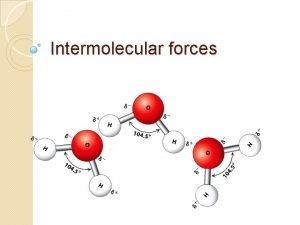
Intermolecular Forces 1 States of Matter What do





- Slides: 30

Intermolecular Forces 1

States of Matter • What do you remember about gases? No attractive or repulsive forces • Not all solids and liquids are equal: Viscosity, conductivity, surface tension, volume, density, melting point, boiling point, hardness • Variables: Size and shape of the molecules/atoms The intra- and intermolecular forces 2

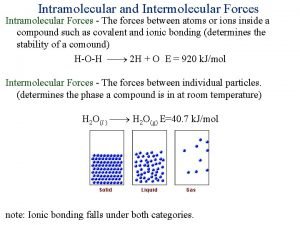
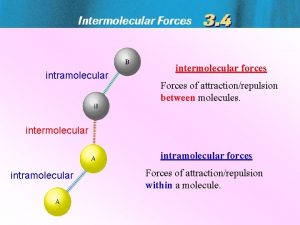
Intramoelcular vs Intermolecular • Intramolecular forces – exist within each molecule – influence the chemical properties of the substance – ionic, covalent, and metallic bonds • Intermolecular forces – exist between the molecules – influence the physical properties of the substance – Responsible for the state of the system: gas, liquid, solid 3

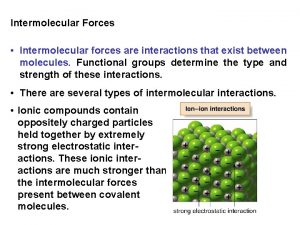
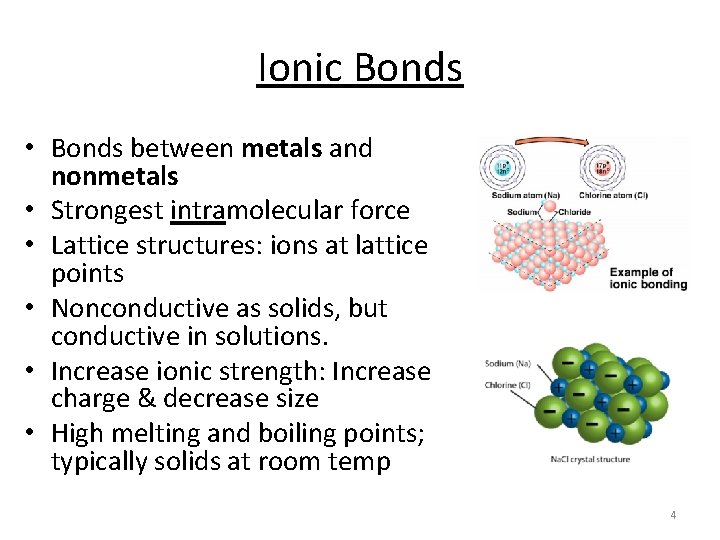
Ionic Bonds • Bonds between metals and nonmetals • Strongest intramolecular force • Lattice structures: ions at lattice points • Nonconductive as solids, but conductive in solutions. • Increase ionic strength: Increase charge & decrease size • High melting and boiling points; typically solids at room temp 4

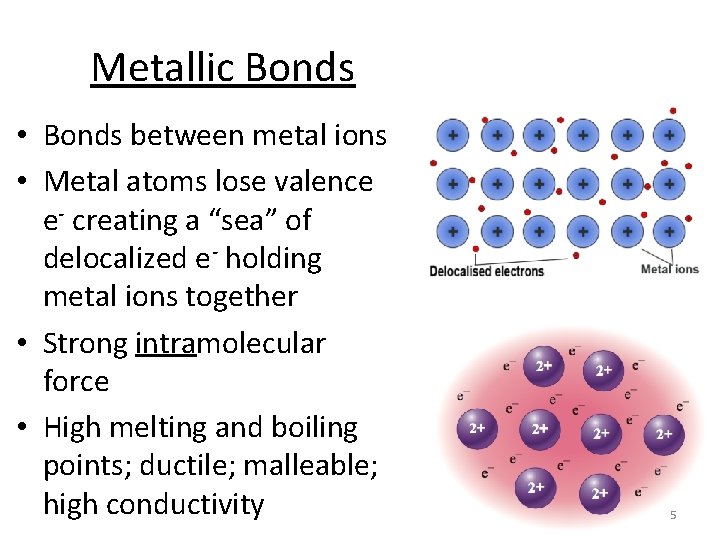
Metallic Bonds • Bonds between metal ions • Metal atoms lose valence e- creating a “sea” of delocalized e- holding metal ions together • Strong intramolecular force • High melting and boiling points; ductile; malleable; high conductivity 5

