Chemistry Do Now 3 1 18 Directions Take












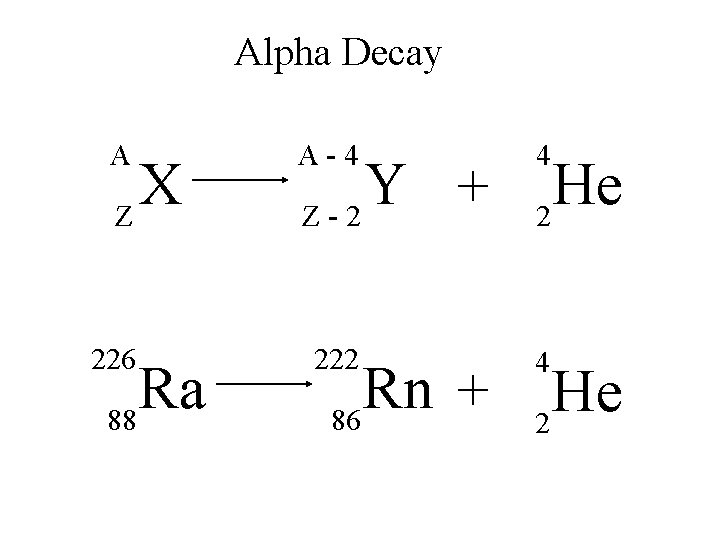
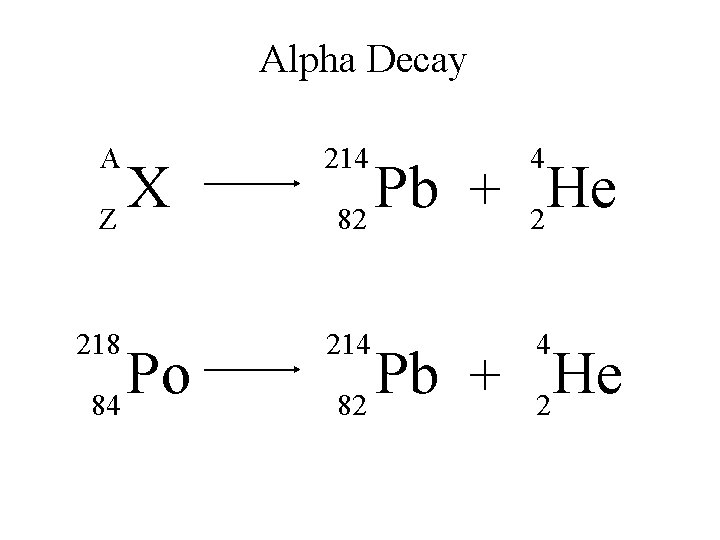
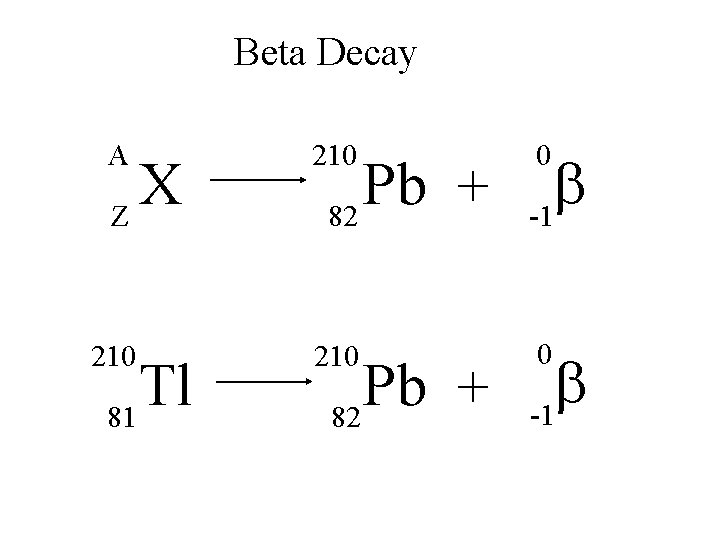
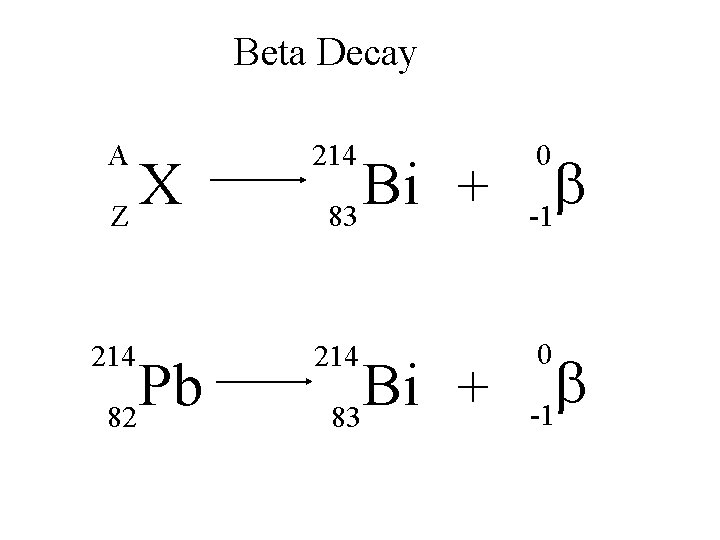


- Slides: 40

Chemistry Do Now 3 -1 -18 • Directions: Take out your Do Now sheet and begin. • • If Uranium 238 undergoes 5 alpha decays, what will be the final element? • If Uranium 238 undergoes 5 beta decays, what will be the final element? • If Uranium 238 undergoes 5 gamma decays, what will be the final element?

