Intermolecular Forces and Liquids and Solids Chapter 11











- Slides: 50

Intermolecular Forces and Liquids and Solids Chapter 11

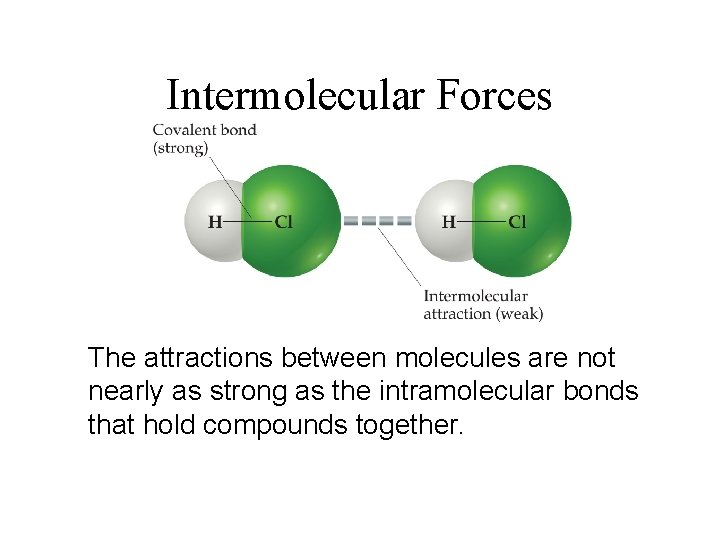
Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular bonds that hold compounds together.

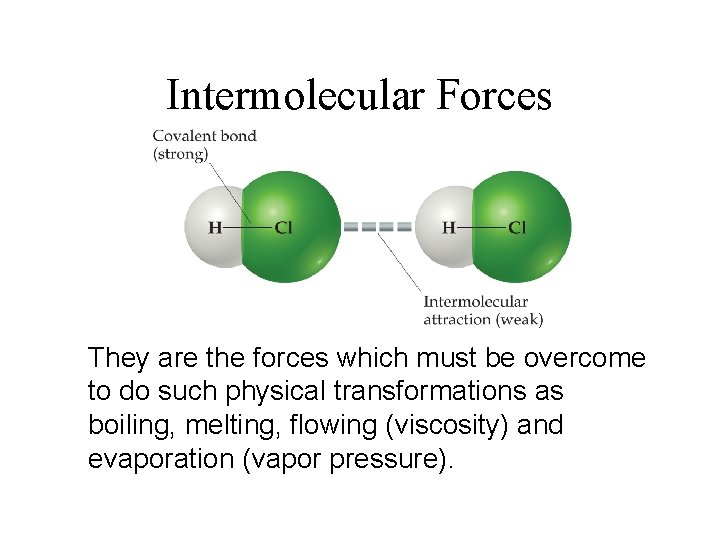
Intermolecular Forces They are the forces which must be overcome to do such physical transformations as boiling, melting, flowing (viscosity) and evaporation (vapor pressure).

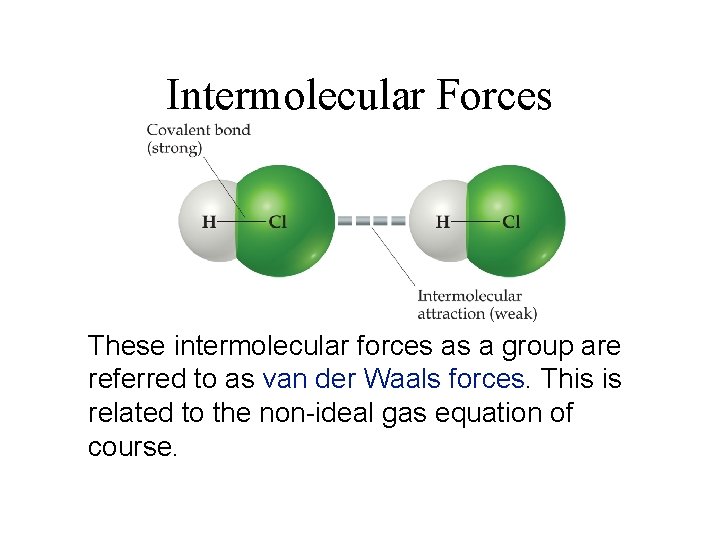
Intermolecular Forces These intermolecular forces as a group are referred to as van der Waals forces. This is related to the non-ideal gas equation of course.

van der Waals Forces • For a pure substance • Strongest to weakest – Hydrogen bonding – Dipole-dipole interactions – London dispersion forces

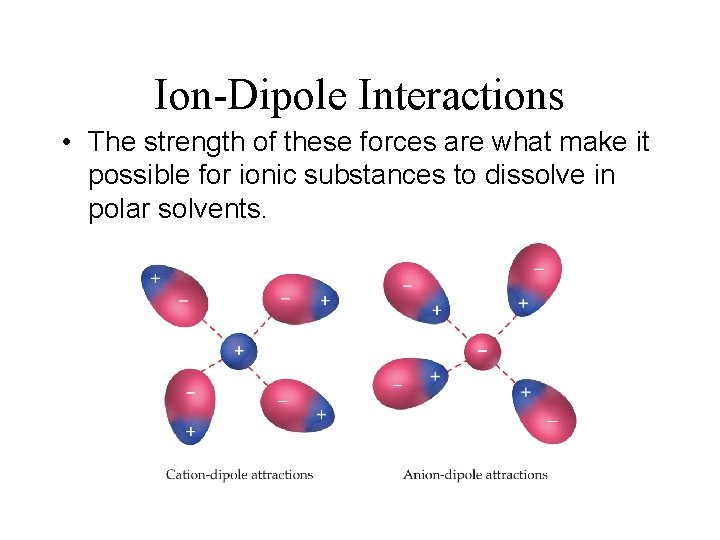
Intermolecular Forces Ion-Dipole Forces • A fourth type of force, ion-dipole interactions are an important force in solutions of ions. • Attractive forces between an ion and a polar molecule Ion-Dipole Interaction 11. 2

Ion-Dipole Interactions • The strength of these forces are what make it possible for ionic substances to dissolve in polar solvents.

