Intermolecular Forces Intramolecular Forces vs Intermolecular Forces Intramolecular





















- Slides: 30

Intermolecular Forces

Intramolecular Forces vs. Intermolecular Forces Intramolecular Forces Chemical bonds Intermolecular Forces Attractive forces between molecules

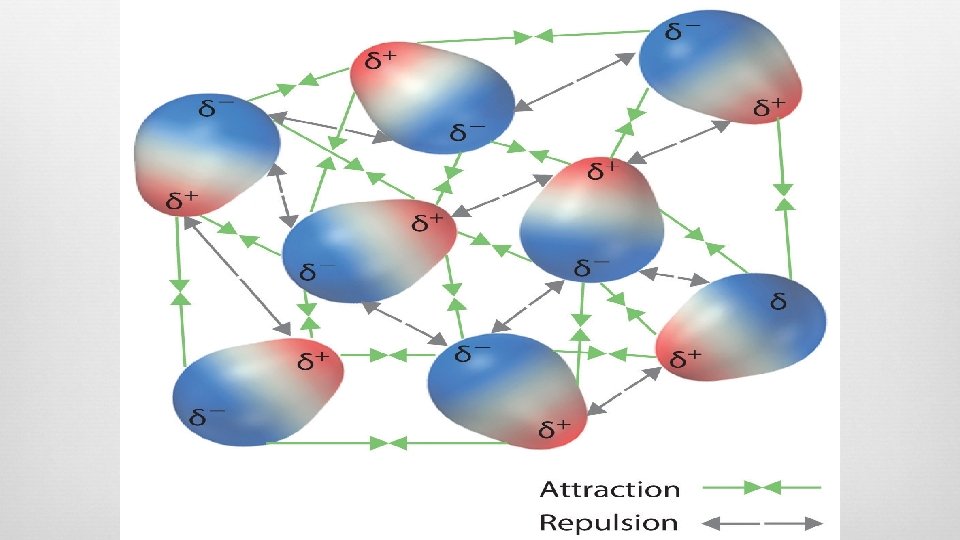
What are intermolecular forces? NOT chemical bonds, less strength Attractive forces between molecules involved in covalent bonding Molecular level, not individual atoms Develop solid and liquid physical properties Types London Dispersion Dipole-Dipole Ion-Dipole Hydrogen Bonding

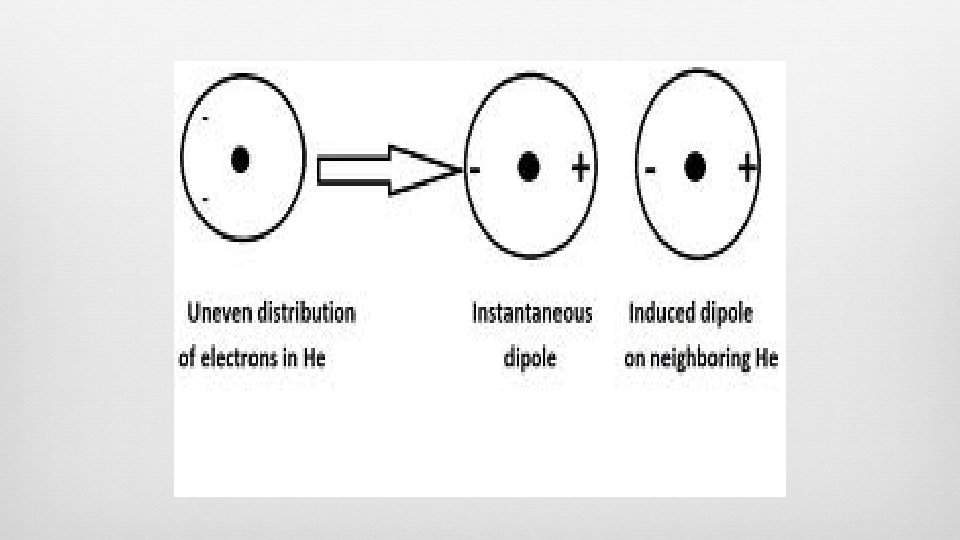
(London) Dispersion Forces Attractive force between dipoles TEMPORARY charge separation in an atom (instantaneous dipole) Sudden dipole in one atom causes “domino” effect— influences electron distribution within adjacent atoms (induced dipoles At SOME point in time, electron density is greater around one atom than the other Induces temporary dipoles in adjacent molecules Found in ALL atoms/molecules


Intermolecular Force Strength Influenced by: 1) Polarizability 2) Molecular Shape

1. Polarizability How easily can a dipole be induced in a molecule or atom How easily can the electron density of an atom/molecule be altered polarizability, strength of intermolecular forces (WHY? ) **Atomic Size and Mass have an influence on polarizability

Example 1: Which molecule is more likely to a gas at room temperature: F 2 or Br 2?

2. Molecular Shape More contact with adjacent molecules creates more dispersion forces Bigger/elongated molecules have this advantage Compact molecules do not have as much surface area

Example 2: Which compound has the strongest intermolecular forces?

