Intermolecular Forces Intermolecular Forces Int RAmolecular Forces Intramolecular







- Slides: 16

Intermolecular Forces, Intermolecular Forces

Int. RAmolecular Forces • Intramolecular Forces (as you recall) were the forces between the atoms IN a molecular, or the bond strength. • Remember – Ionic Bonds have high melting points, Covalent bonds have low melting points etc. Intermolecular Forces

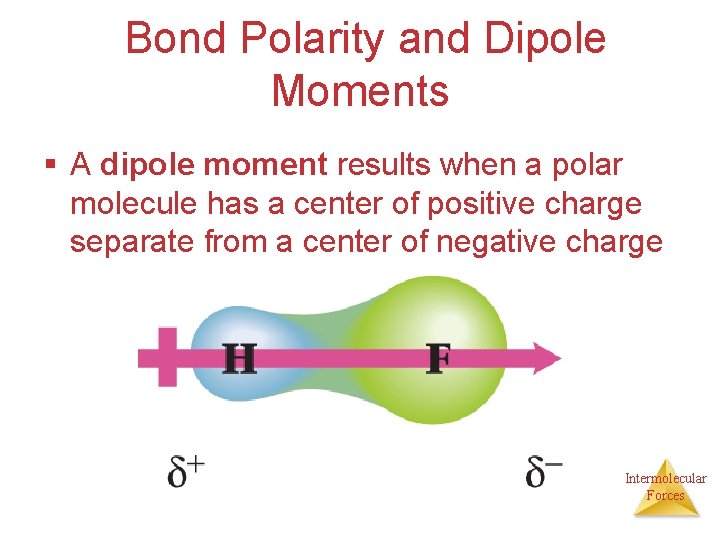
Bond Polarity and Dipole Moments § A dipole moment results when a polar molecule has a center of positive charge separate from a center of negative charge Intermolecular Forces

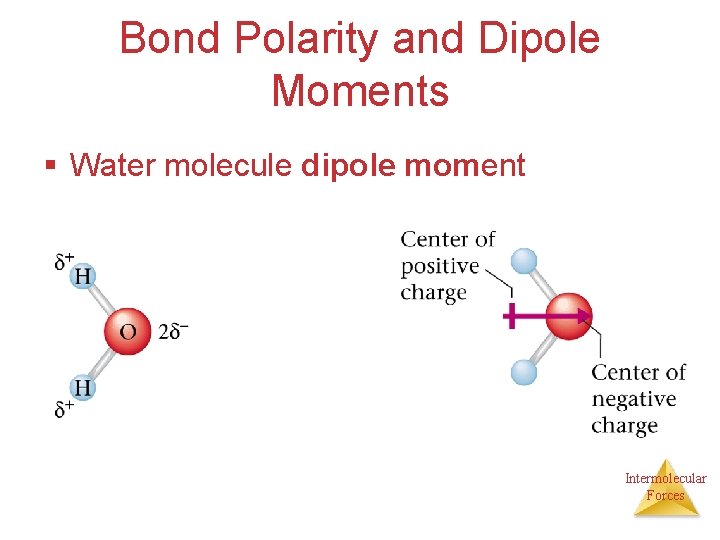
Bond Polarity and Dipole Moments § Water molecule dipole moment Intermolecular Forces

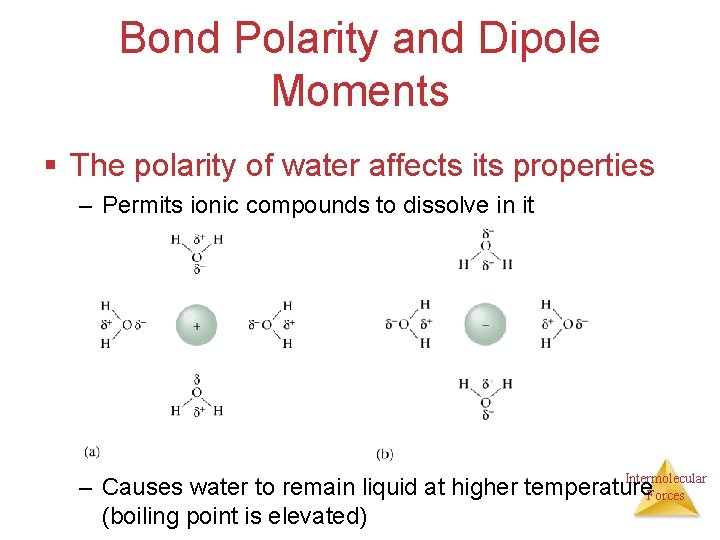
Bond Polarity and Dipole Moments § The polarity of water affects its properties – Permits ionic compounds to dissolve in it Intermolecular – Causes water to remain liquid at higher temperature Forces (boiling point is elevated)