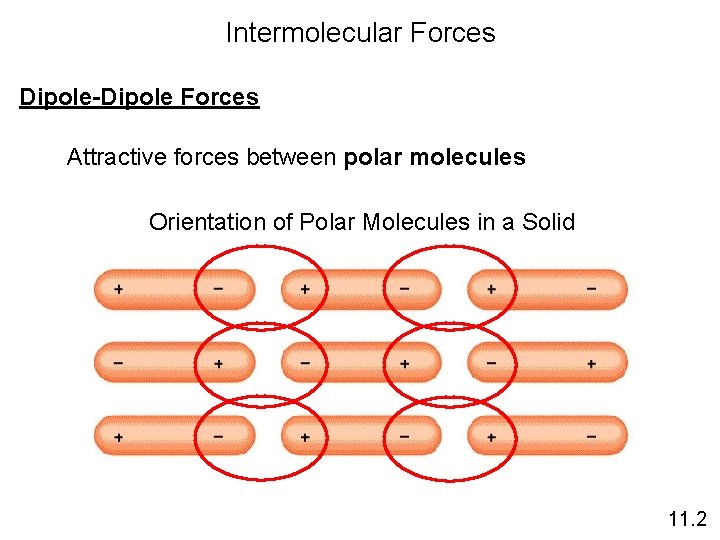
Intermolecular Forces Dipole-Dipole Forces Attractive forces between polar molecules Orientation of Polar Molecules in a Solid 11. 2

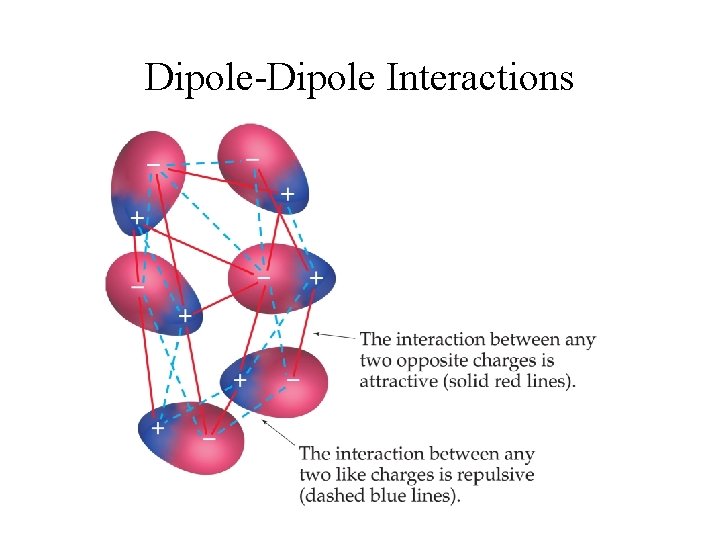
Dipole-Dipole Interactions

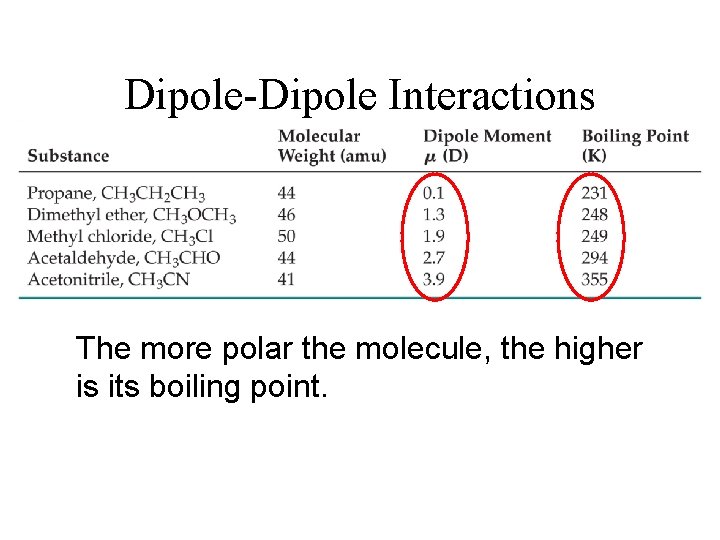
Dipole-Dipole Interactions The more polar the molecule, the higher is its boiling point.

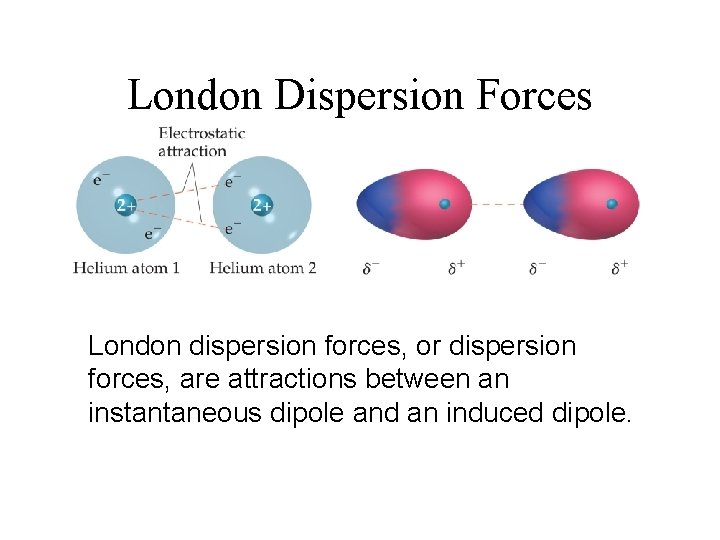
London Dispersion Forces While the electrons in the 1 s orbital of helium tend to be arranged symmetrically, occasionally the electron density is polarized.

London Dispersion Forces At that instant, the excess of electrons on the left side and a shortage on the right side makes the helium atom polar.

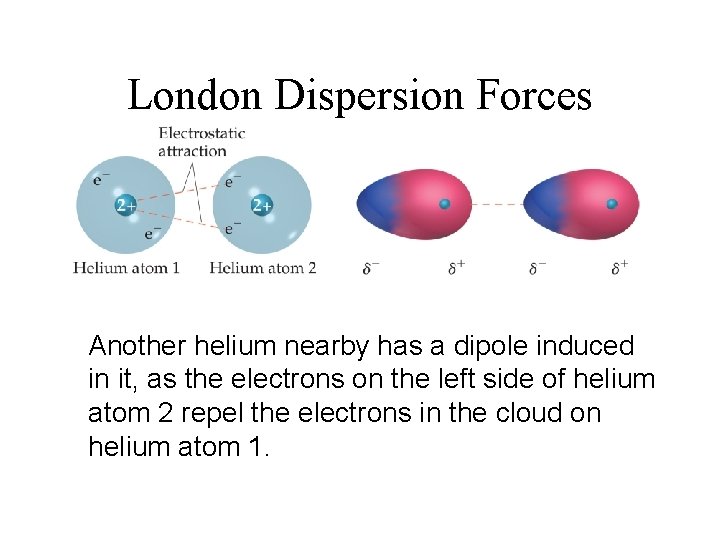
London Dispersion Forces Another helium nearby has a dipole induced in it, as the electrons on the left side of helium atom 2 repel the electrons in the cloud on helium atom 1.

London Dispersion Forces London dispersion forces, or dispersion forces, are attractions between an instantaneous dipole and an induced dipole.

