Chemistry Do Now 12 3 18 Directions Take
























- Slides: 34

Chemistry Do Now 12 -3 -18 Directions: Take out your Do Now sheet and begin. What is a transition metal? Where are transition metals located on the Periodic Table of Elements? Write the formula for sodium bicarbonate. What is the name of Cu. SO 4? Write the formula for silver acetate. What is the name of Fe 2 S 3? Write the formula for ammonium phosphate. How do you indicate the charge on transition metals?

Chemistry Do Now 12 -3 -18 KEY What is a transition metal? Metals with more than one valence or charge. Where are transition metals located on the Periodic Table of Elements? Groups 3 -12 Write the formula for sodium bicarbonate. Na. HCO 3 What is the name of Cu. SO 4? Copper (II) sulfate Write the formula for silver acetate. Ag. CH 3 COO or Ag. C 2 H 3 O 2 What is the name of Fe 2 S 3? Iron (III) sulfide Write the formula for ammonium phosphate. (NH 4)3 PO 4 How do you indicate the charge on transition metals? By the roman numerals

Reminders i-Ready testing starts on Today, 12 -3 -18 during 3 rd period classes. PARCC testing starts on Monday, 12 -10 -18 Starting tomorrow (12 -4 -18) MXLab will be visiting NEA’s science classrooms (Richards’ & Oji’s) to conduct experiments with you. In order to legally participate in the labs, your parent/guardian must sign and return a consent form. Please return them on Monday. Otherwise you will not be able to participate.

Objective Students will know the parts of a chemical reaction and how the gaining, losing or sharing of valence electrons determines the reactivity of molecules by reading informational text, taking notes, completing a POGIL and answering text-dependent questions. Mastery Level: 4/6 on TDQs and 17/24 on Chemical Reactions POGIL

Unit IV Vocabulary Words Chemical reaction Reactant Product Forward reaction Reverse reaction Catalyst Coefficient Subscript Energy of activation Energy hill Valence electrons Synthesis Decomposition Combustion Acid-base reaction/neutralization Single replacement/displacement Double replacement/displacement Monatomic ion Polyatomic ion

Engage What is a chemical reaction? Can you think of an example of a chemical reaction? How do you think the valence electrons relate to the chemical reactivity of a reaction?

Explore Chemical Reaction You. Tube videos: – https: //www. youtube. com/watch? v=p 9 agy. Xxbso – https: //www. youtube. com/watch? v=8 vybo. Vwy zf. U – https: //www. youtube. com/watch? v=0 Bt 6 RPP 2 ANI In what ways are these chemical reactions? How do you know a chemical reaction has occurred?

Explain Transition Metals and Polyatomic Ions Mini -lesson Introduction to Chemical Reactions PPT will be presented to students while they take notes.

Transition Metals/Group B Metals Transition metals are any of the metallic elements occupying (Groups 3– 12) in the periodic table, e. g. , iron, manganese, chromium, and copper. Transition metals usually have more than 1 charge. In other words, most transition metals can lose different numbers of valence electrons (from 1 -7 electrons). EXCEPTIONS : Zinc and Silver only have 1 charge.

Transition Metals/Group B Metals We use roman numerals to indicate the charge on a transition metal ion. Roman numerals you need to know: – – – – I (one) II (two) III (three) IV (four) V (five) VI (six) VII (seven) Examples: – Copper I Cu+ – Copper II Cu+2 – Iron II Fe+2 – Iron III Fe+3 – Gold I Au+ – Gold III Au+3