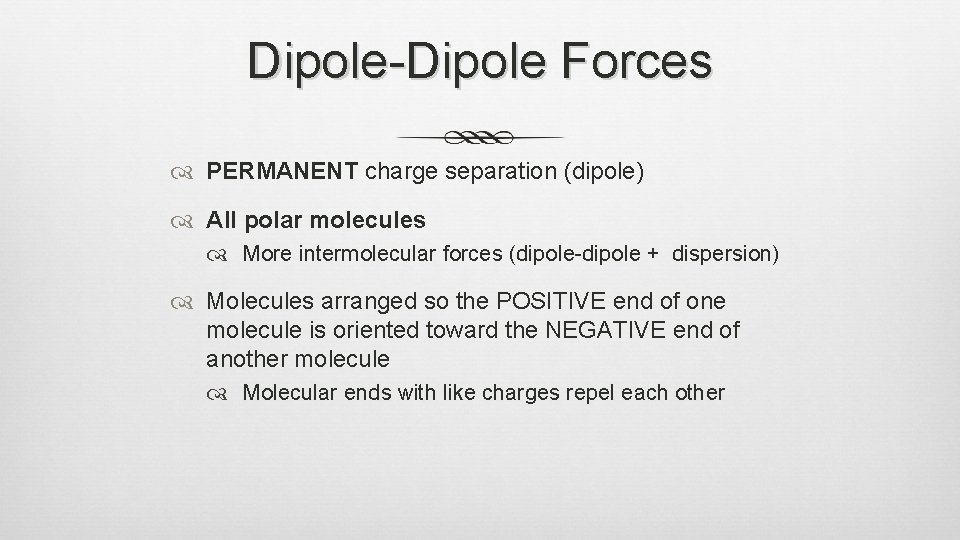
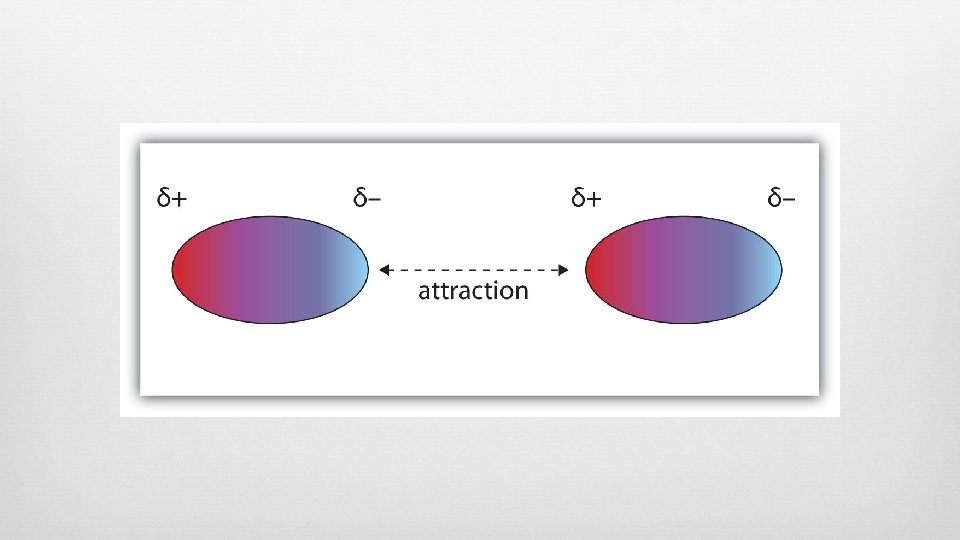
Dipole-Dipole Forces PERMANENT charge separation (dipole) All polar molecules More intermolecular forces (dipole-dipole + dispersion) Molecules arranged so the POSITIVE end of one molecule is oriented toward the NEGATIVE end of another molecule Molecular ends with like charges repel each other



Example 3: Propane vs. acetaldehyde Which has stronger intermolecular forces?


Example 4 Based on intermolecular forces, arrange the following in order from decreasing to increasing boiling point: CBr 4 CH 3 CH 2 CH 3 F 2 CH 3 CHO

Example 5 Examine the following 2 chemical compounds: Br. Cl and IBr. Which compound is a gas at room temperature? A solid?

Ion-Dipole Attractive forces between ions and polar molecules Results from soluble ionic compounds in polar solvent (ex. Water) Ions split up in solvent and are surrounded by water molecules Negative portion of water molecule surrounds POSITIVE ion Positive portion of water molecule surrounds NEGATIVE ion