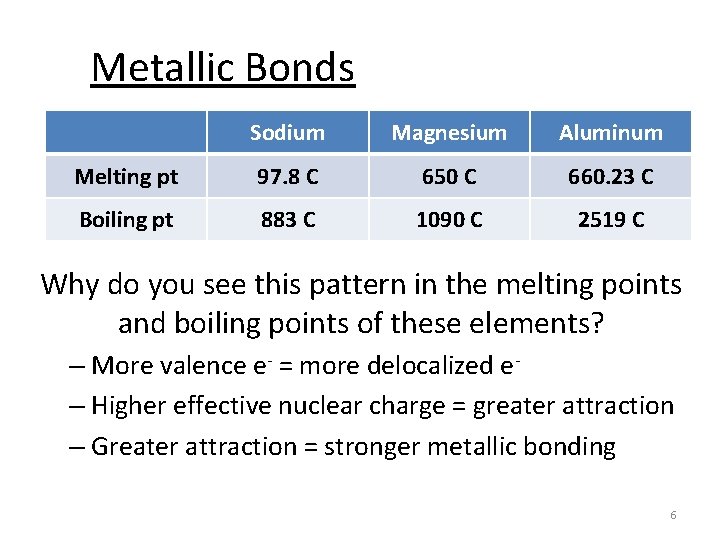
Metallic Bonds Sodium Magnesium Aluminum Melting pt 97. 8 C 650 C 660. 23 C Boiling pt 883 C 1090 C 2519 C Why do you see this pattern in the melting points and boiling points of these elements? – More valence e- = more delocalized e– Higher effective nuclear charge = greater attraction – Greater attraction = stronger metallic bonding 6

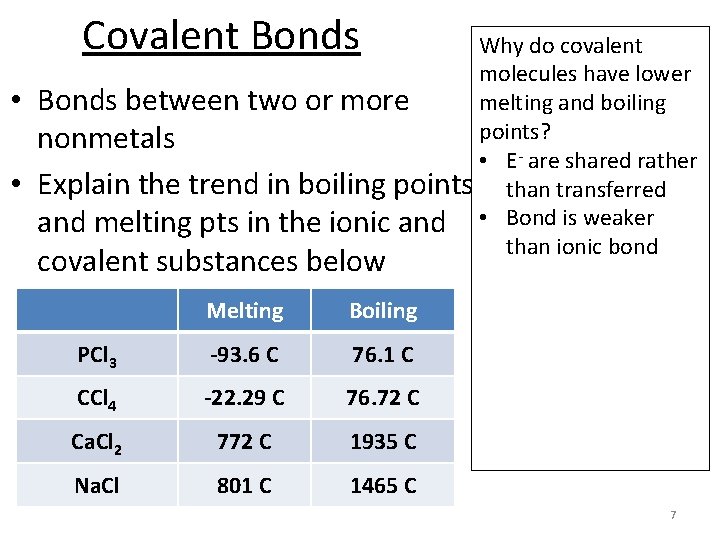
Covalent Bonds • • Why do covalent molecules have lower Bonds between two or more melting and boiling points? nonmetals • E- are shared rather Explain the trend in boiling points than transferred and melting pts in the ionic and • Bond is weaker than ionic bond covalent substances below Melting Boiling PCl 3 -93. 6 C 76. 1 C CCl 4 -22. 29 C 76. 72 C Ca. Cl 2 772 C 1935 C Na. Cl 801 C 1465 C 7