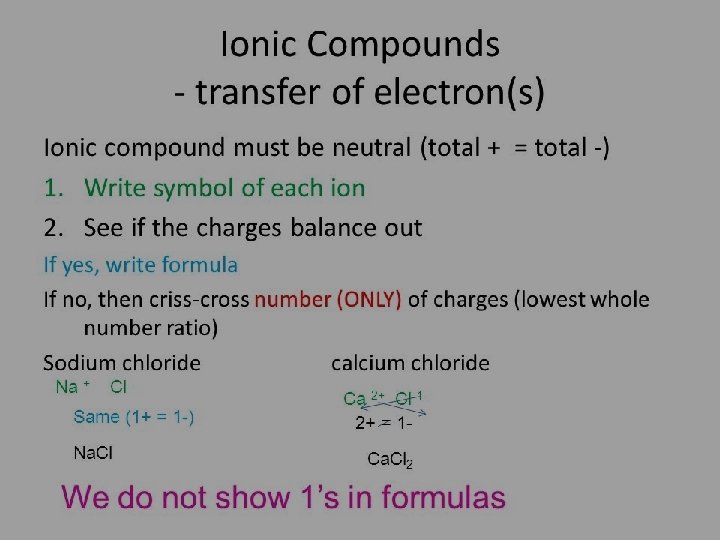
Write the formula for Magnesium Bromide

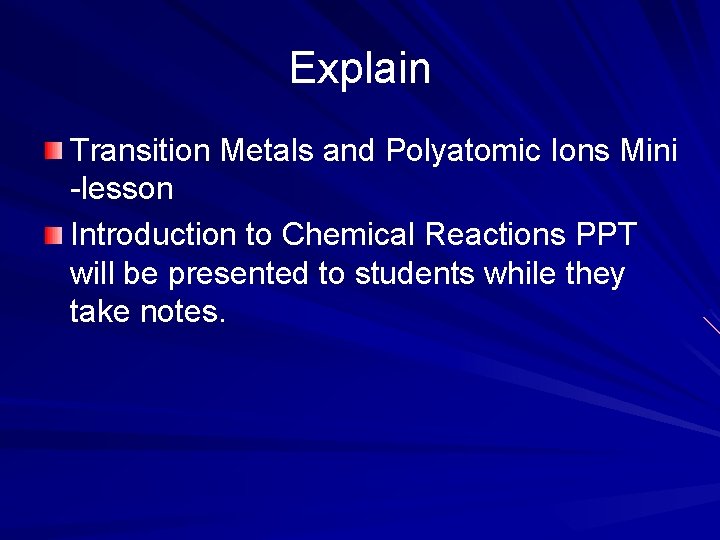
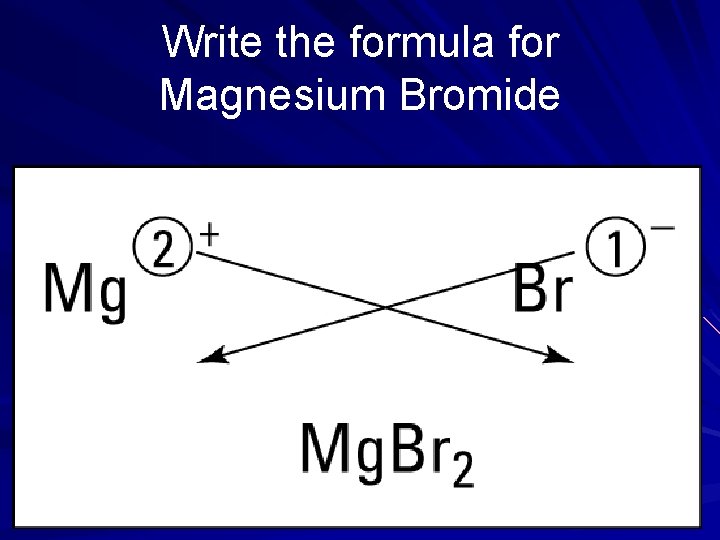
Writing formulas with polyatomic ions is simple!

