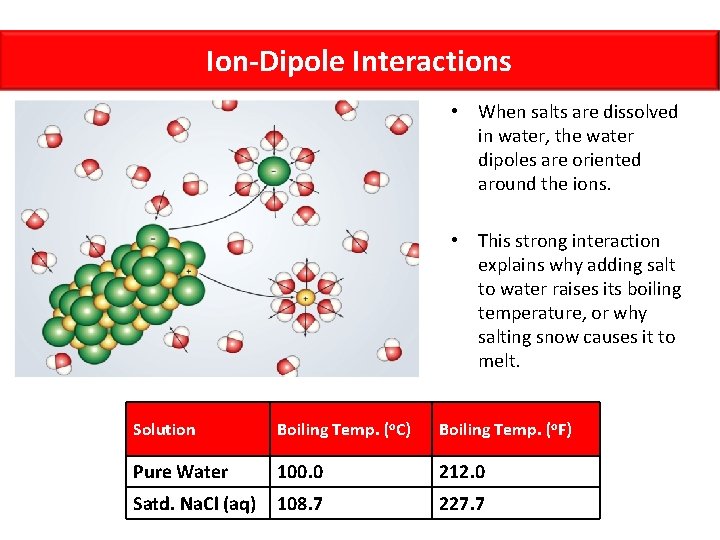
Ion-Dipole Interactions • When salts are dissolved in water, the water dipoles are oriented around the ions. • This strong interaction explains why adding salt to water raises its boiling temperature, or why salting snow causes it to melt. Solution Boiling Temp. (o. C) Boiling Temp. (o. F) Pure Water 100. 0 212. 0 Satd. Na. Cl (aq) 108. 7 227. 7

Ok, time for a…

Take out a sheet of paper and answer. • 1) What is an ion? • 6) What effect does the Coulombic attractive force have • 2) How does an ion form? on boiling point? Why? • 3) How does an ion get its • 7) Draw or describe the dipolecharge? dipole attraction. • 4) What is a cation? Anion? • 8) What is a polar molecule? • 5) What is the difference What effect does a molecules between INTERmolecular forces polarity have on boiling point? and INTRAmolecular forces? Why? • 9) Describe the ion-dipole attraction.