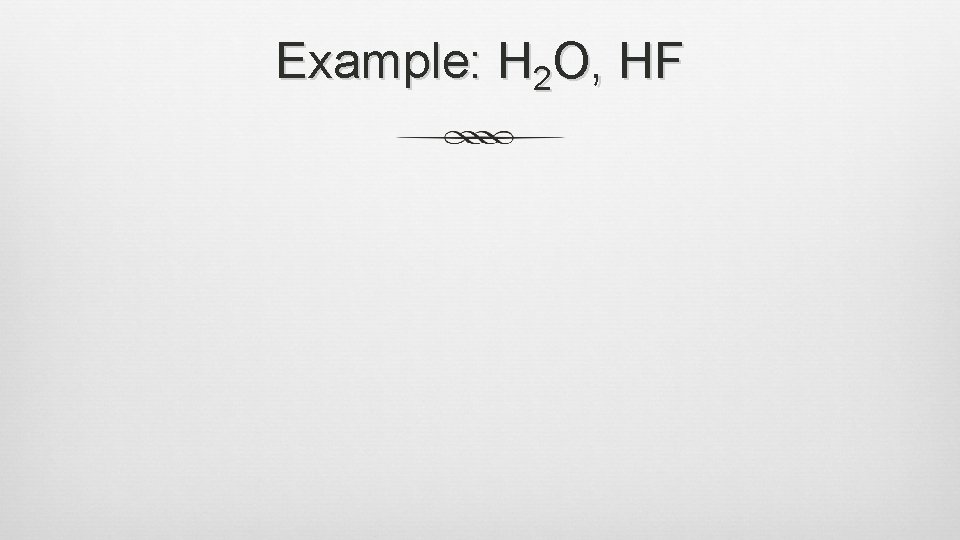
Hydrogen Bonding Type of intermolecular force, strong dipole-dipole force H atom is bonded to an electronegative (nonmetal) atom and is attracted to the electronegative (nonmetal) atom in a neighboring molecule Majority of H-bonding occurs among small, very electronegative, nonmetal atoms (N, O, F) Dotted lines represent hydrogen bonding

Hydrogen Bonding (cont. ) Considered a FORCE, not a type of bond Force holding water molecules together Results from a negative charge on an atom and a positive charge on hydrogen H-X format

Example: H 2 O, HF

Unique Properties of Water due to Hydrogen Bonding Freezes and expands as solid Solid is less dense than liquid (ex. Ice floats) High melting point High boiling point H-bonding strength greater than other intermolecular forces Highest surface tension (Hg only exception) Other compounds can easily dissolve in it High specific heat

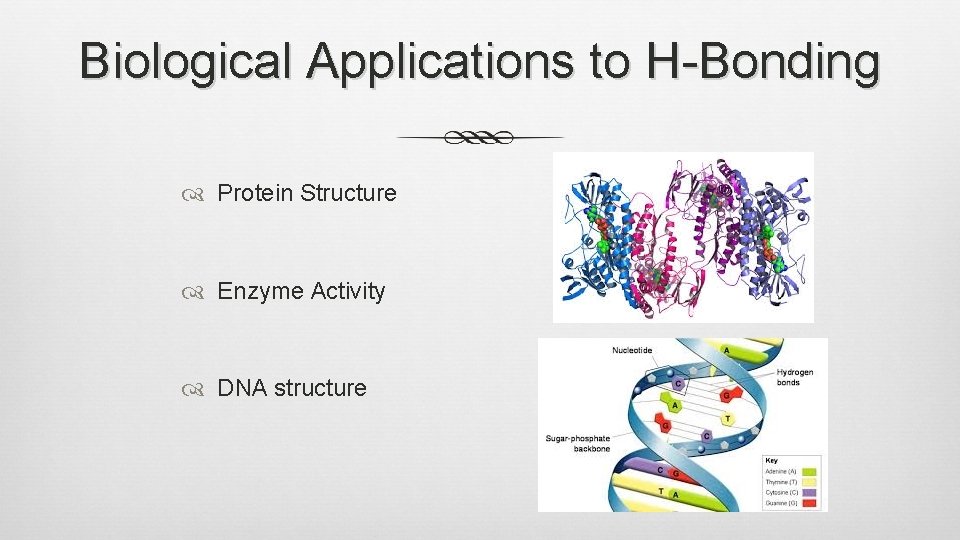
Biological Applications to H-Bonding Protein Structure Enzyme Activity DNA structure

ALL DUE TO HYDROGEN BONDING !

Intermolecular Force Strength All chemical compounds have dispersion forces Other intermolecular forces are dependent on molecular structure Generally, increasing molecular weight indicates an increase in intermolecular forces Analyze molecular structure and types of intermolecular forces for compounds of similar molecule weight. Similar forces, look at molecular weight

Strength of Intermolecular Forces

Example 6: The following compounds have similar molecular weights. Arrange in order from DECREASING to INCREASING boiling point. Formaldehyde (H 2 C=O) Methanol (CH 3—OH) Ethan (CH 3)

Example 7: Arrange in order from DECREASING to INCREASING boiling point. Carbon tetrachloride (CCl 4) Acetone (C 3 H 6 O) Tetrabromobutane (C 4 H 6 Br 4)

Example 8: Arrange in order from INCREASING to DECREASING boiling point. Octanoic acid—CH 3(CH 2)6 COOH Decane—CH 3(CH 2)8 CH 3 Nonanal—CH 3(CH 2)7 CHO