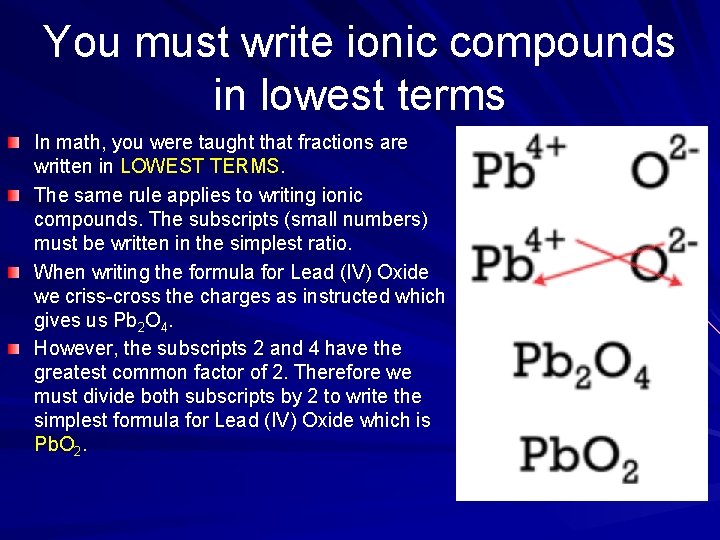
You must write ionic compounds in lowest terms In math, you were taught that fractions are written in LOWEST TERMS. The same rule applies to writing ionic compounds. The subscripts (small numbers) must be written in the simplest ratio. When writing the formula for Lead (IV) Oxide we criss-cross the charges as instructed which gives us Pb 2 O 4. However, the subscripts 2 and 4 have the greatest common factor of 2. Therefore we must divide both subscripts by 2 to write the simplest formula for Lead (IV) Oxide which is Pb. O 2.

Here are the most common transition metals… Fe 3+ iron (III) ion Fe 2+ iron (II) ion Cu 2+ copper (II) ion Cu+ copper (I) ion Cr 3+ chromium (III) ion Cr 2+ chromium (II) ion Ni 3+ nickel (III) ion Ni 2+ nickel (II) ion Pb 4+ lead (IV) ion Pb 2+ lead (II) ion Hg 2+ mercury (II) ion Hg+ mercury (I) ion Au 3+ gold (III) ion Au+ gold (I) ion Zn 2+ zinc ion Ag+ silver ion

Try these! Copper (I) oxide Iron (II) sulfide Iron (III) sulfide Chromium (III) nitride Chromium (II) nitride Calcium hydroxide Potassium acetate Copper (I) cyanide Iron (III) sulfate Mercury (II) nitrate

Answer Key Copper (I) oxide (Cu 2 O) Iron (II) sulfide (Fe. S) Iron (III) sulfide (Fe 2 S 3) Chromium (III) nitride (Cr. N) Chromium (II) nitride (Cr 3 N 2) Calcium hydroxide [Ca(OH)2] Potassium acetate [KCH 3 COO] Copper (I) cyanide [Cu. CN] Iron (III) sulfate [Fe 2(SO 4)3] Mercury (II) nitrate [Hg(NO 3)2]

Go to your internet browser and type in kahoot. it TIME TO PLAY KAHOOT ON IONIC NOMENCLATURE (NAMES)

POLYATOMIC IONS POGIL

Introduction to Chemical Reactions and Equations Unit IV: Reaction Behaviors November 30, 2018

Recognizing Chemical Reactions A CHEMICAL REACTION is a process in which one or more substances are changed into new* substances. “New” simply means substance that were not there before the reaction!

Chemical Equations A chemical reaction cannot be seen; it occurs on the SUBMICROSCOPIC LEVEL! Since reactions can’t be seen, we use a CHEMICAL EQUATION to represent a CHEMICAL REACTION.