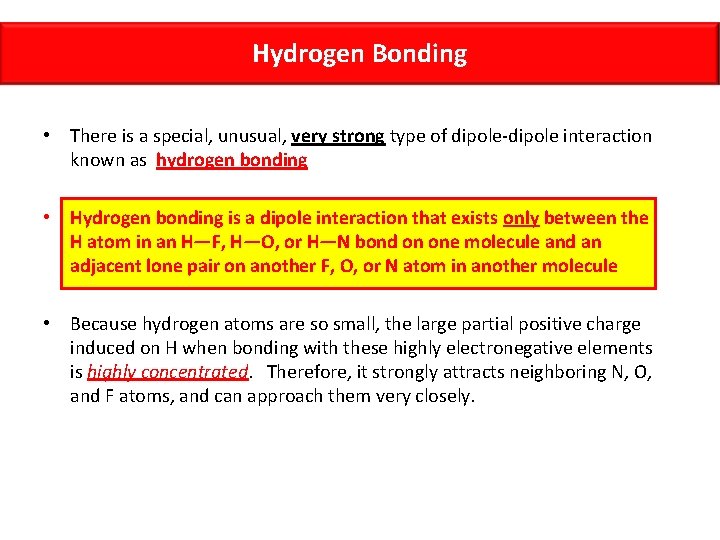
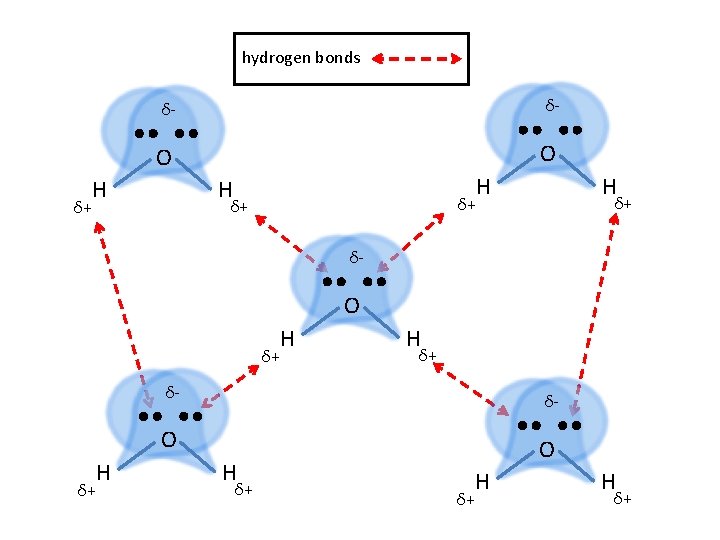
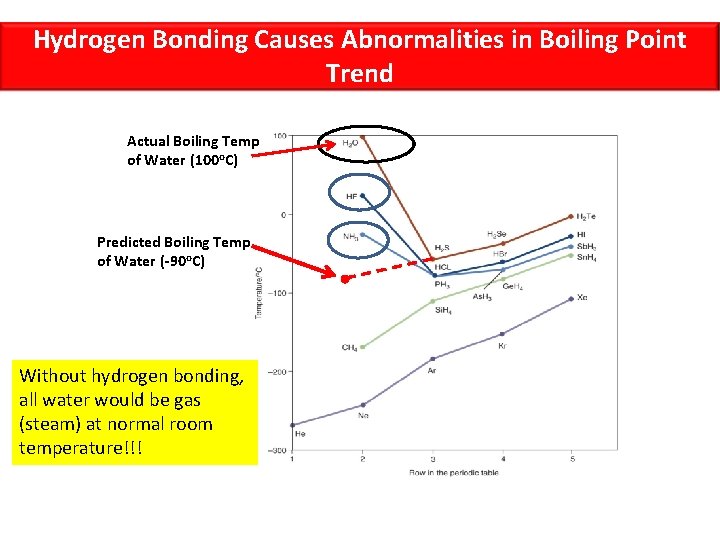
Hydrogen Bonding • There is a special, unusual, very strong type of dipole-dipole interaction known as hydrogen bonding • Hydrogen bonding is a dipole interaction that exists only between the H atom in an H—F, H—O, or H—N bond on one molecule and an adjacent lone pair on another F, O, or N atom in another molecule • Because hydrogen atoms are so small, the large partial positive charge induced on H when bonding with these highly electronegative elements is highly concentrated. Therefore, it strongly attracts neighboring N, O, and F atoms, and can approach them very closely.


Hydrogen Bonding Causes Abnormalities in Boiling Point Trend Actual Boiling Temp of Water (100 o. C) Predicted Boiling Temp of Water (-90 o. C) Without hydrogen bonding, all water would be gas (steam) at normal room temperature!!!

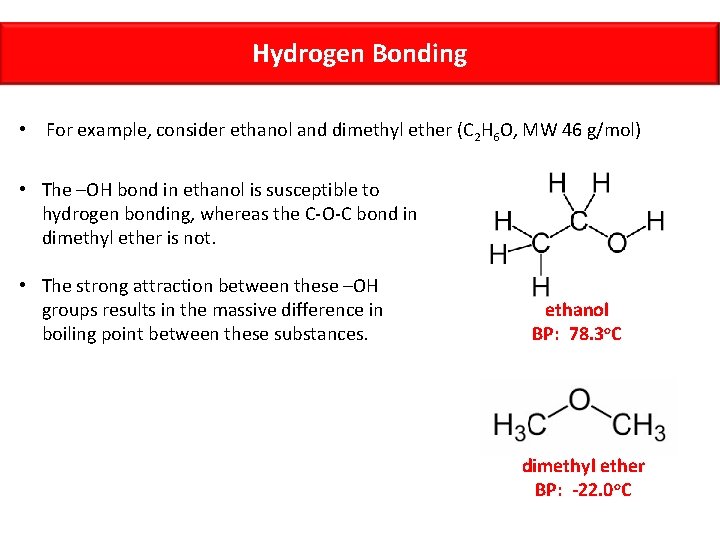
Hydrogen Bonding • For example, consider ethanol and dimethyl ether (C 2 H 6 O, MW 46 g/mol) • The –OH bond in ethanol is susceptible to hydrogen bonding, whereas the C-O-C bond in dimethyl ether is not. • The strong attraction between these –OH groups results in the massive difference in boiling point between these substances. ethanol BP: 78. 3 o. C dimethyl ether BP: -22. 0 o. C