“Like Dissolves Like” • Polar compounds dissolve in polar liquids • Non-polar compounds dissolve in nonpolar liquids Like Dissolves Like Video Intermolecular Forces

Intermolecular Forces • Glue that holds matter together • Melting and boiling points measure the relative strength • Ionic forces – strongest ØFound in salts ØNa. Cl melts at 800°C Chapter 5 Intermolecular Forces 7

Intermolecular Forces § Dipole- Dipole (strongest) § Hydrogen Bonding § London Dispersion Chapter 6 Intermolecular Forces 8

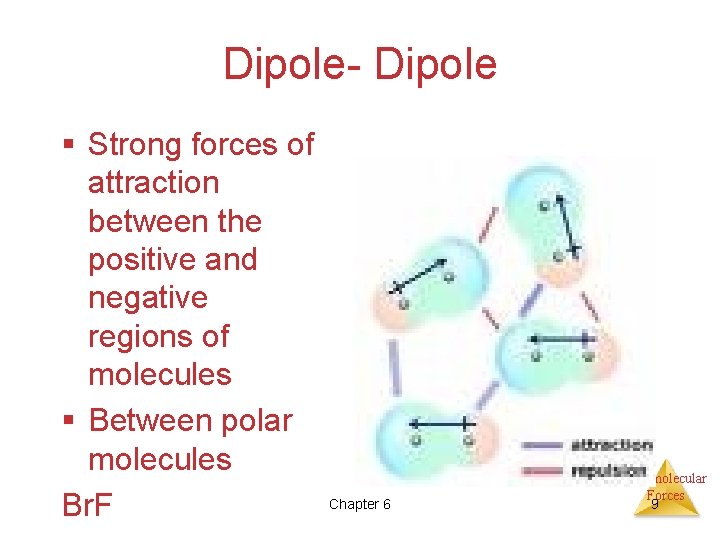
Dipole- Dipole § Strong forces of attraction between the positive and negative regions of molecules § Between polar molecules Br. F Chapter 6 Intermolecular Forces 9

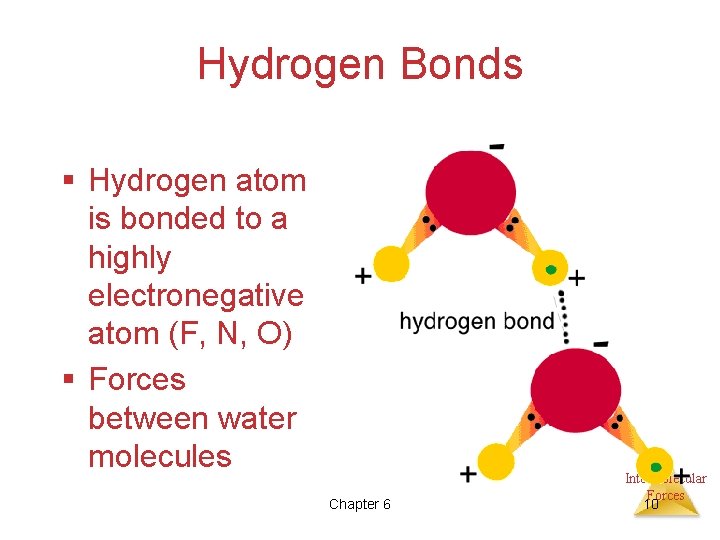
Hydrogen Bonds § Hydrogen atom is bonded to a highly electronegative atom (F, N, O) § Forces between water molecules Chapter 6 Intermolecular Forces 10

London dispersion forces § Weak intermolecular forces § Instantaneous and induced dipoles § Caused by electrons moving around in atom § Important with non-polar molecules Chapter 6 Intermolecular Forces 11

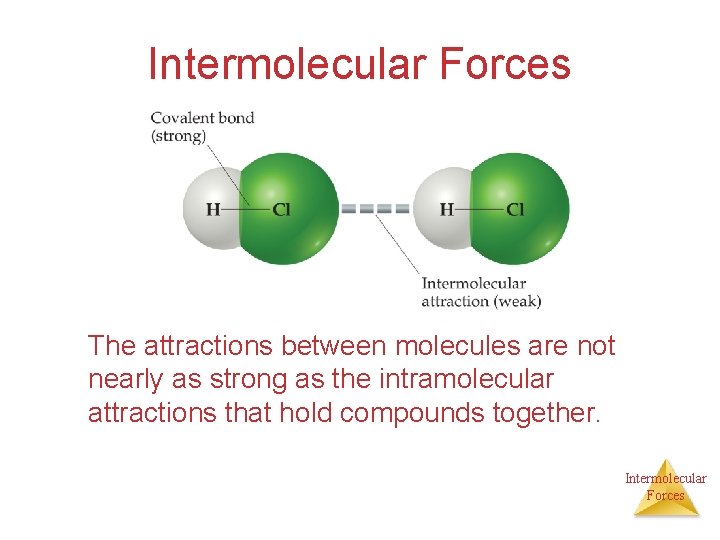
Intermolecular Forces The attractions between molecules are not nearly as strong as the intramolecular attractions that hold compounds together. Intermolecular Forces