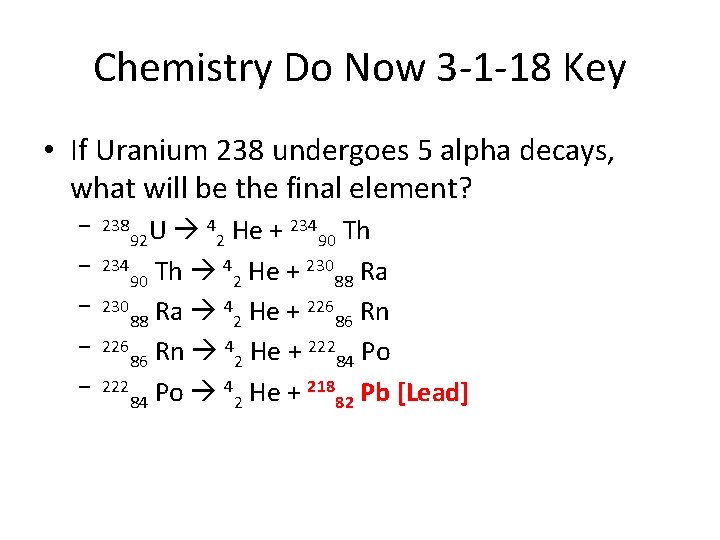
Chemistry Do Now 3 -1 -18 Key • If Uranium 238 undergoes 5 alpha decays, what will be the final element? 4 He + 234 Th U 92 2 90 234 Th 4 He + 230 Ra 90 2 88 230 Ra 4 He + 226 Rn 88 2 86 226 Rn 4 He + 222 Po 86 2 84 222 Po 4 He + 218 Pb [Lead] 84 2 82 – 238 – –

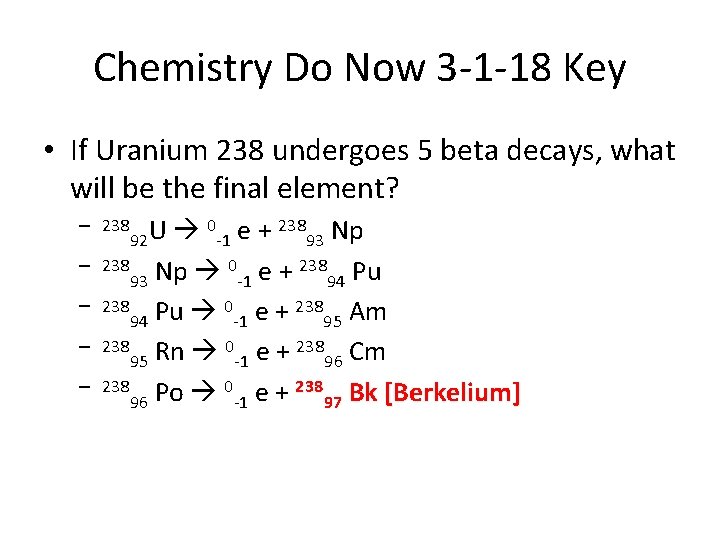
Chemistry Do Now 3 -1 -18 Key • If Uranium 238 undergoes 5 beta decays, what will be the final element? 0 e + 238 Np U 92 -1 93 238 Np 0 e + 238 Pu 93 -1 94 238 Pu 0 e + 238 Am 94 -1 95 238 Rn 0 e + 238 Cm 95 -1 96 238 Po 0 e + 238 Bk [Berkelium] 96 -1 97 – 238 – –

Chemistry Do Now 3 -1 -18 Key • If Uranium 238 undergoes 5 gamma decays, what will be the final element? * 0 γ + 238 U U 92 0 92 – 238 U 0 γ + 238 U [stable form of uranium] 92 0 92 – * Least stable form of uranium – 238

Objective • (a) Students will know the three major types of nuclear decay that may occur in the atomic nucleus to reach stability by completing a nuclear decay worksheet. – Mastery Level: 70% of nuclear decay worksheet. • (b) Students will know the meaning of words starting with the prefixes aer(o)- and anem(o)- by completing vocabulary worksheets. – Mastery Level: 70% (18/26) or better

Engage – Nuclear Decay • Students will watch the video “Alpha, Beta and Gamma Decay | Types of Radioactive Decay | Physics 4 Students” on You. Tube • Source: https: //www. youtube. com/watch? v=YYnf. Lc. H Db. Z 8

Engage – Alpha & Beta Nuclear Decay • Show the students the You. Tube video “Alpha Decay” • Source: https: //www. youtube. com/watch? v=Cw. Exbn. Ozc 4 o • Show the students the You. Tube video “Beta Decay” • Source: https: //www. youtube. com/watch? v=uq. AA_D 9 Mi _I

Explore – Close Read • Students will perform a close read on the article titled RADIATION • Source: http: //www. ducksters. com/science/chemistry /radiation_and_radioactivity. php

Nuclear Decay Vocabulary Words • • • Alpha decay Alpha particle Helium nuclei Beta decay Beta particle Electron Gamma decay Gamma radiation Radioisotope Isotope Atomic Mass

EXPLAIN Nuclear Decay (Students will take notes. )