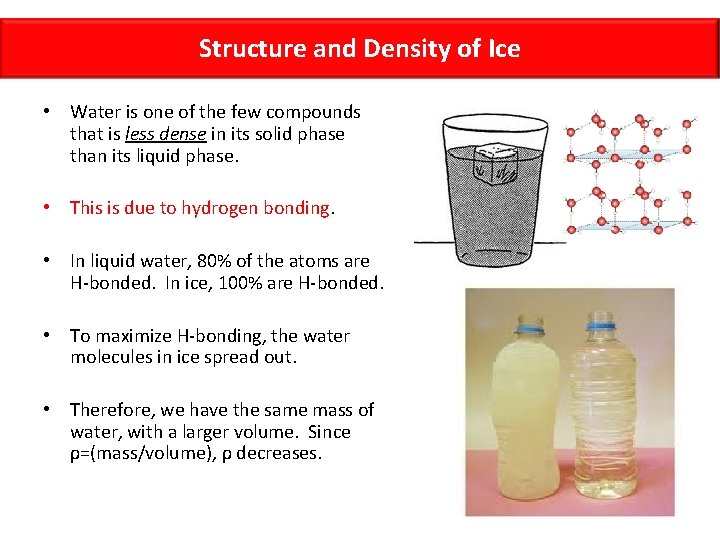
Structure and Density of Ice • Water is one of the few compounds that is less dense in its solid phase than its liquid phase. • This is due to hydrogen bonding. • In liquid water, 80% of the atoms are H-bonded. In ice, 100% are H-bonded. • To maximize H-bonding, the water molecules in ice spread out. • Therefore, we have the same mass of water, with a larger volume. Since ρ=(mass/volume), ρ decreases.

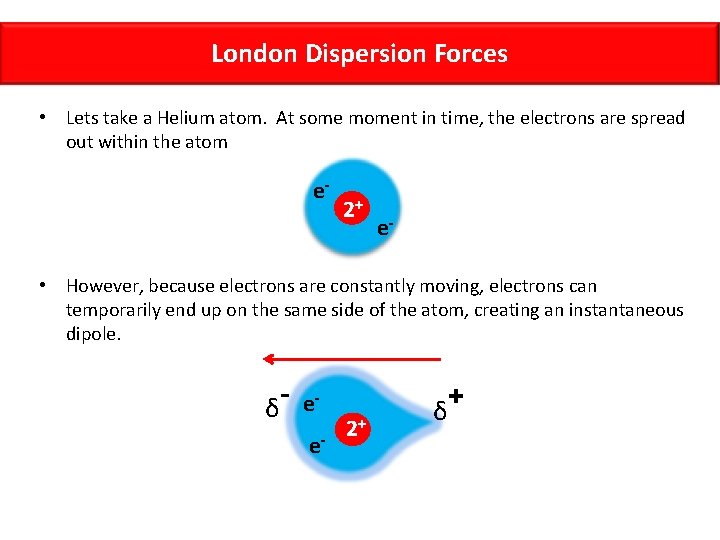
London Dispersion Forces • With nonpolar molecules, there are no dipoles, so we would not expect to see dipole-dipole interactions. Despite this, intermolecular interactions have still been observed. • For example, nonpolar gases like Helium can be liquified, but how can this happen? What force brings the He atoms together? • Fritz London, a physicist, proposed that the motion of electrons in a nonpolar molecule can create instantaneous dipoles

London Dispersion Forces • Lets take a Helium atom. At some moment in time, the electrons are spread out within the atom e- 2+ e- • However, because electrons are constantly moving, electrons can temporarily end up on the same side of the atom, creating an instantaneous dipole. δ ee- 2+ δ +