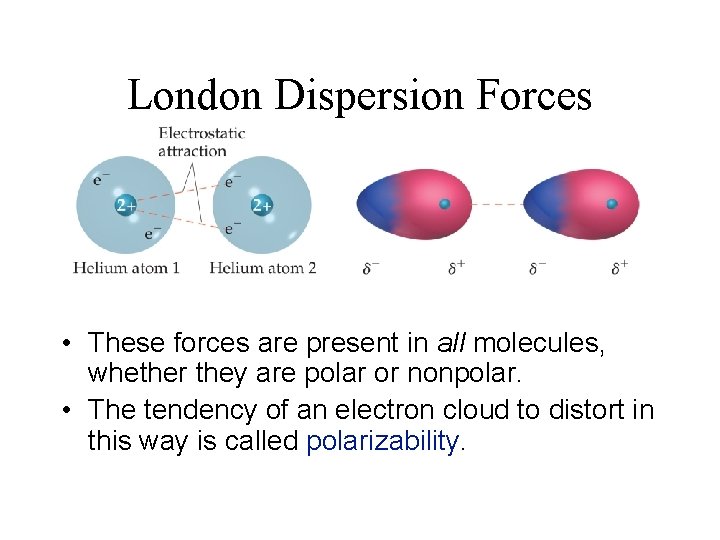
London Dispersion Forces • These forces are present in all molecules, whether they are polar or nonpolar. • The tendency of an electron cloud to distort in this way is called polarizability.

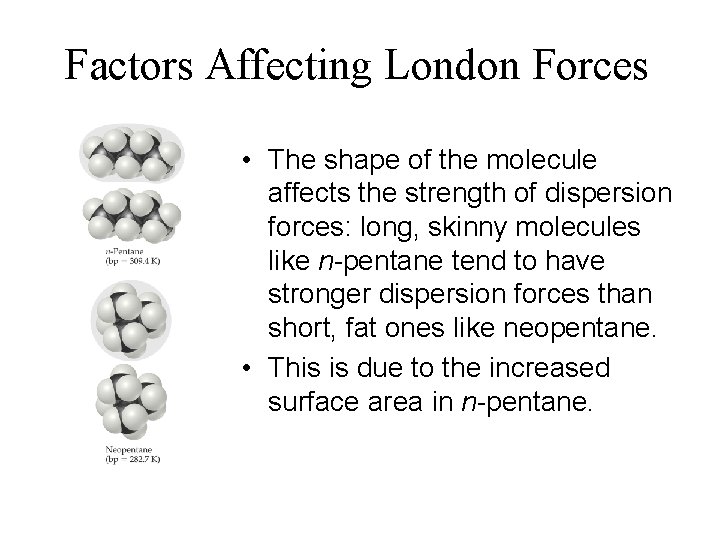
Factors Affecting London Forces • The shape of the molecule affects the strength of dispersion forces: long, skinny molecules like n-pentane tend to have stronger dispersion forces than short, fat ones like neopentane. • This is due to the increased surface area in n-pentane.

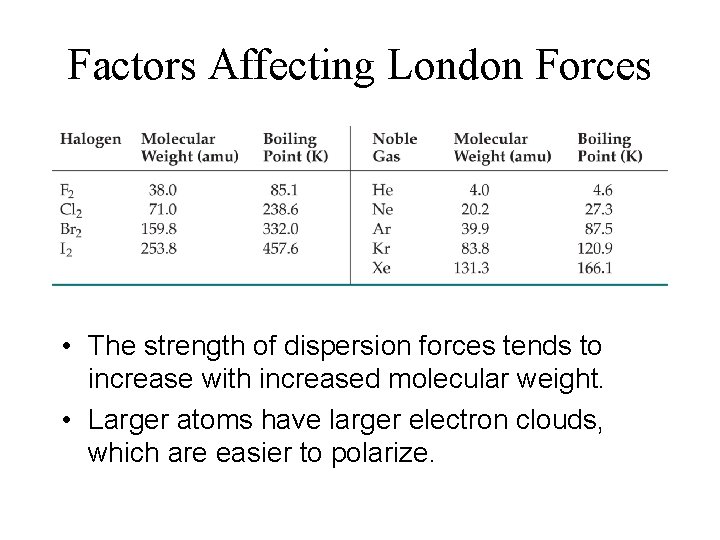
Factors Affecting London Forces • The strength of dispersion forces tends to increase with increased molecular weight. • Larger atoms have larger electron clouds, which are easier to polarize.

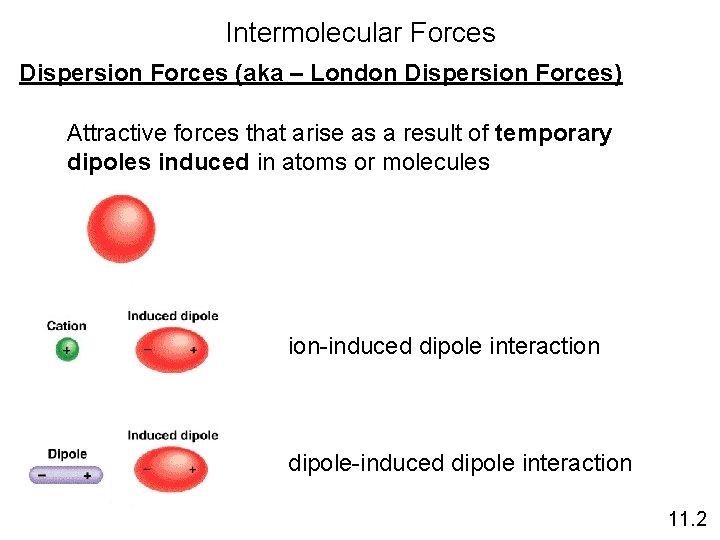
Intermolecular Forces Dispersion Forces (aka – London Dispersion Forces) Attractive forces that arise as a result of temporary dipoles induced in atoms or molecules ion-induced dipole interaction dipole-induced dipole interaction 11. 2

Which Have a Greater Effect: Dipole-Dipole Interactions or Dispersion Forces? • If two molecules are of comparable size and shape, dipole-dipole interactions will likely be the dominating force. • If one molecule is much larger than another, dispersion forces will likely determine its physical properties.

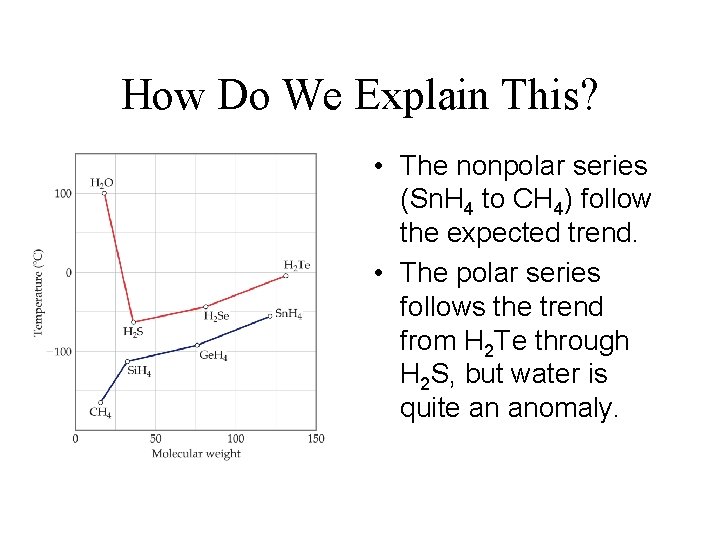
How Do We Explain This? • The nonpolar series (Sn. H 4 to CH 4) follow the expected trend. • The polar series follows the trend from H 2 Te through H 2 S, but water is quite an anomaly.