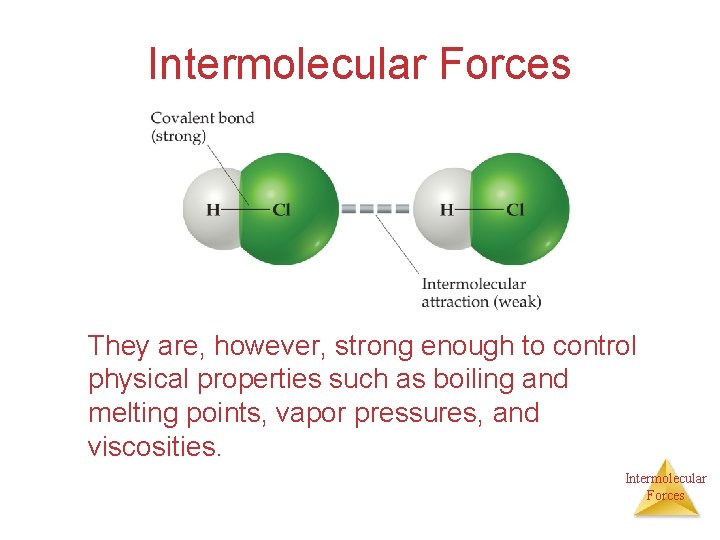
Intermolecular Forces They are, however, strong enough to control physical properties such as boiling and melting points, vapor pressures, and viscosities. Intermolecular Forces

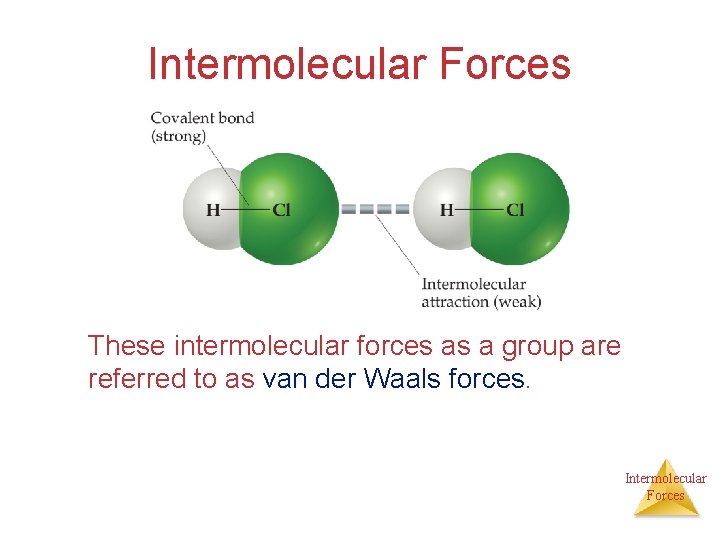
Intermolecular Forces These intermolecular forces as a group are referred to as van der Waals forces. Intermolecular Forces

van der Waals Forces • Dipole-dipole interactions • Hydrogen bonding • London dispersion forces Intermolecular Forces

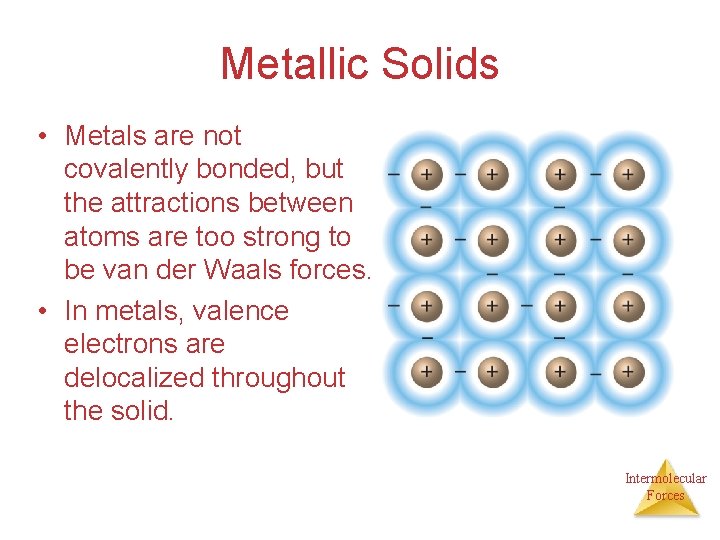
Metallic Solids • Metals are not covalently bonded, but the attractions between atoms are too strong to be van der Waals forces. • In metals, valence electrons are delocalized throughout the solid. Intermolecular Forces