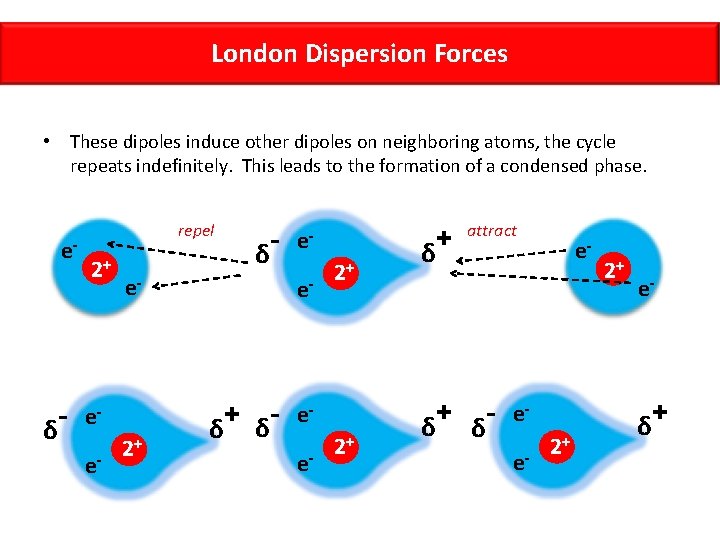
London Dispersion Forces • These dipoles induce other dipoles on neighboring atoms, the cycle repeats indefinitely. This leads to the formation of a condensed phase. e- δ repel 2+ e- ee- δ 2+ ee- + δ δ 2+ ee- 2+ + δ attract + δ δ- e- ee- 2+ 2+ e- δ +

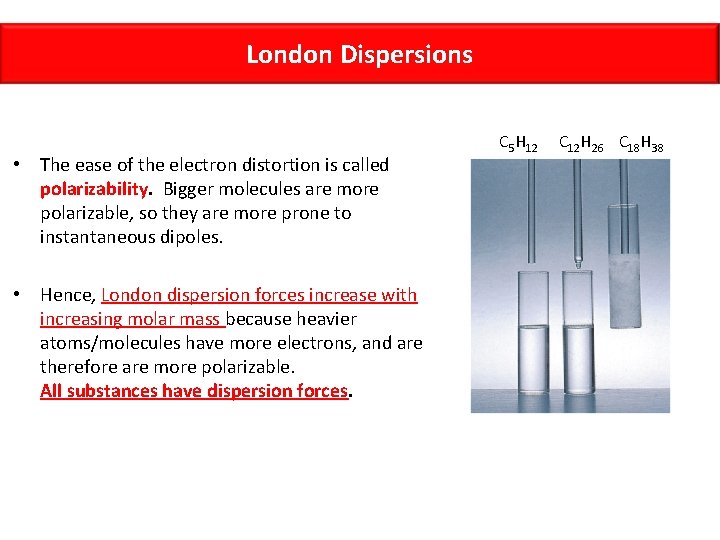
London Dispersions • The ease of the electron distortion is called polarizability. Bigger molecules are more polarizable, so they are more prone to instantaneous dipoles. • Hence, London dispersion forces increase with increasing molar mass because heavier atoms/molecules have more electrons, and are therefore are more polarizable. All substances have dispersion forces. C 5 H 12 C 12 H 26 C 18 H 38

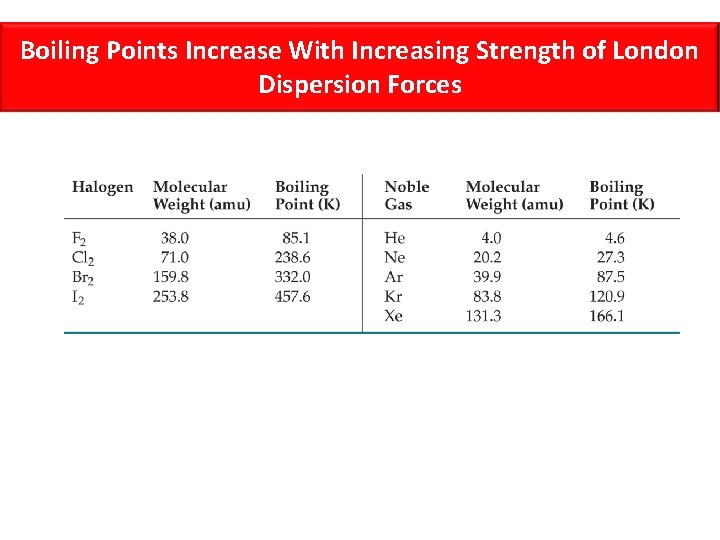
Boiling Points Increase With Increasing Strength of London Dispersion Forces

