Intermolecular Forces of Organic Molecules Dr C Yau










- Slides: 17

Intermolecular Forces of Organic Molecules Dr. C. Yau Fall 2014 1

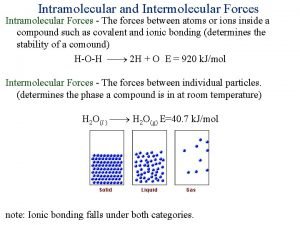
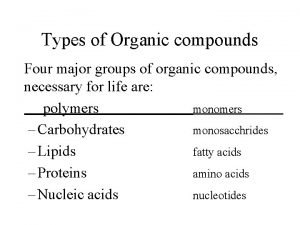
Review of Organic Molecules What are considered “organic compounds? ” They are compounds containing C. What are hydrocarbons? They are compounds containing only C & H. In a previous lecture we talked about several of the classes of organic compounds: • hydrocarbons • carboxylic acids • amines • alcohols It would be wise to review what distinguish these classes of compounds from each other. 2

Intermolecular Forces of Hydrocarbons are nonpolar. You should know why. Intermolecular forces of hydrocarbons can only be London forces, but don’t underestimate them! The larger the molecule, the stronger are the London forces (molecules become more polarizable). What is the significance of this fact? 3

Intermolecular Forces of Hydrocarbons with 1 to 5 carbons are gases, 5 to 13 carbons are liquids, and >13 carbons are solids. You should be able to explain why. What does the number of carbon atoms have to do with the physical state of a hydrocarbon? Think about how intermolecular forces affect the physical state of a substance. 4

Oil Refinery: Distillation Towers 5

6

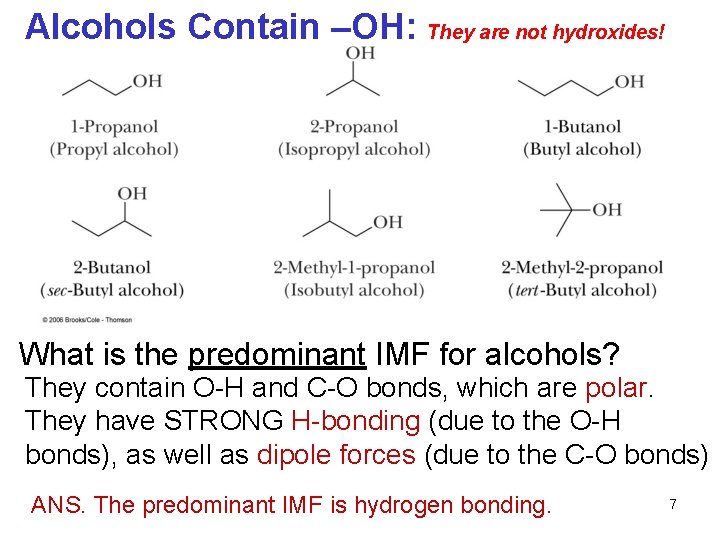
Alcohols Contain –OH: They are not hydroxides! What is the predominant IMF for alcohols? They contain O-H and C-O bonds, which are polar. They have STRONG H-bonding (due to the O-H bonds), as well as dipole forces (due to the C-O bonds) ANS. The predominant IMF is hydrogen bonding. 7

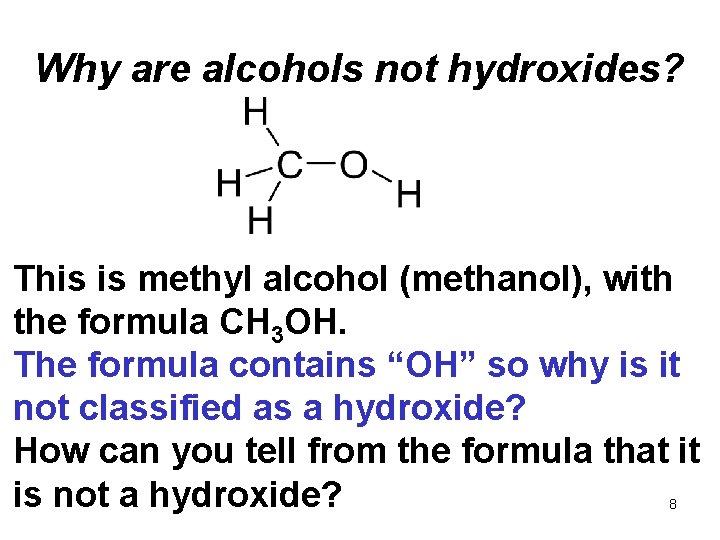
Why are alcohols not hydroxides? This is methyl alcohol (methanol), with the formula CH 3 OH. The formula contains “OH” so why is it not classified as a hydroxide? How can you tell from the formula that it is not a hydroxide? 8