Copy every note and example into your composition notebook or chemistry notes’ section. Nuclear Reactions Alpha, Beta, and Gamma Decay Unit II: Nuclear Chemistry Tuesday, February 27, 2018

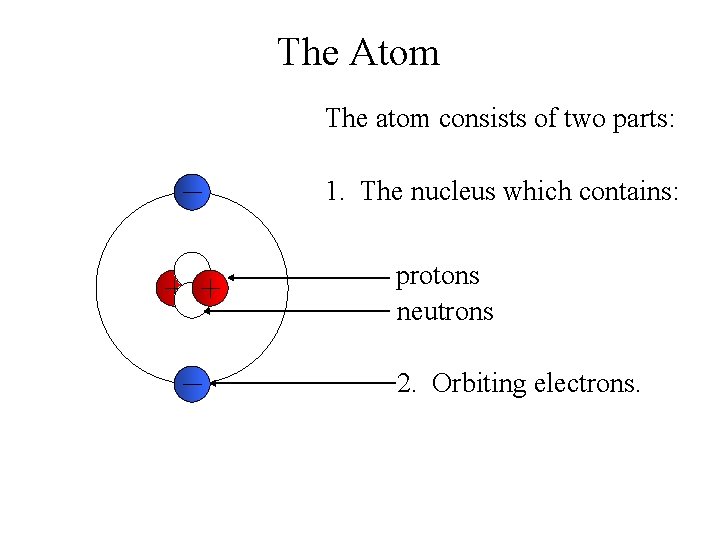
The Atom The atom consists of two parts: 1. The nucleus which contains: protons neutrons 2. Orbiting electrons.

The Atom All matter is made up of elements (e. g. carbon, hydrogen, etc. ). The smallest part of an element is called an atom. Atom of different elements contain different numbers of protons. The mass of an atom is almost entirely due to the number of protons and neutrons.

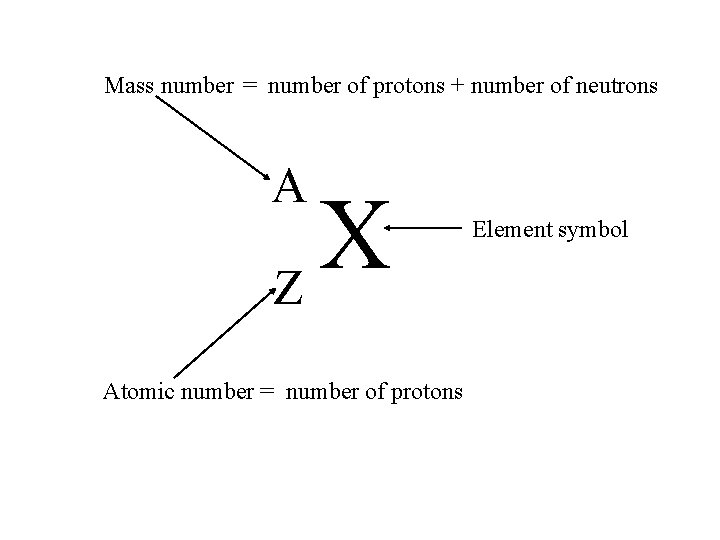
Mass number = number of protons + number of neutrons A X Z Atomic number = number of protons Element symbol

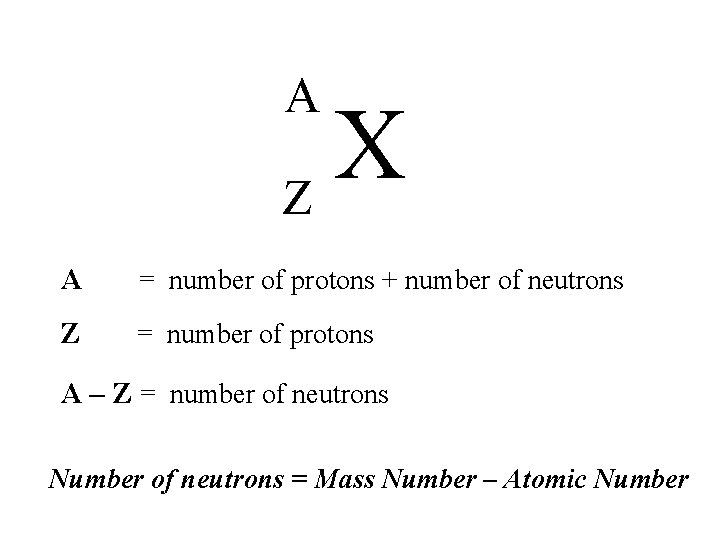
A X Z A = number of protons + number of neutrons Z = number of protons A – Z = number of neutrons Number of neutrons = Mass Number – Atomic Number

There are many types of uranium: 235 238 A A Z Z Number of protons Number of neutrons U 92