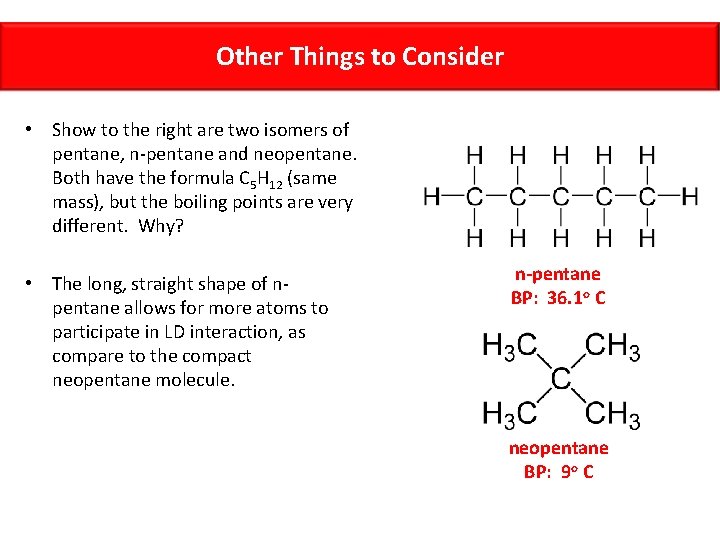
Other Things to Consider • Show to the right are two isomers of pentane, n-pentane and neopentane. Both have the formula C 5 H 12 (same mass), but the boiling points are very different. Why? • The long, straight shape of npentane allows for more atoms to participate in LD interaction, as compare to the compact neopentane molecule. n-pentane BP: 36. 1 o C neopentane BP: 9 o C

Like Substances Mix • Polar substances are soluble in polar substances. • Nonpolar substances are soluble in nonpolar substances. • Polar and nonpolar substances DO NOT MIX (ex. oil and water) • E. G. Oil and water; Dipole-dipole and H-bond forces between water molecules are MUCH stronger than the London dispersion forces between oil and water • Water molecules would rather associate with other water molecules than to associate with oil. • Salts are soluble in water and other highly polar solvents, but insoluble in nonpolar and weakly polar solvents.

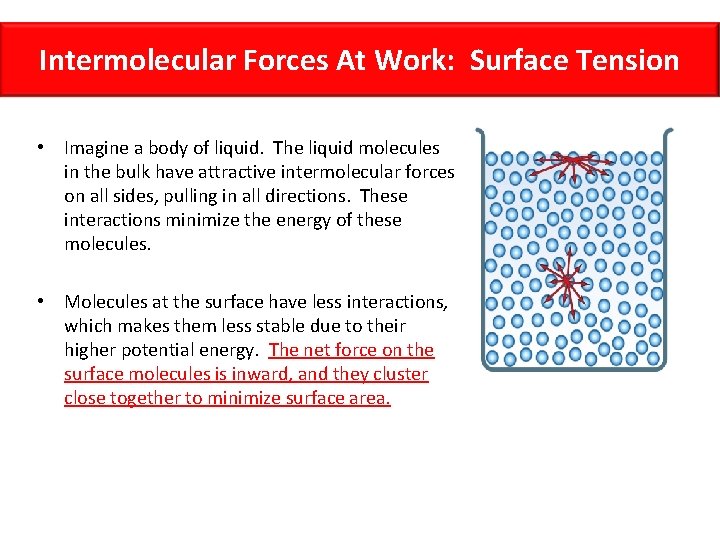
Intermolecular Forces At Work: Surface Tension • Imagine a body of liquid. The liquid molecules in the bulk have attractive intermolecular forces on all sides, pulling in all directions. These interactions minimize the energy of these molecules. • Molecules at the surface have less interactions, which makes them less stable due to their higher potential energy. The net force on the surface molecules is inward, and they cluster close together to minimize surface area.