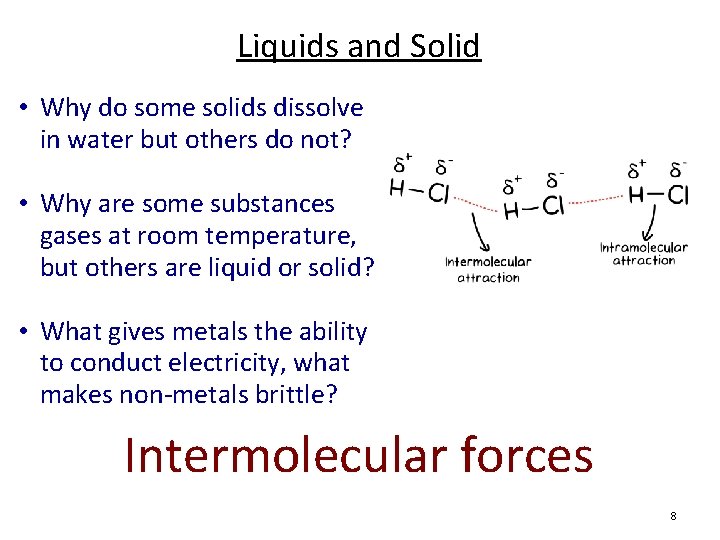
Liquids and Solid • Why do some solids dissolve in water but others do not? • Why are some substances gases at room temperature, but others are liquid or solid? • What gives metals the ability to conduct electricity, what makes non-metals brittle? Intermolecular forces 8

Intermolecular Forces 1) Hydrogen Bonding 2) Dipole-Dipole 3) London dispersion forces 9

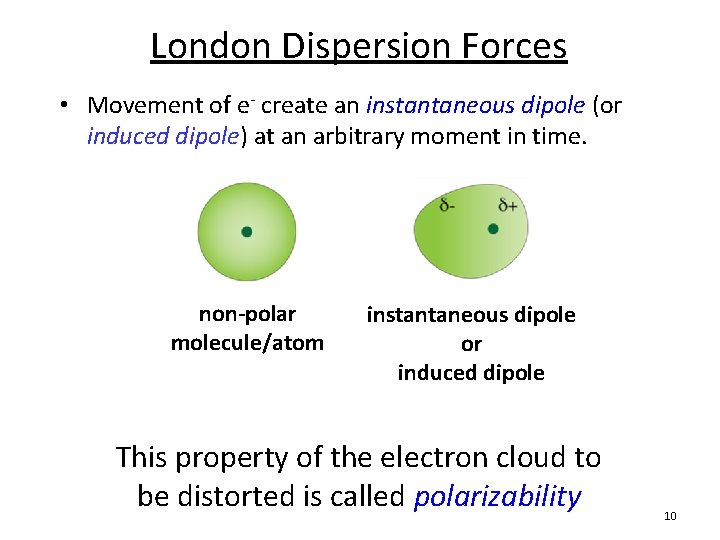
London Dispersion Forces • Movement of e- create an instantaneous dipole (or induced dipole) at an arbitrary moment in time. non-polar molecule/atom instantaneous dipole or induced dipole This property of the electron cloud to be distorted is called polarizability 10

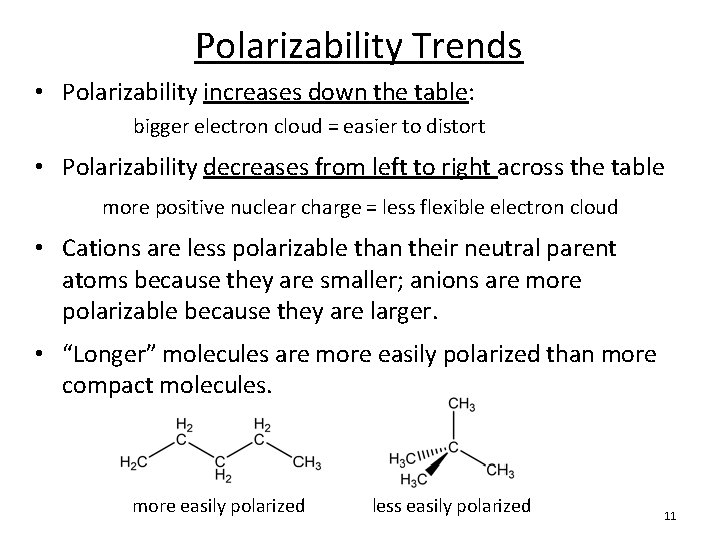
Polarizability Trends • Polarizability increases down the table: bigger electron cloud = easier to distort • Polarizability decreases from left to right across the table more positive nuclear charge = less flexible electron cloud • Cations are less polarizable than their neutral parent atoms because they are smaller; anions are more polarizable because they are larger. • “Longer” molecules are more easily polarized than more compact molecules. more easily polarized less easily polarized 11