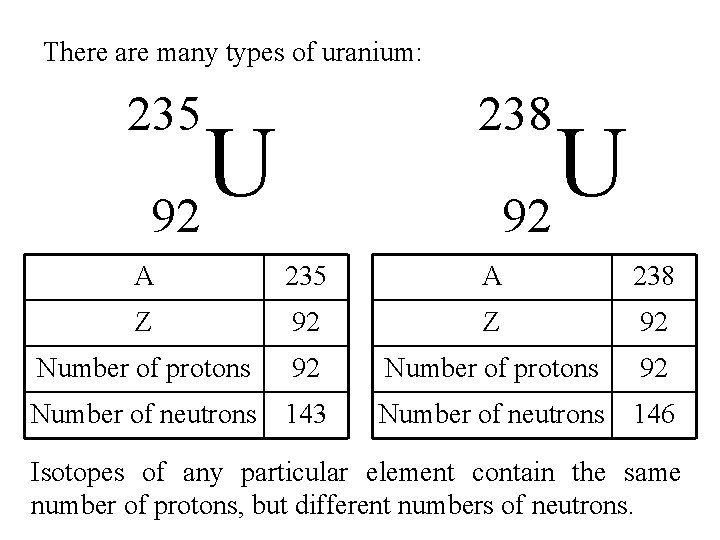
There are many types of uranium: 238 235 U 92 A 235 A 238 Z 92 Number of protons 92 Number of neutrons 143 Number of neutrons 146 Isotopes of any particular element contain the same number of protons, but different numbers of neutrons.

Most of the isotopes which occur naturally are stable. A few naturally occurring isotopes and all of the manmade isotopes are unstable. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/radionuclides.

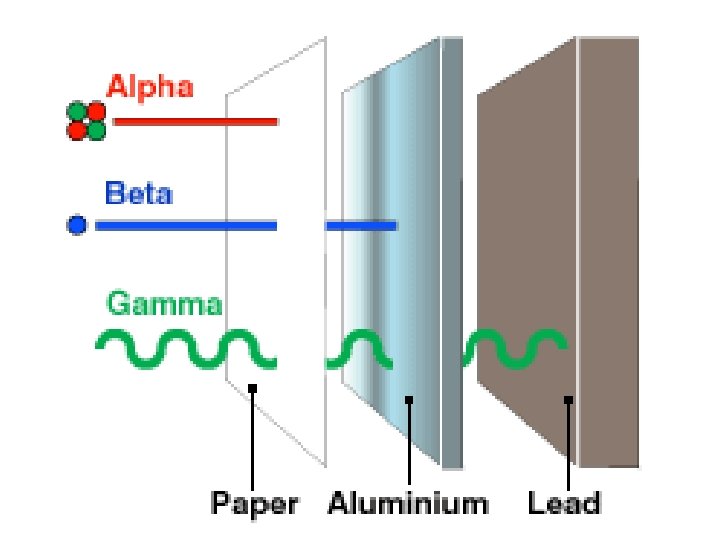
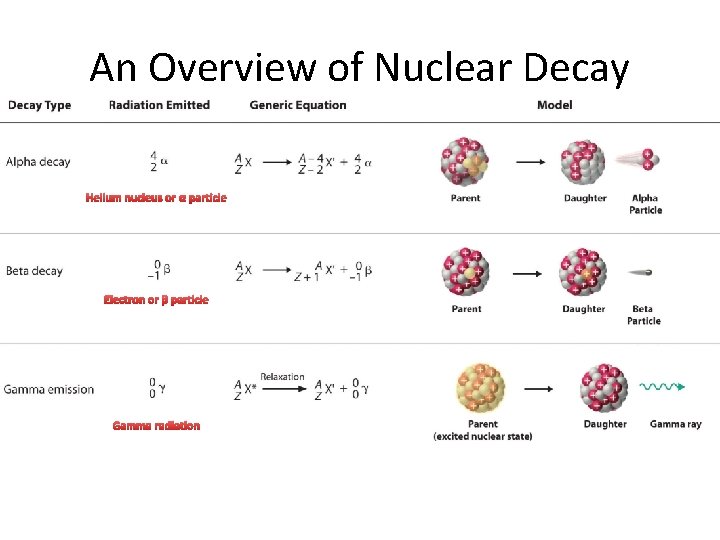
Radioactive Decay Radioactive decay results in the emission of either: • an alpha particle (a), • a beta particle (b), • or a gamma ray(g).