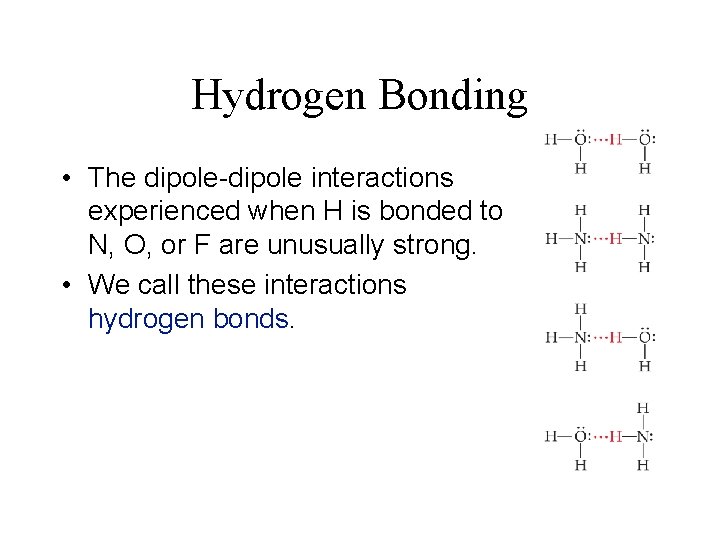
Hydrogen Bonding • The dipole-dipole interactions experienced when H is bonded to N, O, or F are unusually strong. • We call these interactions hydrogen bonds.

Hydrogen Bonding Hydrogen bonding arises from the high electronegativity of nitrogen, oxygen, and fluorine and the small size of the hydrogen atom. When hydrogen is bonded to one of these very electronegative elements, the hydrogen nucleus is exposed. The charge density becomes very high.

Intermolecular Forces Affect Many Physical Properties

Viscosity • Resistance of a liquid to flow is called viscosity. • It is related to the ease with which molecules can move past each other.

Surface Tension Surface tension results from the net inward force experienced by the molecules on the surface of a liquid.

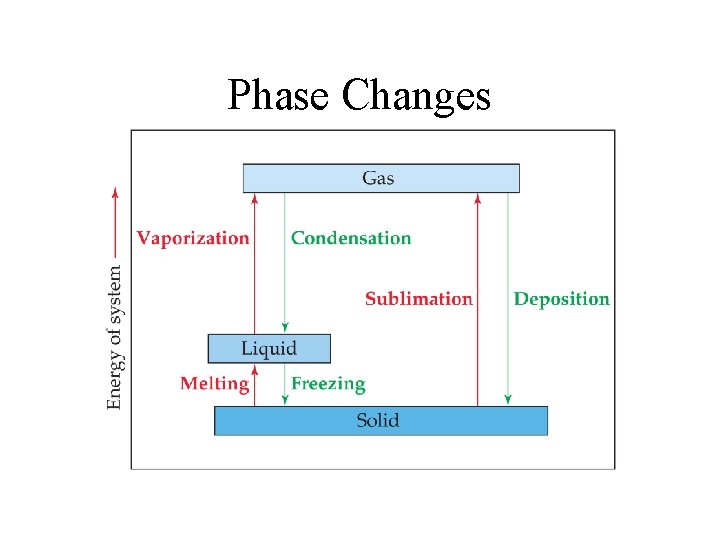
Phase Changes

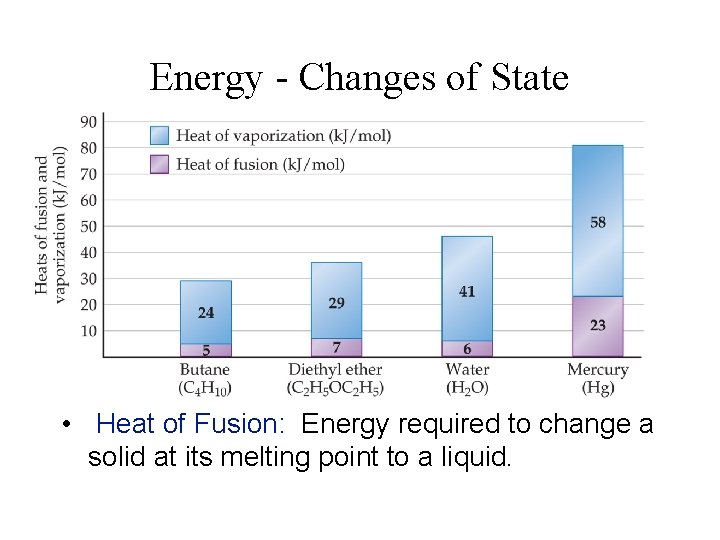
Energy - Changes of State • Heat of Fusion: Energy required to change a solid at its melting point to a liquid.

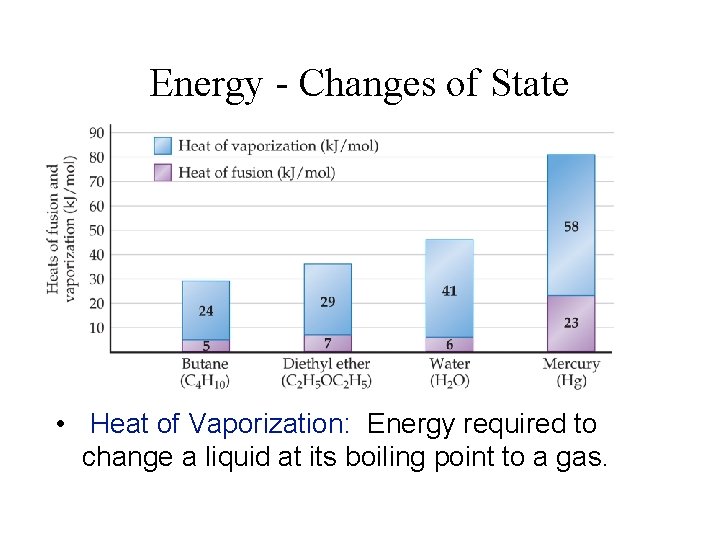
Energy - Changes of State • Heat of Vaporization: Energy required to change a liquid at its boiling point to a gas.