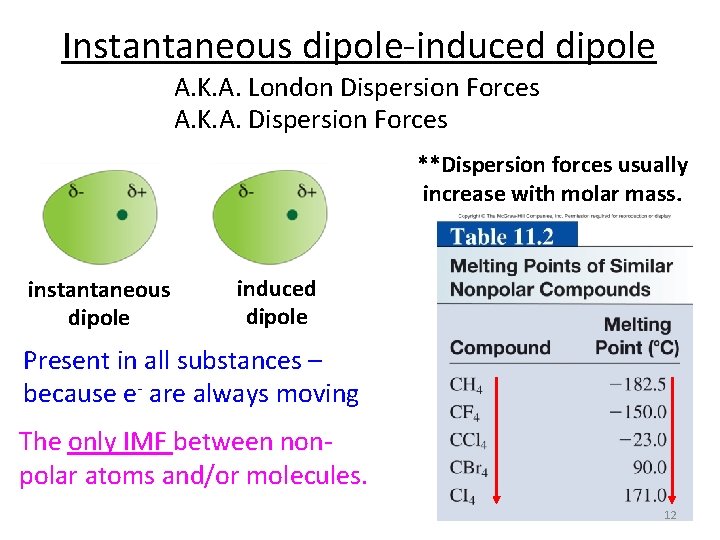
Instantaneous dipole-induced dipole A. K. A. London Dispersion Forces A. K. A. Dispersion Forces **Dispersion forces usually increase with molar mass. instantaneous dipole induced dipole Present in all substances – because e- are always moving The only IMF between nonpolar atoms and/or molecules. 12

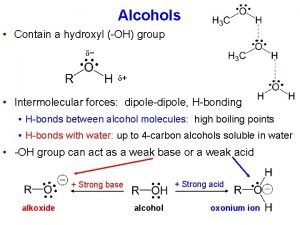
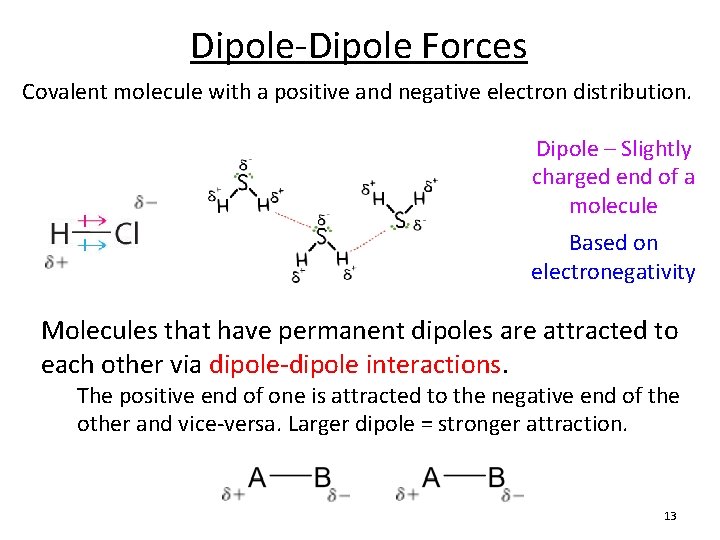
Dipole-Dipole Forces Covalent molecule with a positive and negative electron distribution. Dipole – Slightly charged end of a molecule Based on electronegativity Molecules that have permanent dipoles are attracted to each other via dipole-dipole interactions. The positive end of one is attracted to the negative end of the other and vice-versa. Larger dipole = stronger attraction. 13