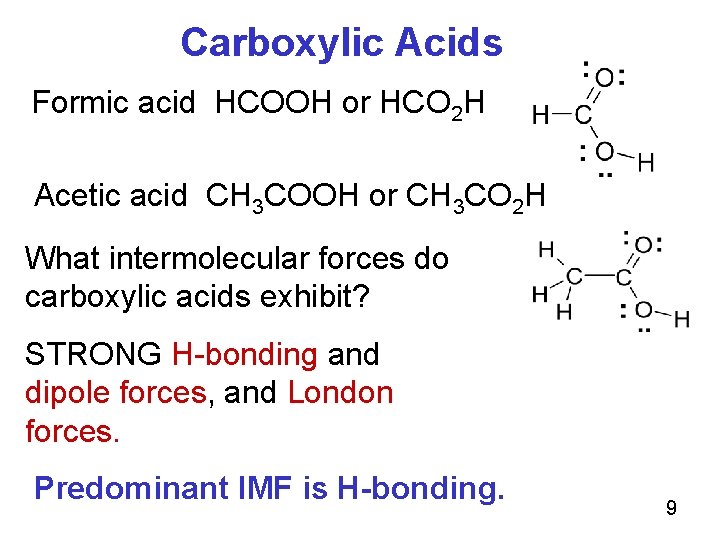
Carboxylic Acids Formic acid HCOOH or HCO 2 H Acetic acid CH 3 COOH or CH 3 CO 2 H What intermolecular forces do carboxylic acids exhibit? STRONG H-bonding and dipole forces, and London forces. Predominant IMF is H-bonding. 9

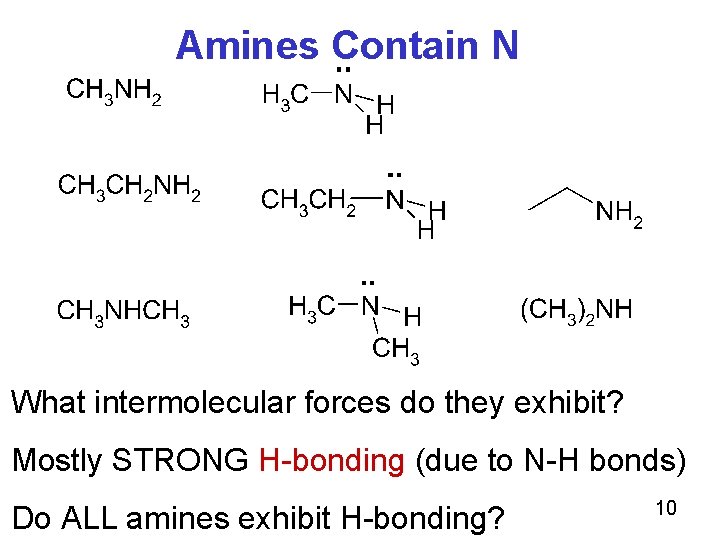
Amines Contain N What intermolecular forces do they exhibit? Mostly STRONG H-bonding (due to N-H bonds) Do ALL amines exhibit H-bonding? 10

Amines Contain N Do they ALL exhibit H-bonding? Decide for yourself: One of the above does NOT exhibit H-bonding. Do you know which one, and why? 11

Note N-H bond here also O-H bonds Trimethylamine exhibit only dipole forces and NOT H-bonding because it does not contain any N-H bonds. 12

Acetone (fingernail polish remover) Acetone belongs in class called ketones. You should have already memorized the structure of acetone. It is a very common organic solvent. CH 3 COCH 3 or (CH 3)2 CO Does it exhibit H-bonding? NO, because it does not contain any O-H bonds. It is polar and exhibits dipole forces (due to C=O). 13

Benzene is another very common organic solvent. (You should have memorized its structure. ) What intermolecular forces does it exhibit? 14