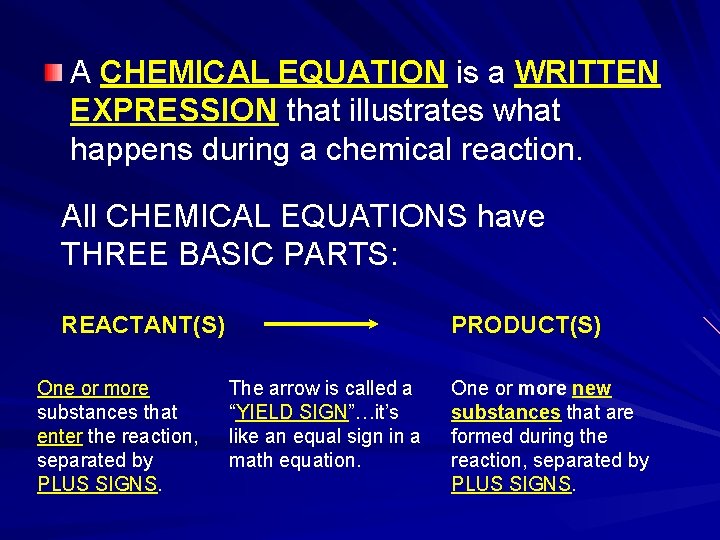
A CHEMICAL EQUATION is a WRITTEN EXPRESSION that illustrates what happens during a chemical reaction. All CHEMICAL EQUATIONS have THREE BASIC PARTS: REACTANT(S) One or more substances that enter the reaction, separated by PLUS SIGNS. PRODUCT(S) The arrow is called a “YIELD SIGN”…it’s like an equal sign in a math equation. One or more new substances that are formed during the reaction, separated by PLUS SIGNS.

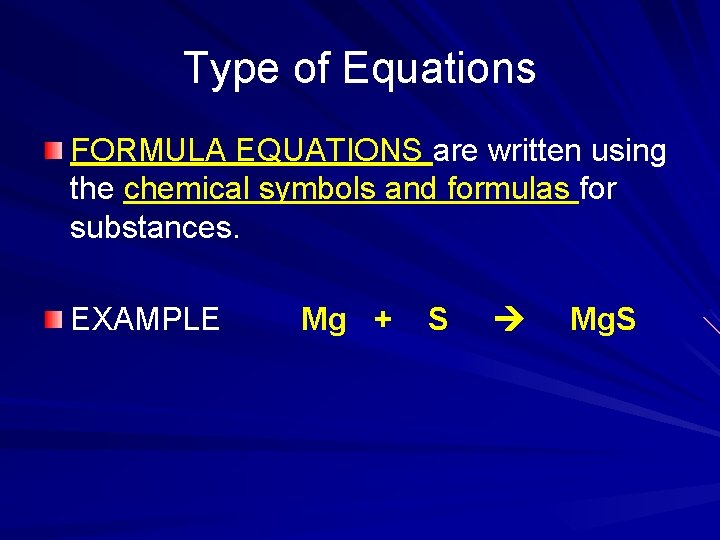
Type of Equations FORMULA EQUATIONS are written using the chemical symbols and formulas for substances. EXAMPLE Mg + S Mg. S

WORD EQUATIONS are written using the names of the elements and compounds involved. EXAMPLE Potassium + Oxygen Potassium Oxide

Equations MUST be BALANCED! q Since a chemical equation illustrates what happens to atoms on the SUBMICROSCOPIC level, it must show exactly what happens to ALL ATOM INVOLVED! The LAW OF CONSERVATION OF MATTER, says MATTER CANNOT BE MADE OR DESTROYED.

• Because of this law, we must write BALANCED equations: equations that have the SAME NUMBER of EACH ATOM on BOTH SIDES of the equation. Consider this equation: Na + Cl 2 Na. Cl Are the SAME NUMBER of SODIUM and CHLORINE atoms on BOTH SIDES of the equation? NO! There are 1 sodium and 2 chlorine atoms on the reactant side, but only 1 sodium and 1 chlorine on the product side…this is NOT BALANCED!

Balancing an Equation Balancing an equation is easy. We can only add numbers BEFORE the formulas to adjust the number of atoms on each side…. we CANNOT change subscripts! These “numbers” are called COEFFICIENTS Coefficients MULTIPLY the number of each kind of atom in a formula!