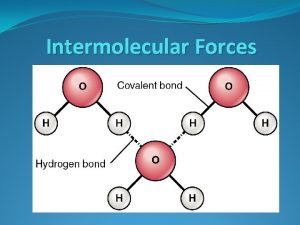
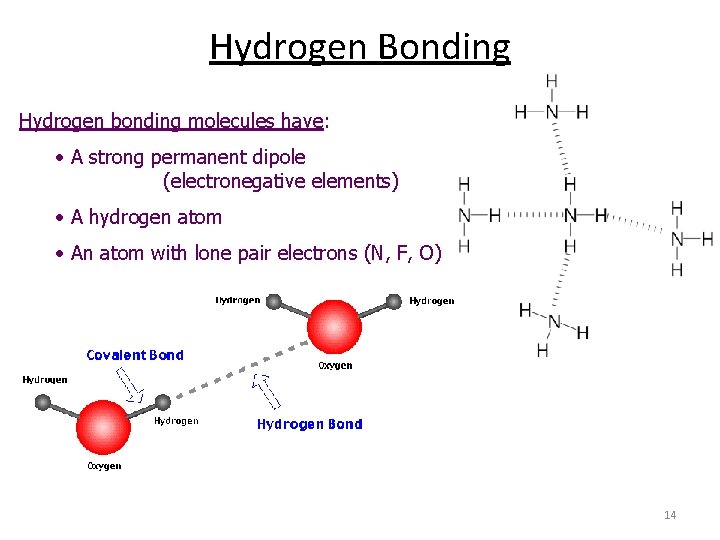
Hydrogen Bonding Hydrogen bonding molecules have: • A strong permanent dipole (electronegative elements) • A hydrogen atom • An atom with lone pair electrons (N, F, O) 14

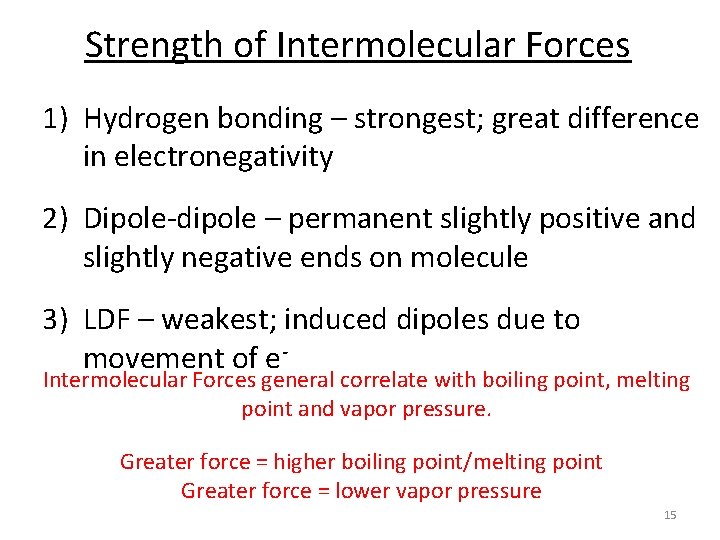
Strength of Intermolecular Forces 1) Hydrogen bonding – strongest; great difference in electronegativity 2) Dipole-dipole – permanent slightly positive and slightly negative ends on molecule 3) LDF – weakest; induced dipoles due to movement of e- Intermolecular Forces general correlate with boiling point, melting point and vapor pressure. Greater force = higher boiling point/melting point Greater force = lower vapor pressure 15

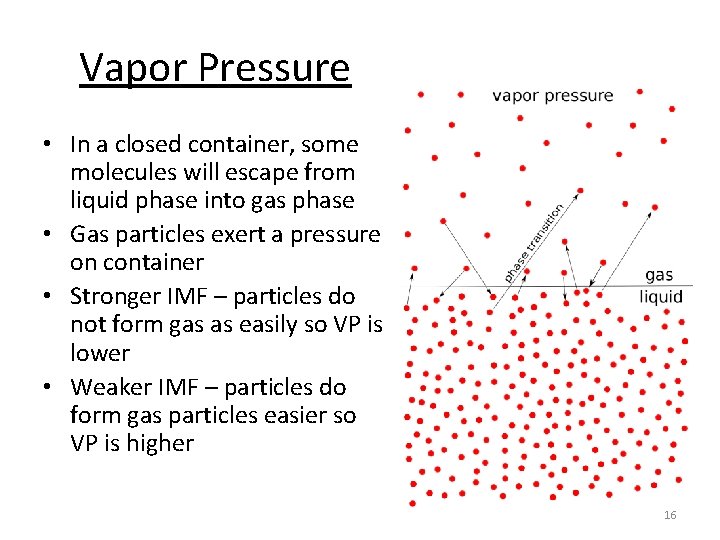
Vapor Pressure • In a closed container, some molecules will escape from liquid phase into gas phase • Gas particles exert a pressure on container • Stronger IMF – particles do not form gas as easily so VP is lower • Weaker IMF – particles do form gas particles easier so VP is higher 16