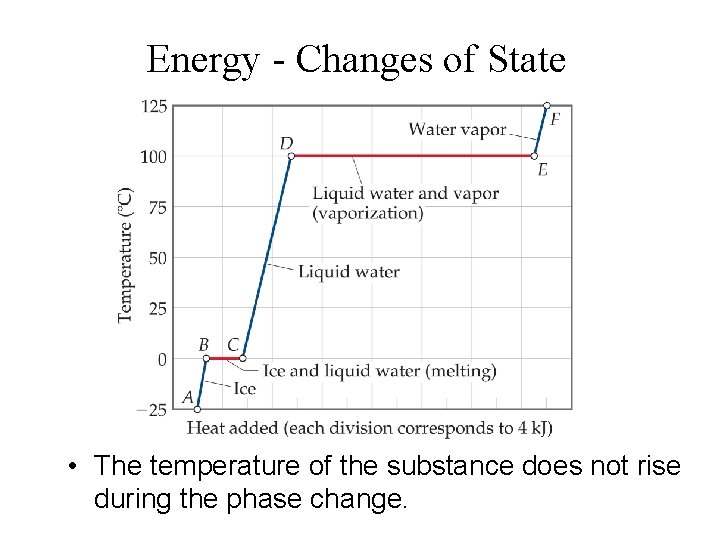
Energy - Changes of State • The temperature of the substance does not rise during the phase change.

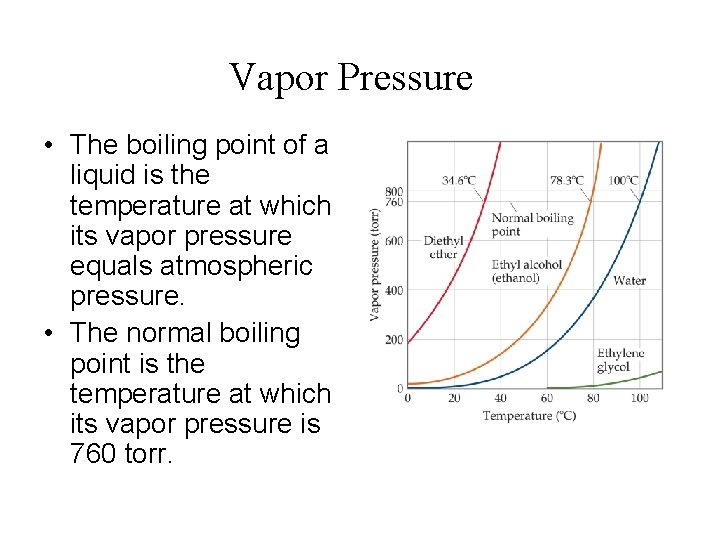
Vapor Pressure • Some molecules in a liquid or solid have enough energy to escape. • Temperature rises, “escapies” increase.

Vapor Pressure • The boiling point of a liquid is the temperature at which its vapor pressure equals atmospheric pressure. • The normal boiling point is the temperature at which its vapor pressure is 760 torr.

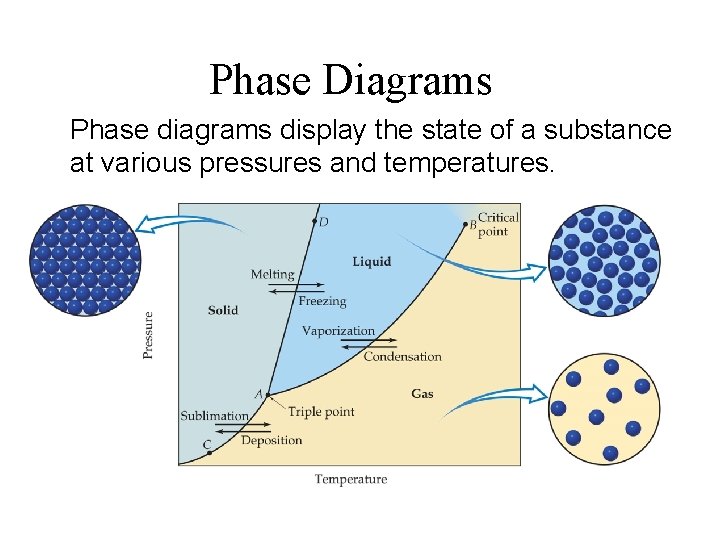
Phase Diagrams Phase diagrams display the state of a substance at various pressures and temperatures.