Intermolecular Forces At Work: Surface Tension • The surface tension of a liquid is the energy required to increase its surface area by a unit amount (J/cm 2 or m. J/m 2). The surface tension of a liquid is what causes its surface to create a “skin” that resists penetration, or assume a droplet shape.

Intermolecular Forces At Work: Viscosity • Viscosity is a measure of a liquid’s resistance to flow and is a temperature dependent property (e. g. Maple syrup is more viscous than water). Stronger intermolecular forces lead to higher viscosity. • C. I. R. L. : Viscosity is an important property of motor oil. It must be thick enough to lubricate and protect the engine, but thin enough not to clog the components. • Multi-grade oils allow drivers to keep the same oil all year long without a loss of performance. • Ex. An oil with a 10 W-30 rating has a viscosity of 10 (thin) in the winter and 30 (thick) in the summer. How?

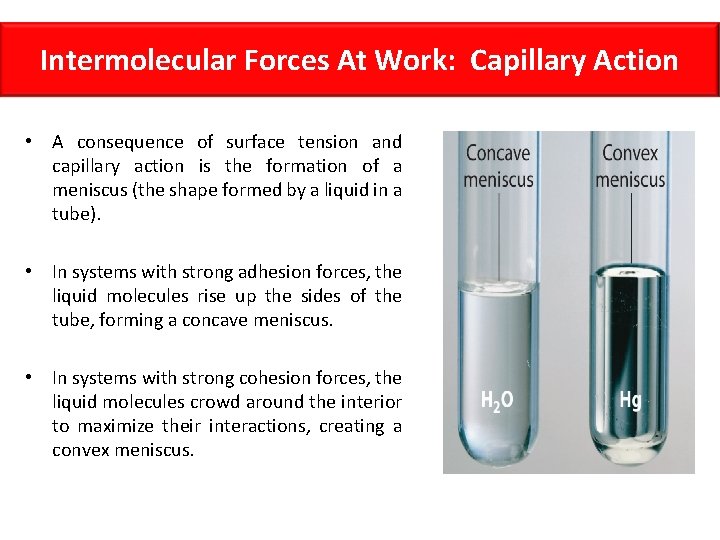
Intermolecular Forces At Work: Capillary Action • Closely related to surface tension, capillary action is the ability of a liquid to flow against gravity up a tube. • This is caused by a combination of intermolecular forces between neighboring liquid molecules (cohesive forces), as well as intermolecular forces between the liquid molecules and the molecules of the surface of the tube (adhesive forces). • If the adhesive forces are stronger than the cohesive forces, the liquid rises.

Intermolecular Forces At Work: Capillary Action • A consequence of surface tension and capillary action is the formation of a meniscus (the shape formed by a liquid in a tube). • In systems with strong adhesion forces, the liquid molecules rise up the sides of the tube, forming a concave meniscus. • In systems with strong cohesion forces, the liquid molecules crowd around the interior to maximize their interactions, creating a convex meniscus.

Summary • We can arrange the intermolecular forces by relative strength: 1. Coulombic attraction 2. Ion-dipole/Hydrogen Bonding 3. Dipole-Dipole 4. *London Dispersion • It is important to note that molecules with large nonpolar portions and polar end groups will not mix with polar solvents (ex. CH 3 CH 2 CH 2 CH 2 OH) because the LD forces dominate. • The list above is a general trend. For very large molecules, LD forces can exceed the strength of dipole-based forces.