Strength of Intermolecular Forces 1. Arrange these substances in order of INCREASING boiling point: Cu, H 2 O, Li. Cl, H 2 S • H 2 – LDF forces • H 2 S – dipole-dipole • H 2 O – hydrogen bonding • Cu – metallic bonding • Li. Cl – ionic bonding 2. Which of the following is expected to have the highest normal boiling point? And why? A. C 2 H 6 B. C 3 H 8 C. C 5 H 12 D. C 4 H 10 E. CH 4 • C 5 H 12 – longest chain and highest molar mass 3. Which of the following is expected to have the highest normal boiling point? And why? A. HF B. HCl C. HBr D. HI E. HAt • HF – strongest IMF (Hydrogen bonding) 17

When in Doubt… • Ask yourself what kind of molecule (ionic, metallic, covalent) • If covalent, is it polar or nonpolar? • Draw the molecule, if needed! Homework – Pages 4 -5 18

Solubility and IMF Why do some substances dissolve in water while others don’t? 19

Follow-up to Lab – discuss with partner! • We tested the following chemicals in Part 2: – Cu. SO 4, NH 3, Na 2 CO 3, Ca. Cl 2, C 3 H 6 O and C 18 H 38 • Identify the dominant force holding each of these substances together. • You should have observed the polar substances and ionic substances dissolved in water. What could explain why those substances dissolved in water? Think about the forces of water as well! 20

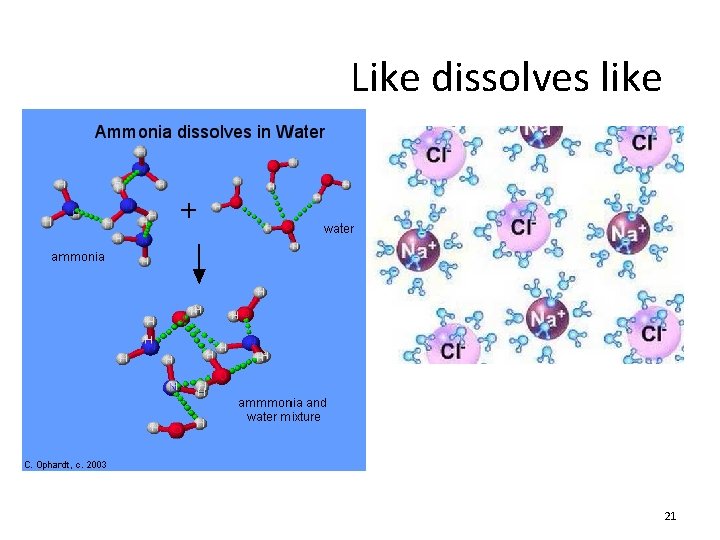
Like dissolves like 21

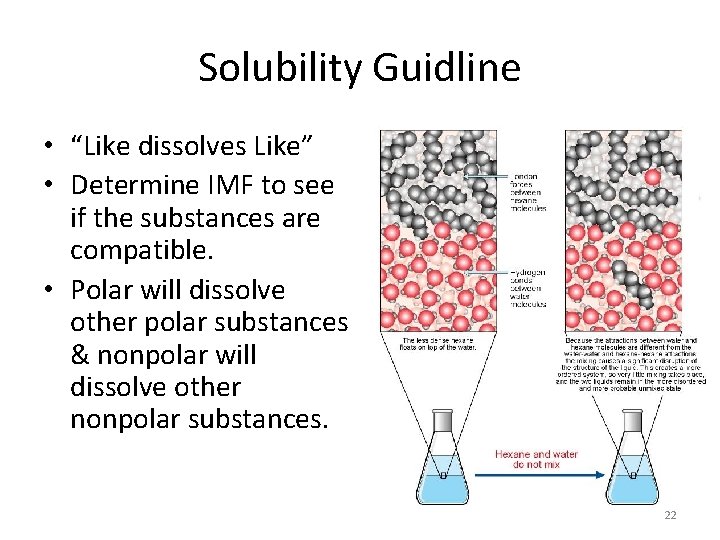
Solubility Guidline • “Like dissolves Like” • Determine IMF to see if the substances are compatible. • Polar will dissolve other polar substances & nonpolar will dissolve other nonpolar substances. 22