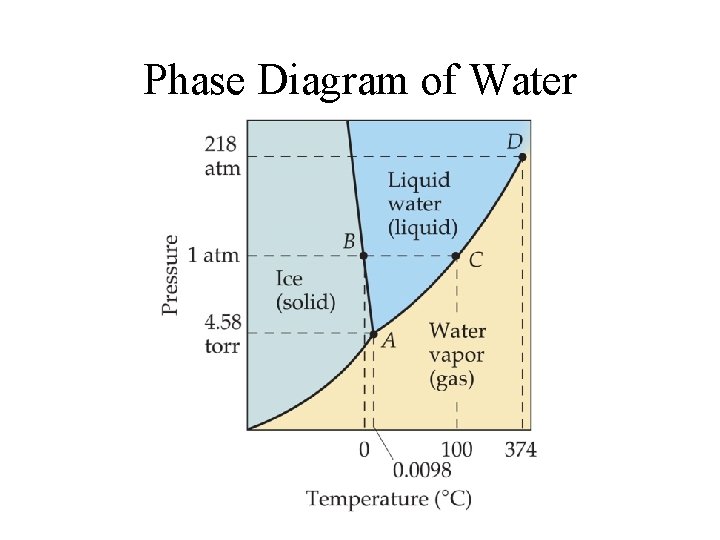
Phase Diagram of Water

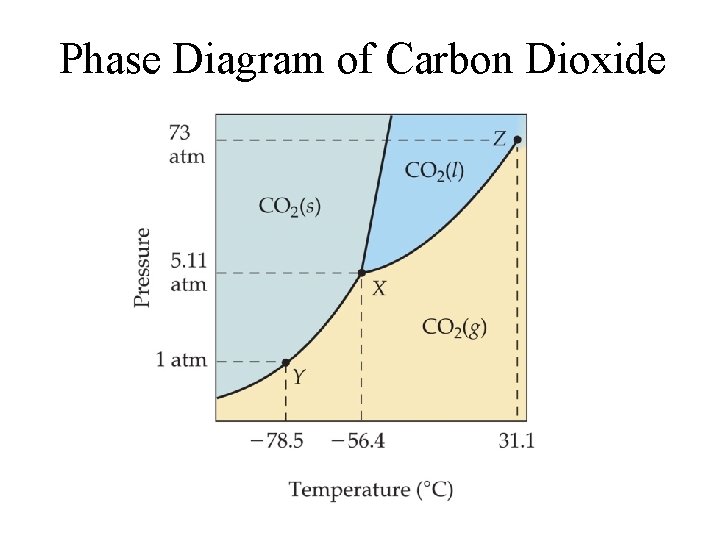
Phase Diagram of Carbon Dioxide

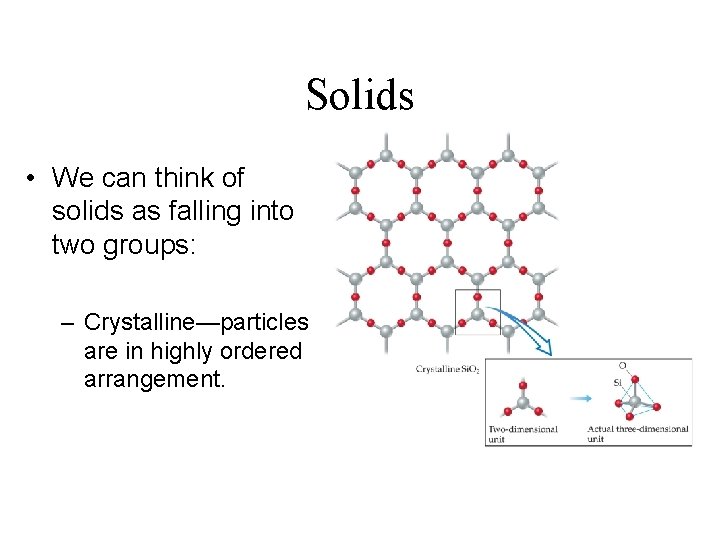
Solids • We can think of solids as falling into two groups: – Crystalline—particles are in highly ordered arrangement.

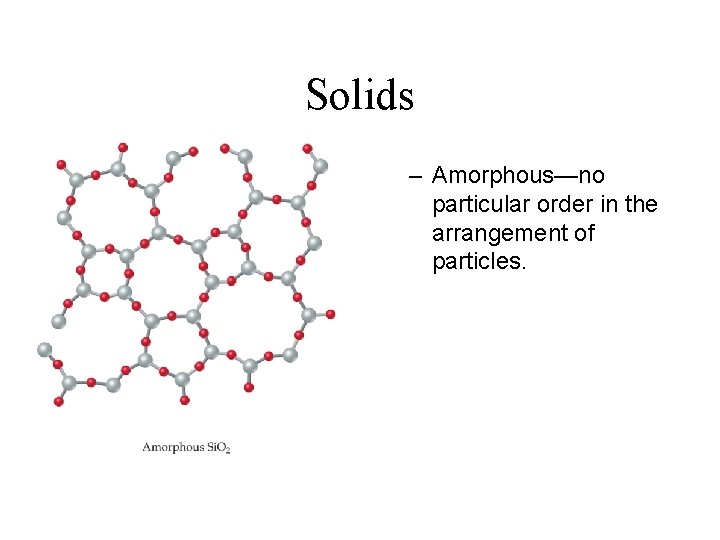
Solids – Amorphous—no particular order in the arrangement of particles.

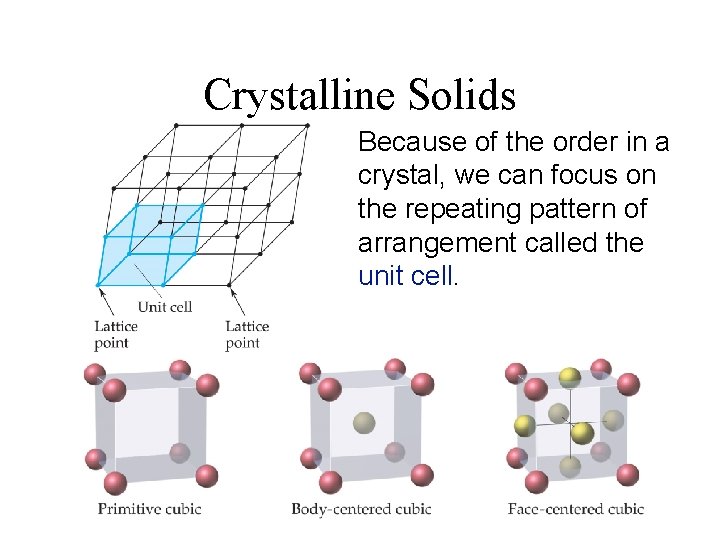
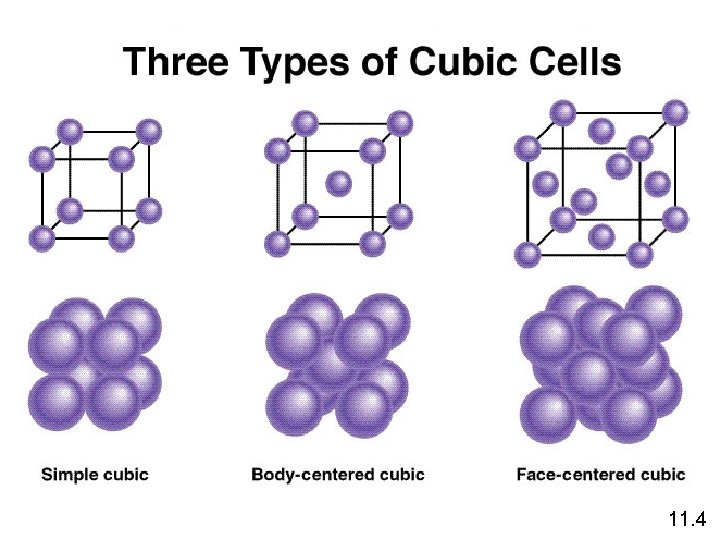
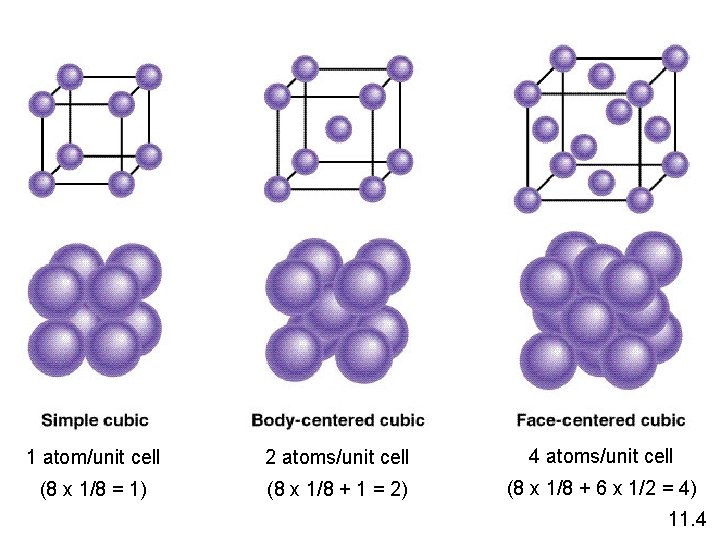
Crystalline Solids Because of the order in a crystal, we can focus on the repeating pattern of arrangement called the unit cell.

