1 List the intermolecular and intramolecular forces Why














- Slides: 21

1 List the intermolecular and intramolecular forces. Why is the term intramolecular sometimes a misnomer? [Hint: it has to do with the ‘molecular’ part of the word] Identify intramolecular and intermolecular forces for each of the following. The difference in electronegativity for Hydrogen and Ge/Si/Sn/C are all less than 0. 5 (quite small). Predict the order in which they would melt. Ge. H 4 Si. H 4 Sn. H 4 CH 4

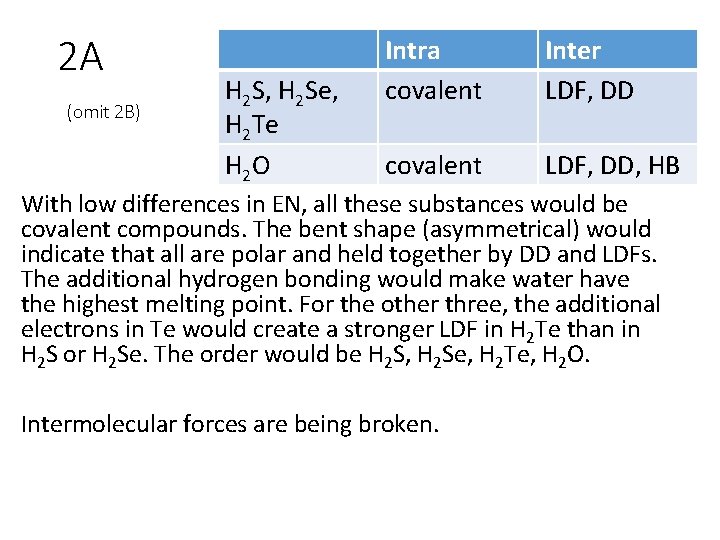
2 A. Identify intramolecular and intermolecular forces for each of the following. The electronegativities of Se, S, and Te are all within 0. 5 of each other which is quite small. Predict the order in which they would melt. When these substances melt, are intermolecular or intramolecular forces being broken? H 2 Te H 2 S H 2 O H 2 Se B. If a mixture contained all four of these substances in the liquid phase, which would be the first to distill? Which would be the last? How does this list compare with your list in part A? Why?

3 Does the following diagram best describe a crystalline solid, liquid, or gas? Explain.

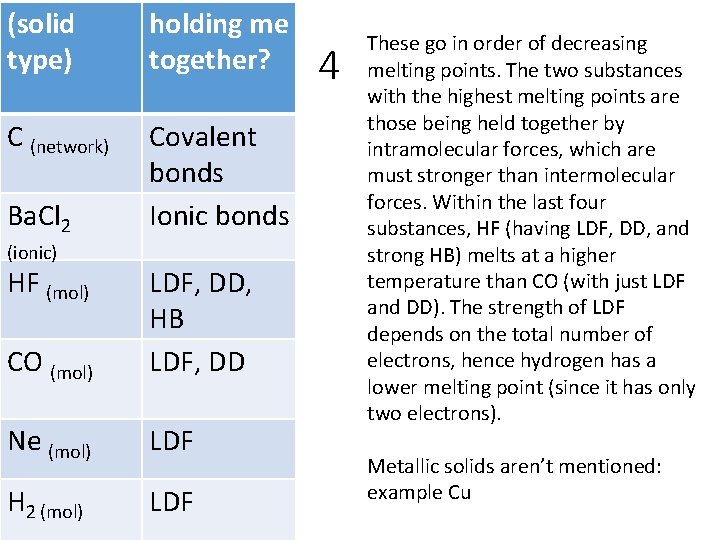
4 The following are in order; do they go in order of increasing or decreasing melting points? Use intramolecular and intermolecular forces to thoroughly justify the order. C, Ba. Cl 2, HF, CO, Ne, H 2 What type of solid would each substance form? Are any solid types not represented? If so, list them and give an example.