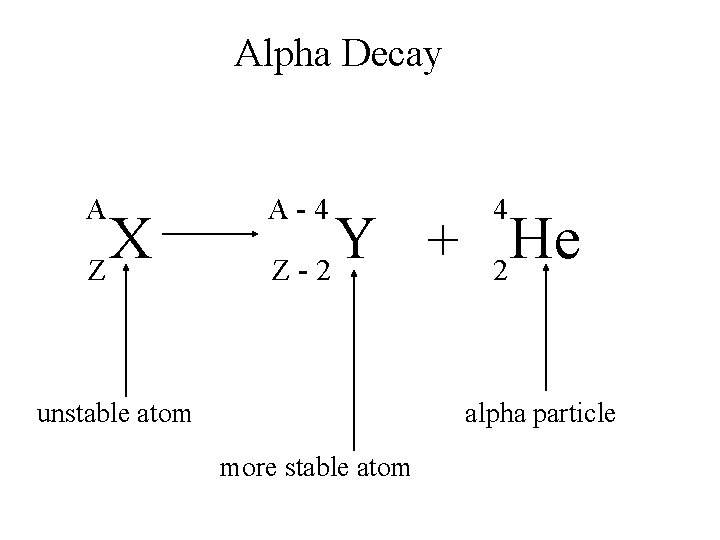
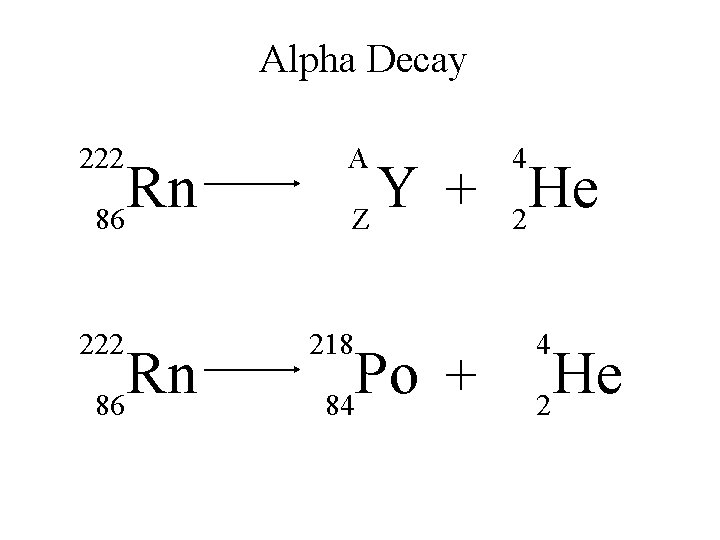
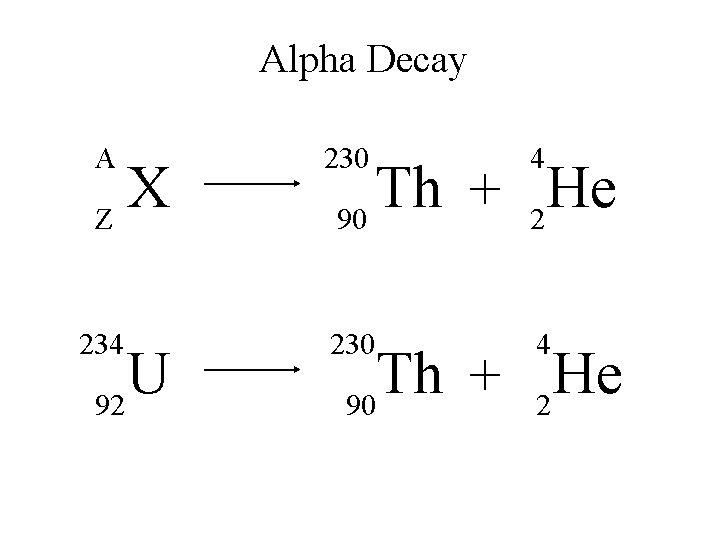
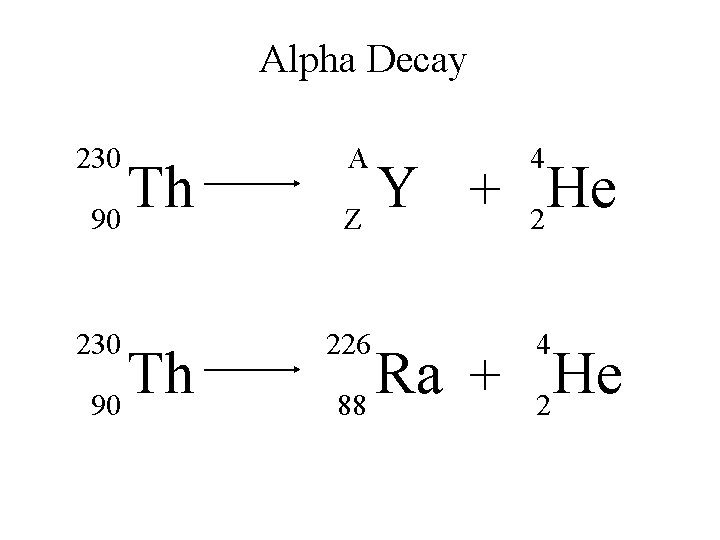
Alpha Decay An alpha particle is identical to that of a helium nucleus. It contains two protons and two neutrons.

Alpha Decay A X Z A-4 4 Y He + Z-2 2 unstable atom alpha particle more stable atom

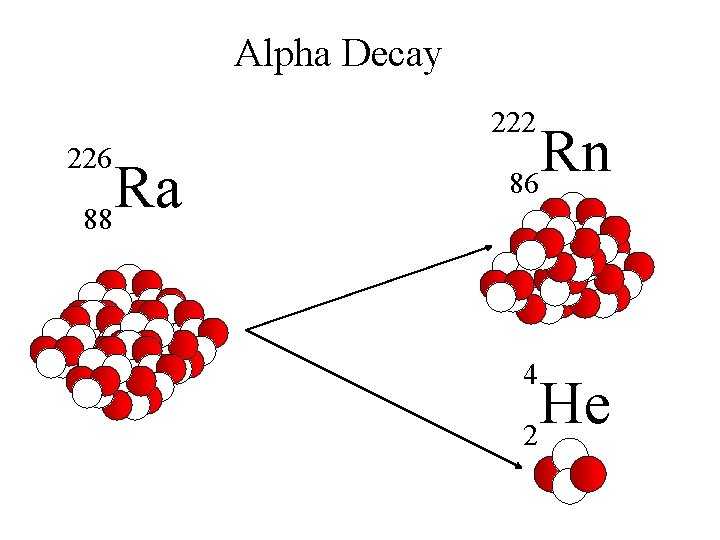
Alpha Decay 222 226 Ra 88 Rn 86 4 He 2
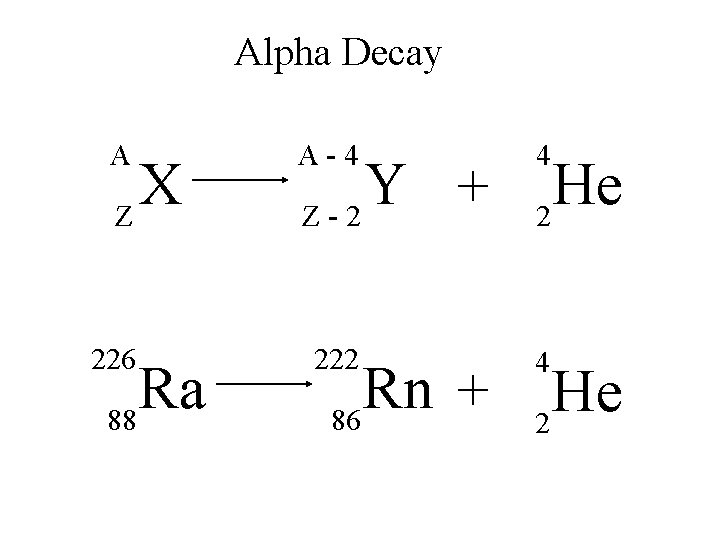
Alpha Decay A A-4 4 226 222 4 X Z Ra 88 Y + Z-2 Rn + 86 He 2