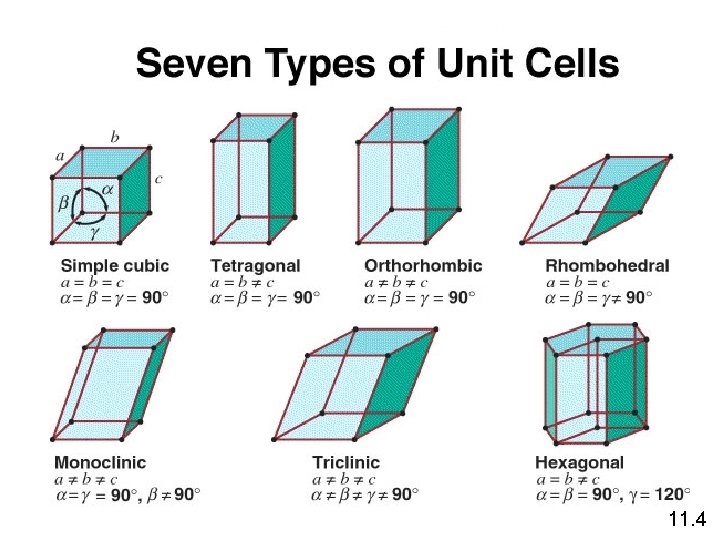
11. 4

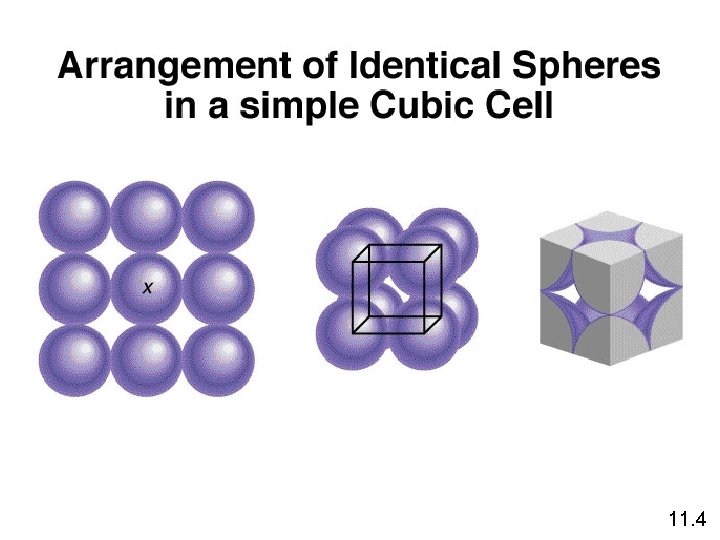
11. 4

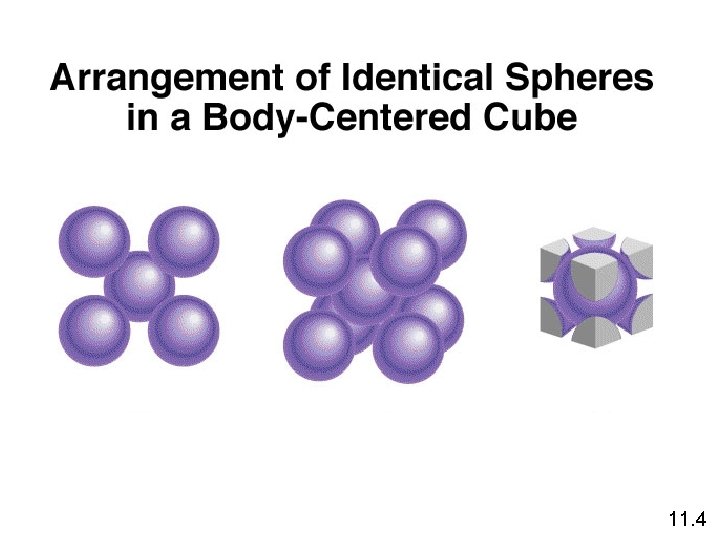
11. 4

11. 4

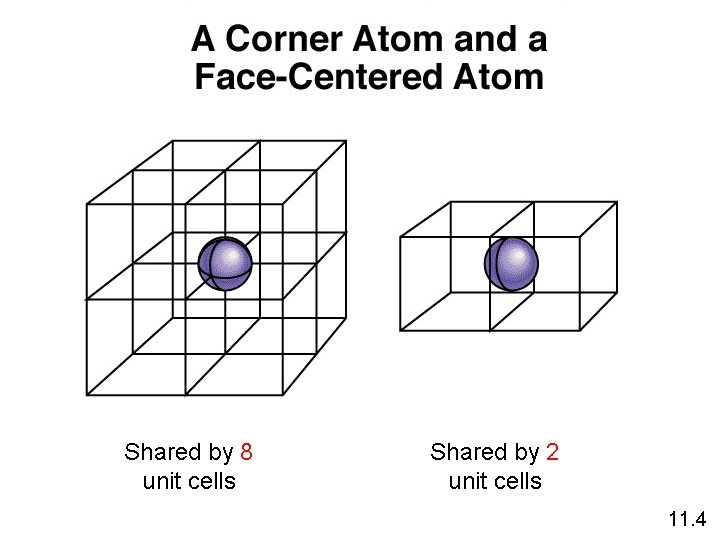
Shared by 8 unit cells Shared by 2 unit cells 11. 4

1 atom/unit cell 2 atoms/unit cell 4 atoms/unit cell (8 x 1/8 = 1) (8 x 1/8 + 1 = 2) (8 x 1/8 + 6 x 1/2 = 4) 11. 4

11. 4

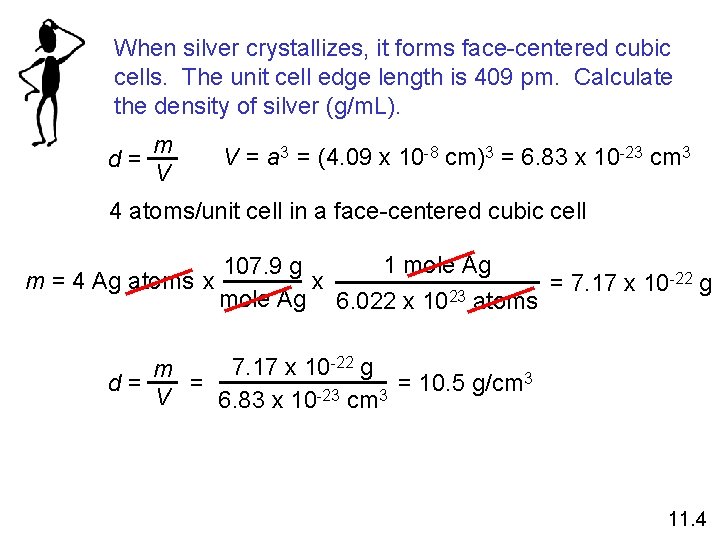
When silver crystallizes, it forms face-centered cubic cells. The unit cell edge length is 409 pm. Calculate the density of silver (g/m. L). d= m V V = a 3 = (4. 09 x 10 -8 cm)3 = 6. 83 x 10 -23 cm 3 4 atoms/unit cell in a face-centered cubic cell 1 mole Ag 107. 9 g -22 g x m = 4 Ag atoms x = 7. 17 x 10 mole Ag 6. 022 x 1023 atoms 7. 17 x 10 -22 g m 3 = = 10. 5 g/cm d= V 6. 83 x 10 -23 cm 3 11. 4