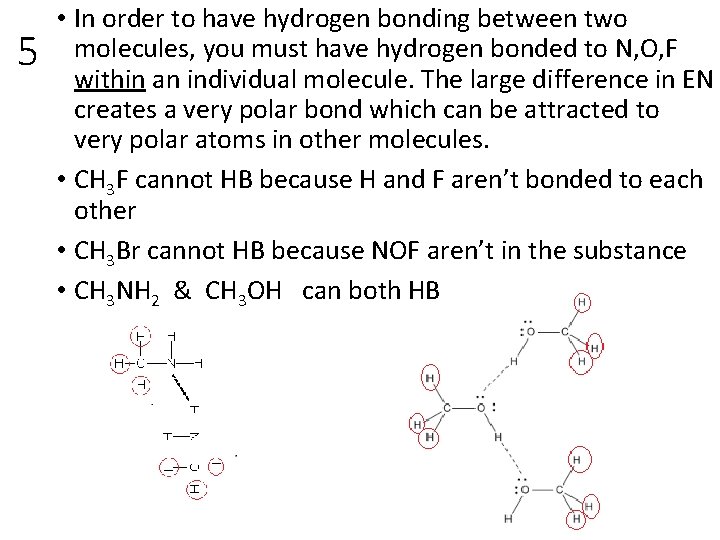
5 Which of the following molecules can form hydrogen bonds with other molecules of the same kind? Draw two molecules for each selection and indicate Hydrogen bonding. Circle covalently-bonded hydrogens that cannot H-bond. CH 3 F CH 3 NH 2 CH 3 OH CH 3 Br

6 As the intermolecular attractive forces between molecules increase in magnitude, do you expect each of the following to increase or decrease in magnitude? boiling point, melting point, viscosity, surface tension, adhesion, cohesion, vapor pressure

7 What kinds of attractive forces exist between particles in molecular solids, network solids, ionic solids, and metallic solids (discuss electrons)? A white substance melts with some decomposition at 730°C. As a solid, it is a nonconductor of electricity, but it dissolves in water to form a conducting solution. Which type of solid might the substance be? How do you know?

8 The state of aggregation of solids can be described as belonging to the following four types: (1) ionic (3) network (2) metallic (4) molecular For each of these types of solids, sketch a diagram of the solid and indicate the kinds of particles that occupy the lattice points and identify forces among these particles. How could each type of solid be identified in the laboratory?

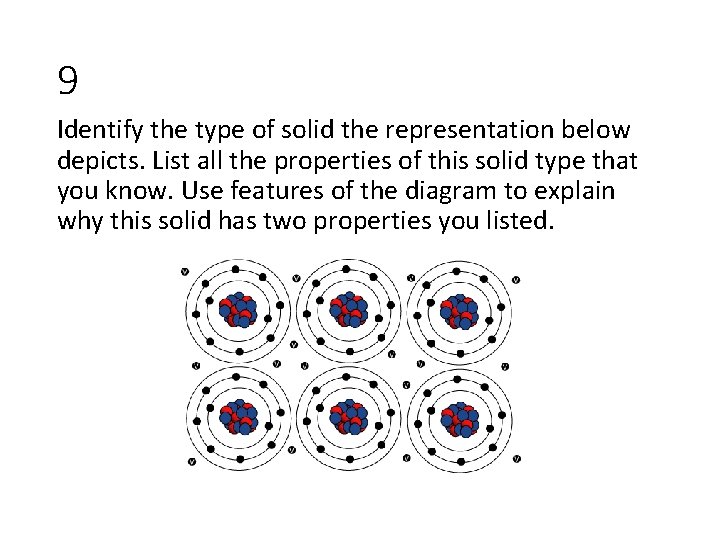
9 Identify the type of solid the representation below depicts. List all the properties of this solid type that you know. Use features of the diagram to explain why this solid has two properties you listed.

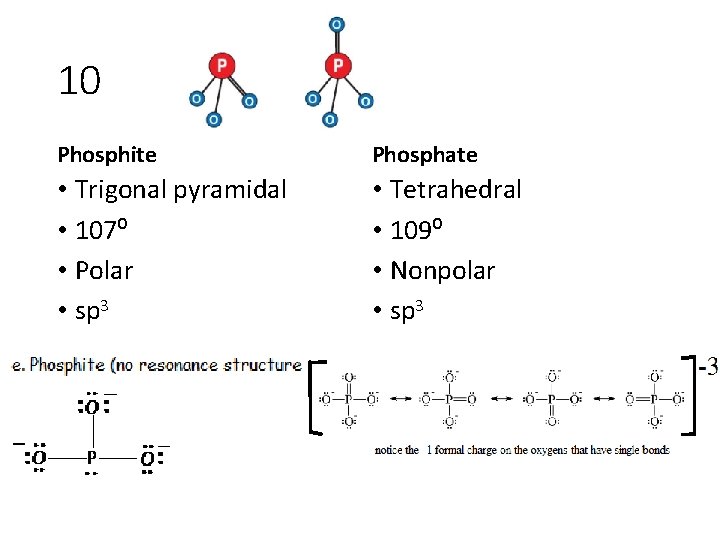
10 For the following: draw the lewis structure (with formal charges), name the shape according to modern bonding theory, identify angles, and indicate polarity and hybridization. Explain similarities and differences: phosphite and phosphate