Alpha Decay 222 Rn 86 A 4 Y He + Z 2 218 Po + 84 4 He 2

Alpha Decay A 230 4 X Z U 92 Th He + 90 2

Alpha Decay 230 Th 90 A 4 226 4 Y He + Z 2 Ra He + 88 2
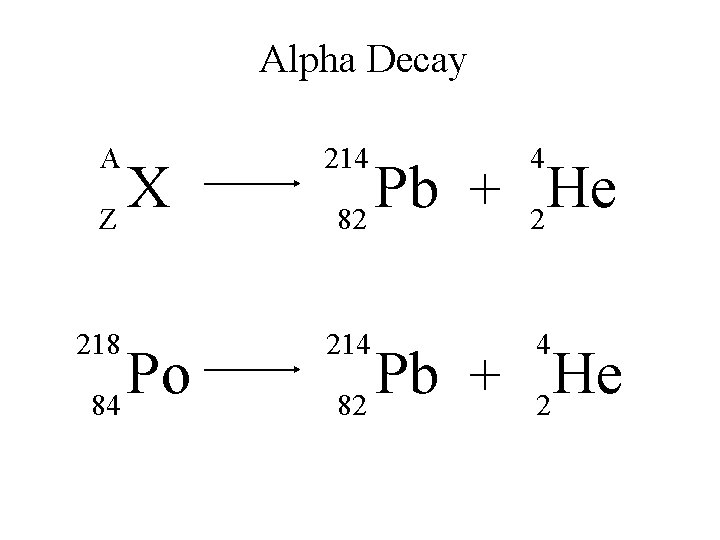
Alpha Decay A 214 4 218 214 4 X Z Po 84 Pb He + 82 2

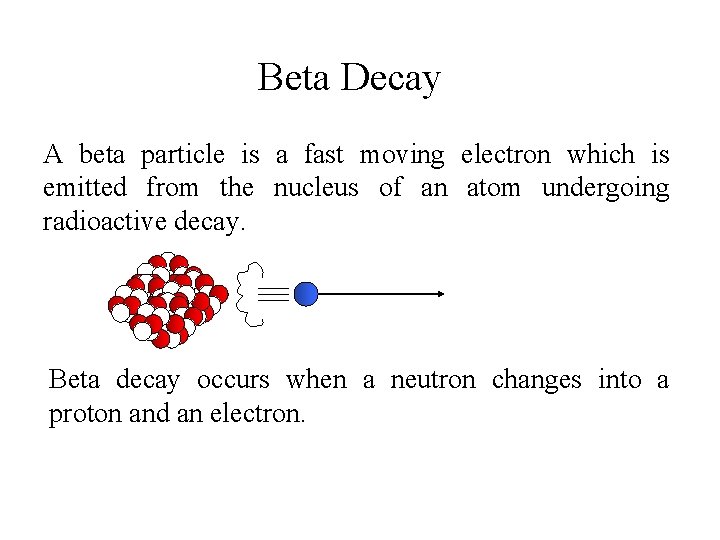
Beta Decay A beta particle is a fast moving electron which is emitted from the nucleus of an atom undergoing radioactive decay. Beta decay occurs when a neutron changes into a proton and an electron.

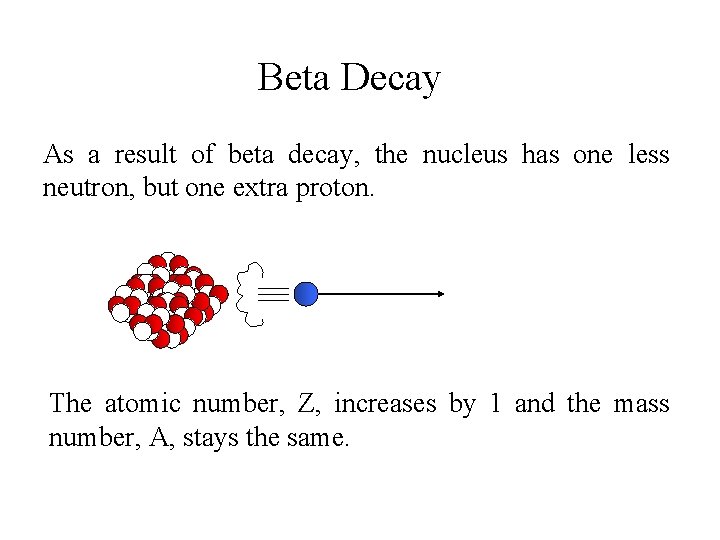
Beta Decay As a result of beta decay, the nucleus has one less neutron, but one extra proton. The atomic number, Z, increases by 1 and the mass number, A, stays the same.