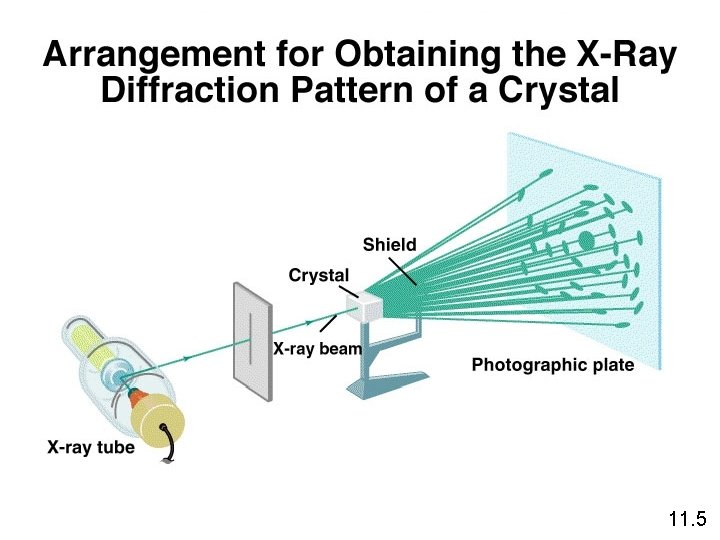
11. 5

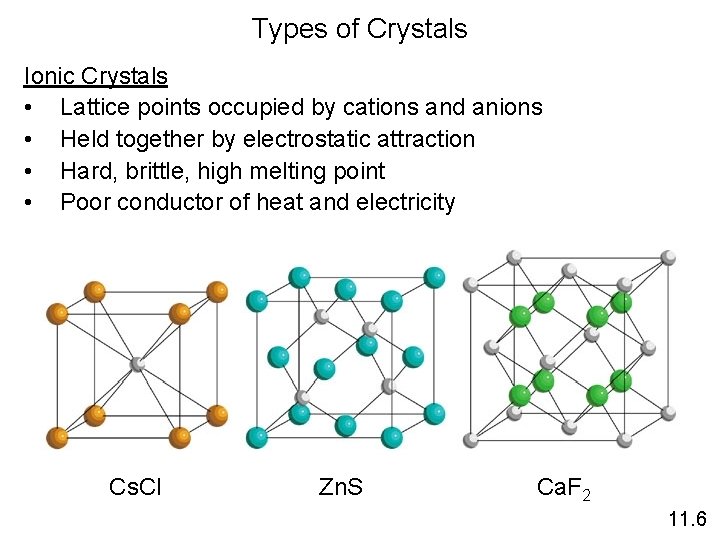
Types of Crystals Ionic Crystals • Lattice points occupied by cations and anions • Held together by electrostatic attraction • Hard, brittle, high melting point • Poor conductor of heat and electricity Cs. Cl Zn. S Ca. F 2 11. 6

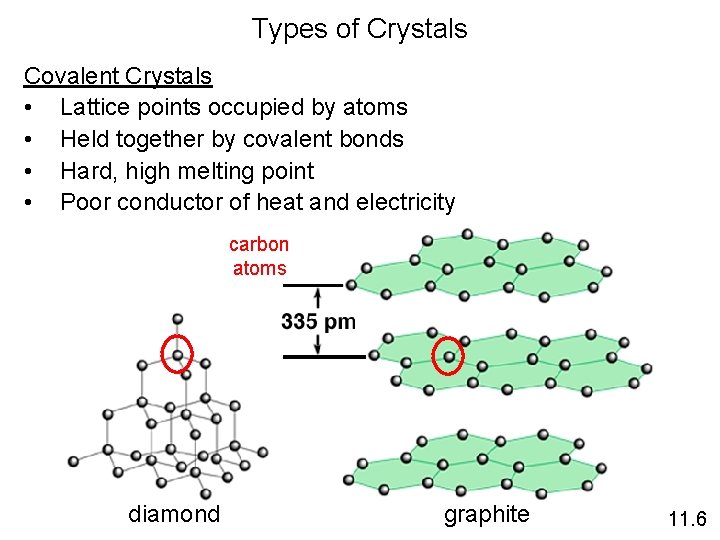
Types of Crystals Covalent Crystals • Lattice points occupied by atoms • Held together by covalent bonds • Hard, high melting point • Poor conductor of heat and electricity carbon atoms diamond graphite 11. 6

Types of Crystals Molecular Crystals • Lattice points occupied by molecules • Held together by intermolecular forces • Soft, low melting point • Poor conductor of heat and electricity 11. 6

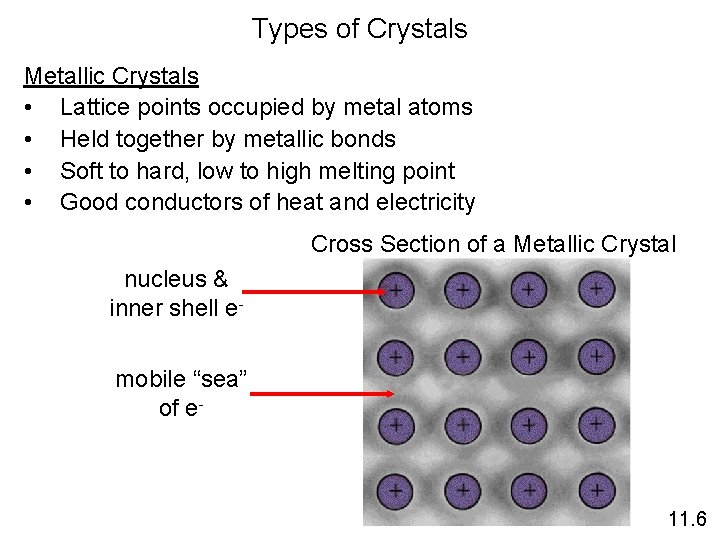
Types of Crystals Metallic Crystals • Lattice points occupied by metal atoms • Held together by metallic bonds • Soft to hard, low to high melting point • Good conductors of heat and electricity Cross Section of a Metallic Crystal nucleus & inner shell emobile “sea” of e- 11. 6