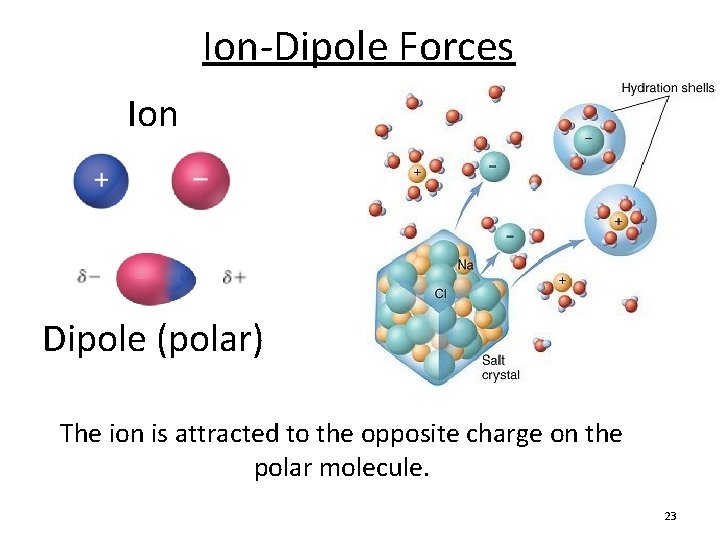
Ion-Dipole Forces Ion Dipole (polar) The ion is attracted to the opposite charge on the polar molecule. 23

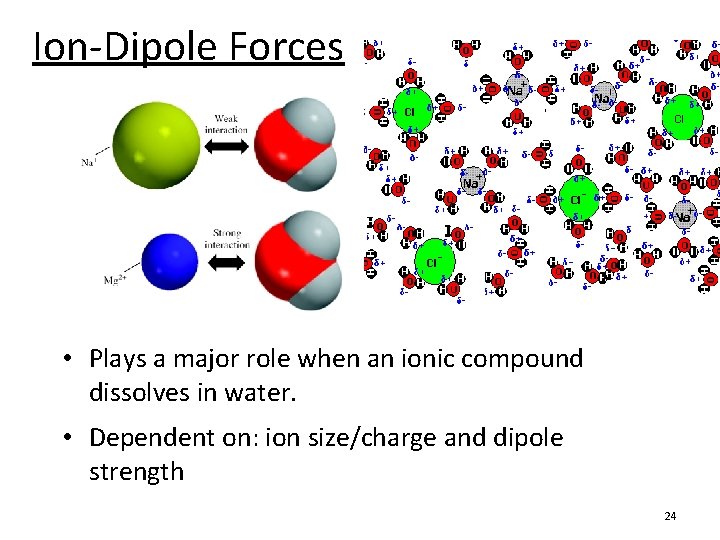
Ion-Dipole Forces • Plays a major role when an ionic compound dissolves in water. • Dependent on: ion size/charge and dipole strength 24

• Potassium chloride and iron (III) chloride are both soluble in water. Draw a particulate diagram showing how both of these dissolve in water. 25

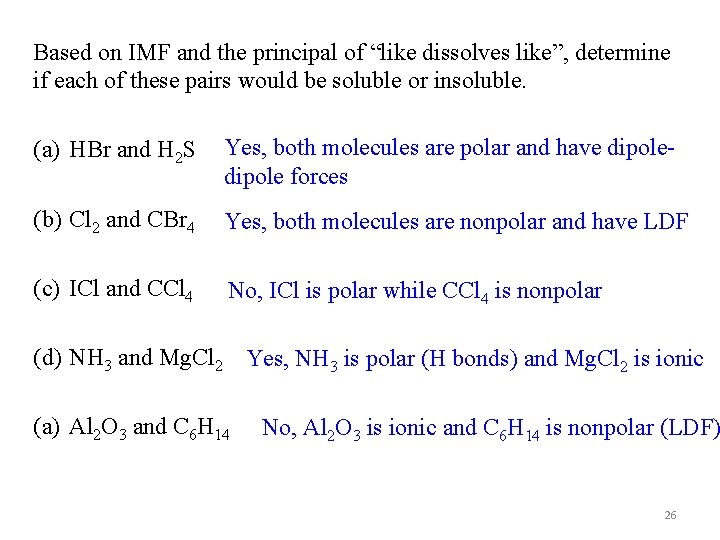
Based on IMF and the principal of “like dissolves like”, determine if each of these pairs would be soluble or insoluble. (a) HBr and H 2 S Yes, both molecules are polar and have dipole forces (b) Cl 2 and CBr 4 Yes, both molecules are nonpolar and have LDF (c) ICl and CCl 4 No, ICl is polar while CCl 4 is nonpolar (d) NH 3 and Mg. Cl 2 Yes, NH 3 is polar (H bonds) and Mg. Cl 2 is ionic (a) Al 2 O 3 and C 6 H 14 No, Al 2 O 3 is ionic and C 6 H 14 is nonpolar (LDF) 26