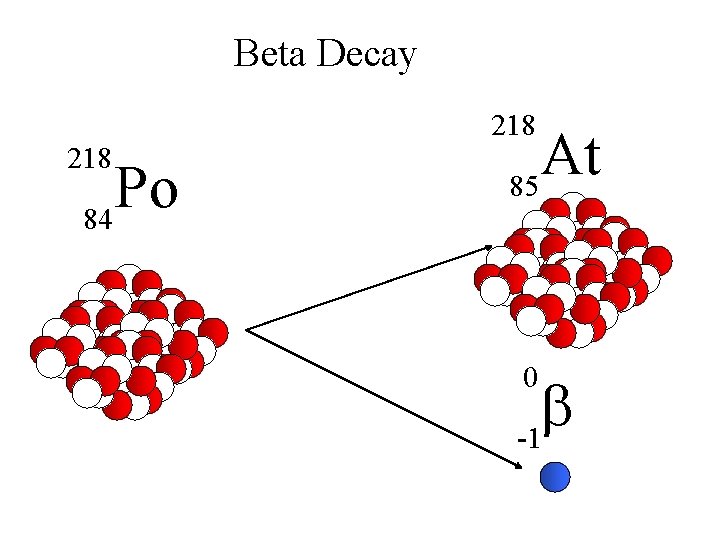
Beta Decay 218 Po 84 At 85 0 b -1

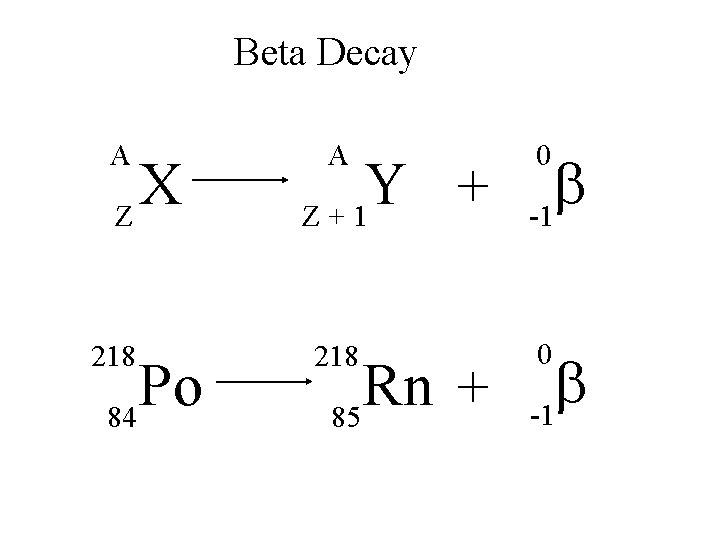
Beta Decay A X Z 218 Po 84 A Y + Z+1 218 Rn + 85 0 b -1

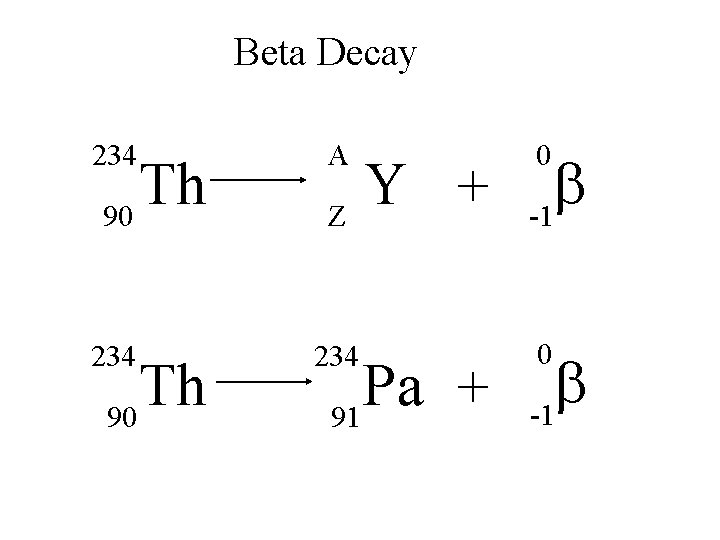
Beta Decay 234 A 234 Th 90 Y + Z Pa + 91 0 b -1
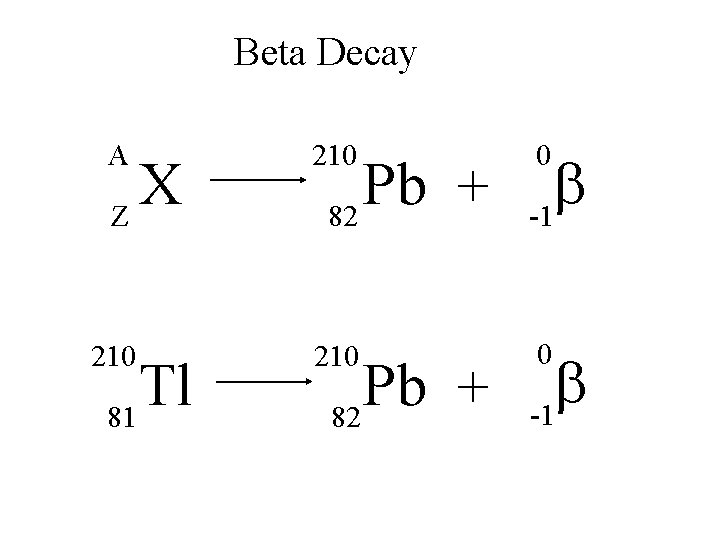
Beta Decay A 210 b -1 210 0 X Z Tl 81 Pb + 82 0 b -1