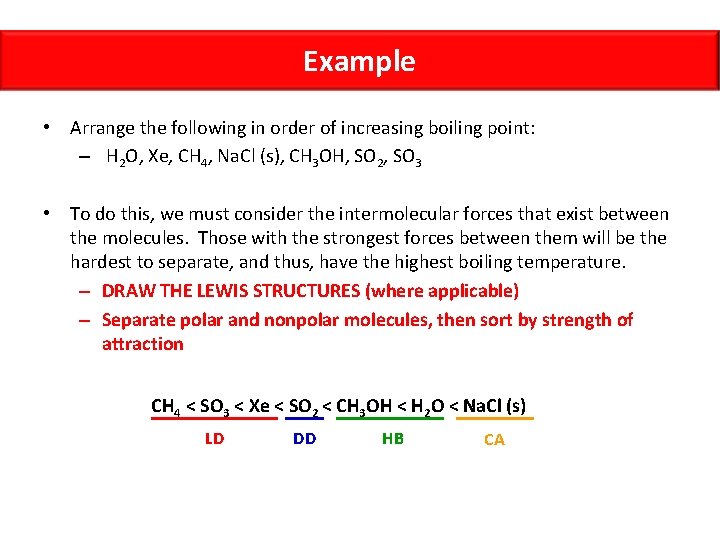
Example • Arrange the following in order of increasing boiling point: – H 2 O, Xe, CH 4, Na. Cl (s), CH 3 OH, SO 2, SO 3 • To do this, we must consider the intermolecular forces that exist between the molecules. Those with the strongest forces between them will be the hardest to separate, and thus, have the highest boiling temperature. – DRAW THE LEWIS STRUCTURES (where applicable) – Separate polar and nonpolar molecules, then sort by strength of attraction CH 4 < SO 3 < Xe < SO 2 < CH 3 OH < H 2 O < Na. Cl (s) LD DD HB CA

Extend – Freezing Depression Point Materials • MATERIALS Ice Cream Bag Ice Bag 1 liter-size (1 quart) Ziplock freezer bag 4 liter-size (1 gallon) Ziplock freezer bag 250 m. L (1 cup) milk 90 m. L (6 tablespoons) table salt 30 m. L (2 tablespoons) sugar, or equivalent 2 double handfuls of ice sweetener for diabetics 250 m. L (1 cup) water 5 m. L (1 teaspoon) vanilla Also Needed cup or bowl spoon

Extend – Exit Ticket Questions on Intermolecular Forces • What is a dipole and how do dipoles determine the type of interactions between atoms or molecules? • Explain each interaction in terms of properties: hydrogen bonding, London. Dispersion, ion-dipole, and dipole-dipole.
 Shot me out of the sky one direction
Shot me out of the sky one direction Take out your notebook
Take out your notebook Take out your homework
Take out your homework Take out your notebooks
Take out your notebooks Checked homework
Checked homework Friction
Friction Take out your homework
Take out your homework Take out your homework
Take out your homework Take out your notebook
Take out your notebook Write down your homework
Write down your homework Take out your notebook
Take out your notebook Take out your notebook
Take out your notebook Take out your homework
Take out your homework Take out your homework
Take out your homework Take out your homework
Take out your homework Take out your notebook
Take out your notebook In your notebook write cs if the sentence
In your notebook write cs if the sentence Take out your homework
Take out your homework Radiant energy spreads out in all directions
Radiant energy spreads out in all directions Now i see it now you don't
Now i see it now you don't Take a bus or take a train
Take a bus or take a train You put your right foot in you put your right foot out
You put your right foot in you put your right foot out Now act out the following dialogue
Now act out the following dialogue Now my soul cries out hallelujah
Now my soul cries out hallelujah Medeatakeout
Medeatakeout Signing naturally unit 9.10 minidialogue 2 answers
Signing naturally unit 9.10 minidialogue 2 answers Take out a sheet of paper
Take out a sheet of paper Take out a sheet of paper
Take out a sheet of paper Translate
Translate Take out a piece of paper
Take out a piece of paper Take out a piece of paper
Take out a piece of paper Take the sh out of it
Take the sh out of it Take out a piece of paper
Take out a piece of paper Take out a piece of paper
Take out a piece of paper Take out a piece of paper
Take out a piece of paper Take out a piece of paper
Take out a piece of paper Conversion ladder metric system
Conversion ladder metric system Please take out some time
Please take out some time Take out a piece of paper
Take out a piece of paper Give us your hungry your tired your poor
Give us your hungry your tired your poor Ib chemistry functional groups
Ib chemistry functional groups