Answers

1 • Inter = LDF, DD, HB • Intra = cov, ionic, metallic • With low differences in EN, all these substances would be covalent compounds. The tetrahedral shape (symmetrical) would indicate that all are nonpolar and held together by LDFs. The order would be CH 4, Si. H 4, Ge. H 4, Sn. H 4, with CH 4 melting first because it has the fewest electrons and the weakest LDFs. Less energy is required to break weaker LDFs.

2 A (omit 2 B) H 2 S, H 2 Se, H 2 Te H 2 O Intra covalent Inter LDF, DD covalent LDF, DD, HB With low differences in EN, all these substances would be covalent compounds. The bent shape (asymmetrical) would indicate that all are polar and held together by DD and LDFs. The additional hydrogen bonding would make water have the highest melting point. For the other three, the additional electrons in Te would create a stronger LDF in H 2 Te than in H 2 S or H 2 Se. The order would be H 2 S, H 2 Se, H 2 Te, H 2 O. Intermolecular forces are being broken.

3 Although a claim could be made for this representation to be a gas, it seems that the molecules are close enough to be a liquid. Their unorganized structure would not indicate a solid.

(solid type) holding me together? C (network) Covalent Decreasing bonds Ba. Cl 2 Ionic bonds (ionic) HF (mol) CO (mol) LDF, DD, HB LDF, DD Ne (mol) LDF H 2 (mol) LDF 4 These go in order of decreasing melting points. The two substances with the highest melting points are those being held together by intramolecular forces, which are must stronger than intermolecular forces. Within the last four substances, HF (having LDF, DD, and strong HB) melts at a higher temperature than CO (with just LDF and DD). The strength of LDF depends on the total number of electrons, hence hydrogen has a lower melting point (since it has only two electrons). Metallic solids aren’t mentioned: example Cu

5 • In order to have hydrogen bonding between two molecules, you must have hydrogen bonded to N, O, F within an individual molecule. The large difference in EN creates a very polar bond which can be attracted to very polar atoms in other molecules. • CH 3 F cannot HB because H and F aren’t bonded to each other • CH 3 Br cannot HB because NOF aren’t in the substance • CH 3 NH 2 & CH 3 OH can both HB

6 • Surface tension – when a liquid resists a change in shape – allows some little animals to ‘walk on water’ • Adhesion – a substance sticking to the container walls • Cohesion – a substance sticking to itself Stronger intermolecular forces would cause an increase in: boiling point, melting point, viscosity, surface tension, adhesion & cohesion Stronger intermolecular forces would cause a decrease in: vapor pressure (the more the molecules stick to each other, the fewer molecules that escape into the gas phase, the lower the VP)

7 • Molecular solids have intermolecular forces • Network solids have covalent bonds (intra; sharing electrons) • Ionic solids have electrostatic attractions (intra; transfer of e) • Metallic solids have nondirectional sharing of e (intra; sea of e) The solid is most likely ionic – the only type of substance that is a nonconductor in the solid state but conducts electricity in the liquid state or when dissolved in water

8 Ionic – lattice points occupies by ions Lab: Doesn’t conduct in solid state but does conduct when dissolved in water Molecular – lattice points occupies by nonmetals/covalent compounds Lab: soft and brittle in the solid state; hit it and it should shatter Metallic– lattice points occupies by metal atoms Lab: conducts electricity; could also try to bend it and it shouldn’t break (it’s malleable) Network – lattice points occupies by C, Si, Si. C, Si. O 2 Lab: crazy hard, won’t be able to break it

9 • Metal • Malleable/ductile • Conducts heat and electricity • Low vapor pressure • Good conductor of heat/electricity • Electrons are delocalized and relatively free to move (they can carry the current and absorb the kinetic energy) • Malleable/Ductile • Deforming the solid doesn’t change the environment immediately surrounding each metal core

10 Phosphite Phosphate • Trigonal pyramidal • 107⁰ • Polar • sp 3 • Tetrahedral • 109⁰ • Nonpolar • sp 3