• Let’s balance our equations now: 2 Na + Cl 2 2 Na. Cl 1. Count the number of EACH atom on both sides: There is 1 Na atom and 2 Cl atoms on the left side. There is 1 Na atoms and 1 Cl atom on the right side. 2. Start with the first unbalanced atom and try adding a coefficient to balance it. 3. Continue adding coefficients as needed until all atoms are balanced in number on both sides. *****USE A PENCIL!!******

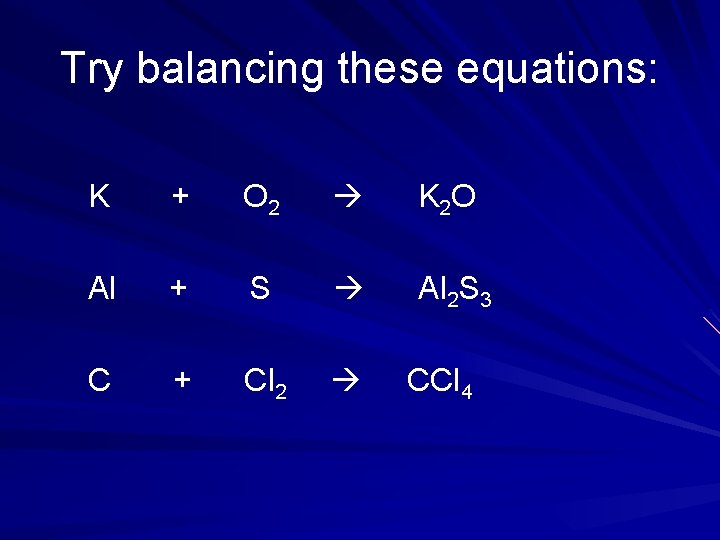
Try balancing these equations: K + O 2 K 2 O Al + S Al 2 S 3 C + Cl 2 CCl 4

4 K + O 2 2 Al + 3 S C + 2 Cl 2 2 K 2 O Al 2 S 3 CCl 4

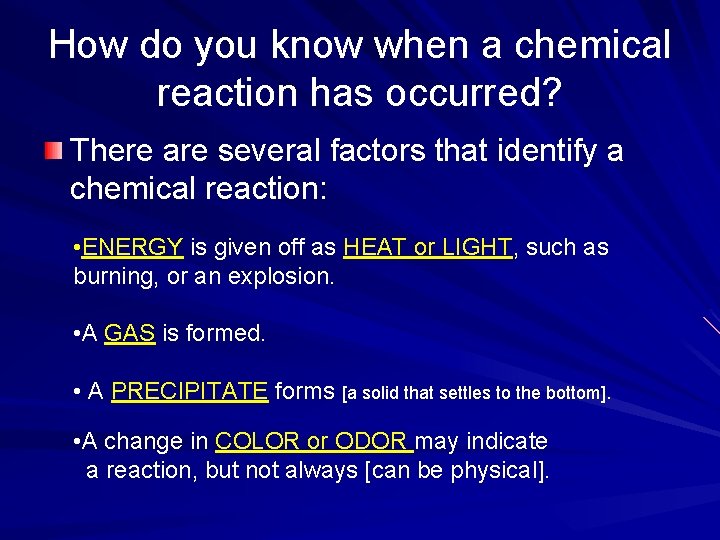
How do you know when a chemical reaction has occurred? There are several factors that identify a chemical reaction: • ENERGY is given off as HEAT or LIGHT, such as burning, or an explosion. • A GAS is formed. • A PRECIPITATE forms [a solid that settles to the bottom]. • A change in COLOR or ODOR may indicate a reaction, but not always [can be physical].

Chemical Reactions POGIL EXTEND

Evaluate 1) Write the formula for potassium permanganate. 2) Name Cu(SO 4)2 3) Name and describe each type of chemical reaction.