Answer the following • Which one of the following substances would be the most soluble in CCl 4? Why? A) H 2 O B) Na. Cl C) CH 3 CH 2 OH D) NH 3 E) C 10 H 22 27

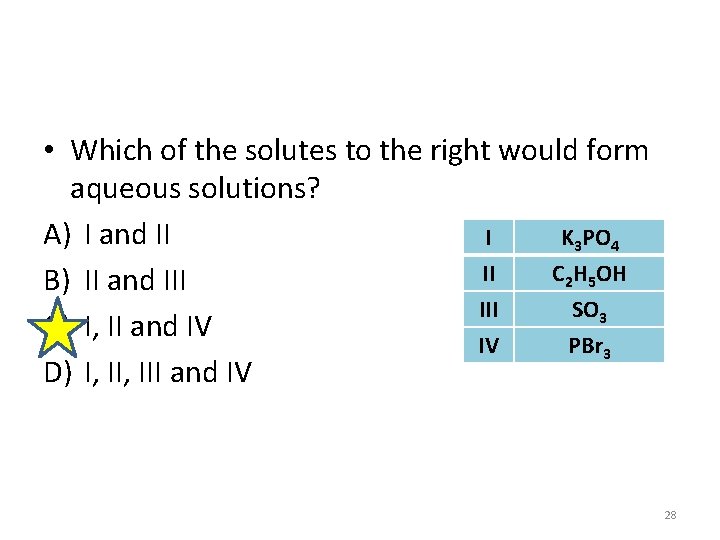
• Which of the solutes to the right would form aqueous solutions? A) I and II I K 3 PO 4 II C 2 H 5 OH B) II and III SO 3 C) I, II and IV IV PBr 3 D) I, III and IV 28

• Of the following, ______ should be insoluble with carbon tetrachloride. A) C 6 H 14 B) Br 2 C) CH 3 CH 2 OH D) C 3 H 8 E) I 2 29

• Which one of the following substances is more likely to dissolve in benzene (C 6 H 6)? A. CH 3 CH 2 OH B. NH 3 C. Na. Cl D. CCl 4 E. HBr 30
 Intermolecular vs intramolecular
Intermolecular vs intramolecular Intermolecular forces in a lava lamp
Intermolecular forces in a lava lamp Intramolecular forces vs intermolecular forces
Intramolecular forces vs intermolecular forces Intermolecular force
Intermolecular force Dipole-dipole interaction example
Dipole-dipole interaction example Interatomic and intermolecular forces
Interatomic and intermolecular forces Ch2cl intermolecular forces
Ch2cl intermolecular forces 3 types of intermolecular forces
3 types of intermolecular forces Gecko feet facts
Gecko feet facts Ion solvent interaction
Ion solvent interaction Bi3 intermolecular forces
Bi3 intermolecular forces Intermolecular forces
Intermolecular forces Sucrose intermolecular forces
Sucrose intermolecular forces Phthatic
Phthatic Intramolecular forces list
Intramolecular forces list London dispersion forces induced dipole
London dispersion forces induced dipole Type of intermolecular forces
Type of intermolecular forces Intermolecular forces london dispersion
Intermolecular forces london dispersion Butanal intermolecular forces
Butanal intermolecular forces Hbr intermolecular forces
Hbr intermolecular forces Carbonyl sulfide intermolecular forces
Carbonyl sulfide intermolecular forces Metallic bond formula
Metallic bond formula Coh2 intermolecular forces
Coh2 intermolecular forces Concept of imfa
Concept of imfa Hybrid bonding
Hybrid bonding Methyl propyl ether
Methyl propyl ether Induced dipole induced dipole interaction
Induced dipole induced dipole interaction Intermolecular forces review
Intermolecular forces review Poem about intermolecular forces
Poem about intermolecular forces Hco2h intermolecular forces
Hco2h intermolecular forces Formaldehyde intermolecular forces
Formaldehyde intermolecular forces