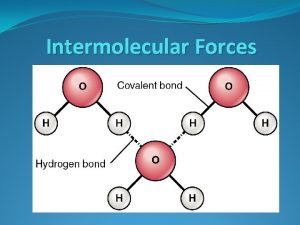
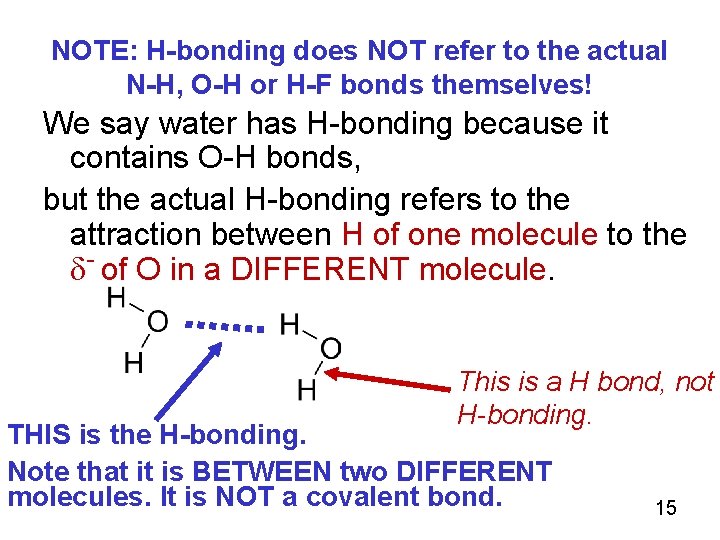
NOTE: H-bonding does NOT refer to the actual N-H, O-H or H-F bonds themselves! We say water has H-bonding because it contains O-H bonds, but the actual H-bonding refers to the attraction between H of one molecule to the - of O in a DIFFERENT molecule. This is a H bond, not H-bonding. THIS is the H-bonding. Note that it is BETWEEN two DIFFERENT molecules. It is NOT a covalent bond. 15

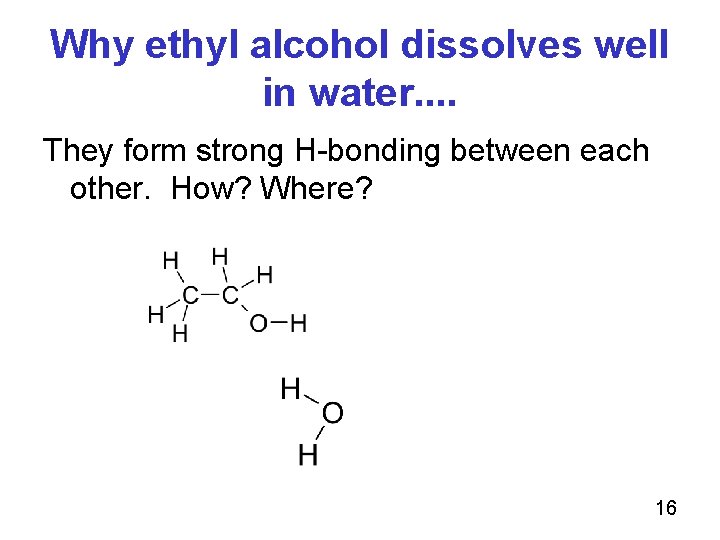
Why ethyl alcohol dissolves well in water. . They form strong H-bonding between each other. How? Where? 16

H-bonding between ethyl alcohol and water H-bonding here There is NO H-bonding here! Why not? Be sure you know why not! 17
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Intramolecular vs intermolecular
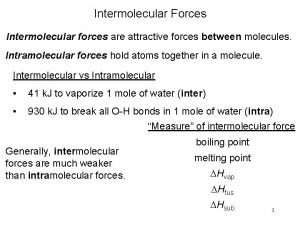
Intramolecular vs intermolecular Intermolecular forces vs intramolecular forces
Intermolecular forces vs intramolecular forces Intramolecular forces vs intermolecular forces
Intramolecular forces vs intermolecular forces Elaine yau
Elaine yau Are unsaturated fats solid at room temperature
Are unsaturated fats solid at room temperature Four major groups of organic compounds
Four major groups of organic compounds Organic vs inorganic molecules
Organic vs inorganic molecules Summary of intermolecular forces
Summary of intermolecular forces Pvc intermolecular forces
Pvc intermolecular forces Hco2h intermolecular forces
Hco2h intermolecular forces Unit 3 intermolecular forces and properties
Unit 3 intermolecular forces and properties Dipole dipole interaction example
Dipole dipole interaction example Intermolecular forces and boiling point
Intermolecular forces and boiling point Intermolecular forces
Intermolecular forces Ion induced dipole examples
Ion induced dipole examples Boiling point with intermolecular forces
Boiling point with intermolecular forces Intermolecular forces
Intermolecular forces