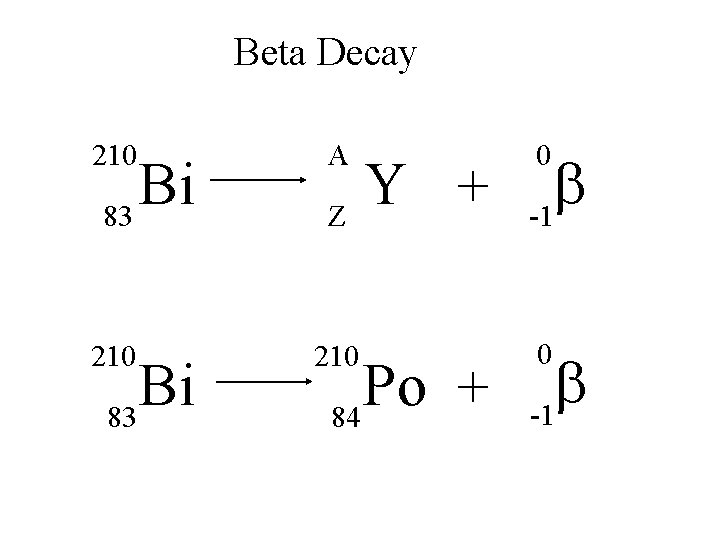
Beta Decay 210 A 210 Bi 83 Y + Z Po + 84 0 b -1
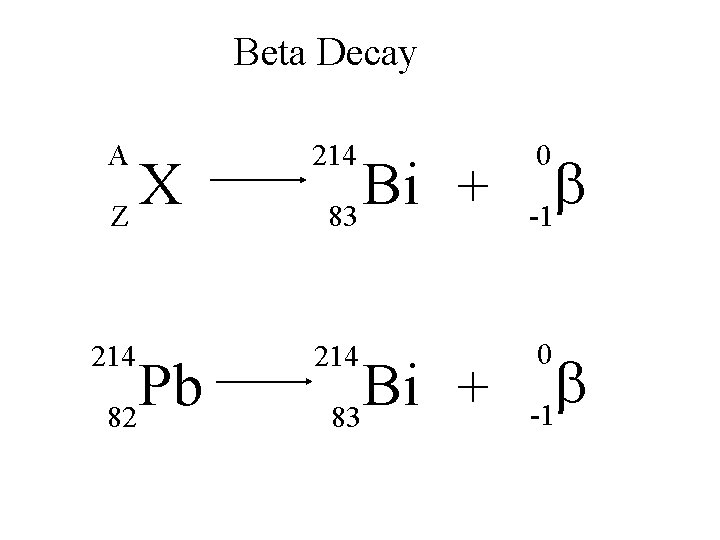
Beta Decay A 214 b -1 214 0 X Z Pb 82 Bi + 83 0 b -1

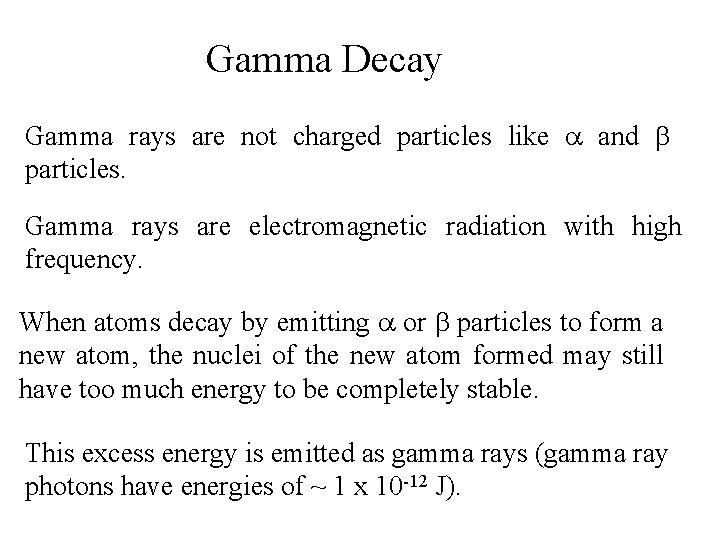
Gamma Decay Gamma rays are not charged particles like a and b particles. Gamma rays are electromagnetic radiation with high frequency. When atoms decay by emitting a or b particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely stable. This excess energy is emitted as gamma rays (gamma ray photons have energies of ~ 1 x 10 -12 J).

An Overview of Nuclear Decay Helium nucleus or α particle Electron or β particle Gamma radiation

Extend/Evaluate – Nuclear Decay Worksheet (Let’s Review it!) • Nuclear Decay Worksheet – Goal: 23 out of 33 correct or more

LET’S PLAY KAHOOT ON PARCC VOCABULARY LIST #2