Questions Why do some solids dissolve in water













- Slides: 27

Questions • Why do some solids dissolve in water but others do not? • Why are some substances gases at room temperature, but others are liquid or solid? • What gives metals the ability to conduct electricity, what makes non-metals brittle? • The answers have to do with … Intermolecular forces

Intermolecular Forces • Distinction between intramolecular bonding and intermolecular forces. • Intermolecular forces: van der Waals’, dipole, hydrogen bonding. • Effect of the intermolecular forces on the boiling point of a covalent substance. • Comparison of the boiling points of H 2 and O 2, of C 2 H 4 and HCHO, and of H 2 O and H 2 S

Intermolecular forces Overview • There are 2 types of attraction in molecules: intramolecular bonds & intermolecular forces • We have already looked at intramolecular bonds (ionic, polar, non-polar) • Intermolecular forces (IMF) have to do with the attraction between molecules • IMFs come in 3 flavours: 1 dipole - dipole, ) H -bonding, 3) van der waals

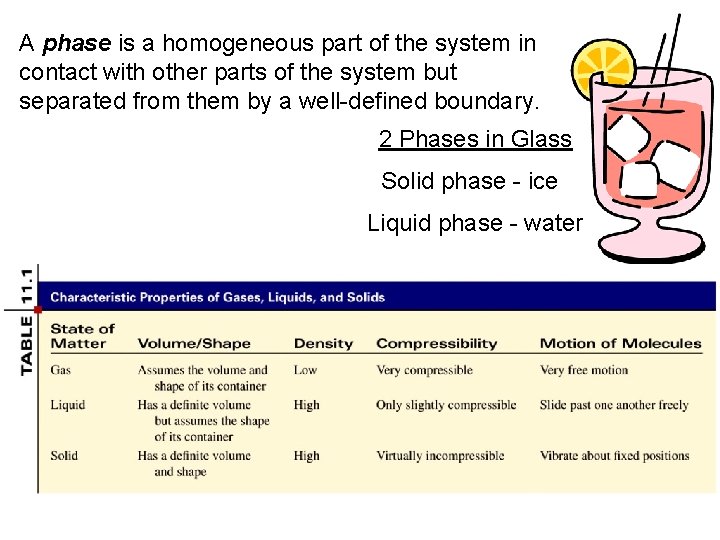
A phase is a homogeneous part of the system in contact with other parts of the system but separated from them by a well-defined boundary. 2 Phases in Glass Solid phase - ice Liquid phase - water

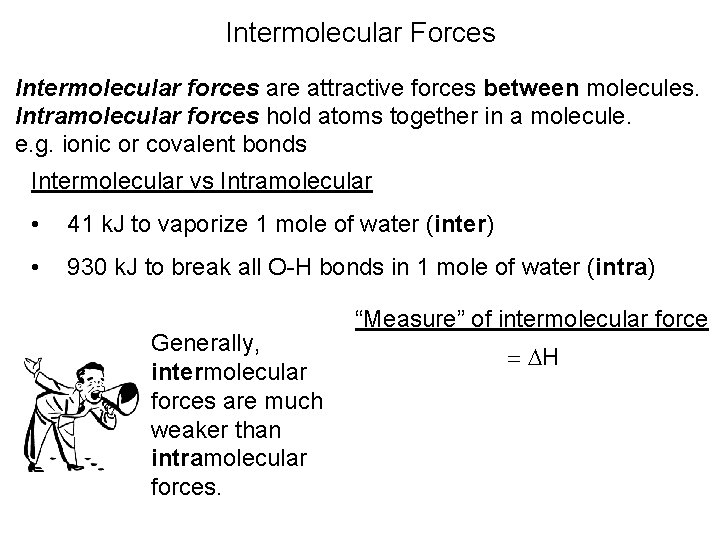
Intermolecular Forces Intermolecular forces are attractive forces between molecules. Intramolecular forces hold atoms together in a molecule. e. g. ionic or covalent bonds Intermolecular vs Intramolecular • 41 k. J to vaporize 1 mole of water (inter) • 930 k. J to break all O-H bonds in 1 mole of water (intra) Generally, intermolecular forces are much weaker than intramolecular forces. “Measure” of intermolecular force = H

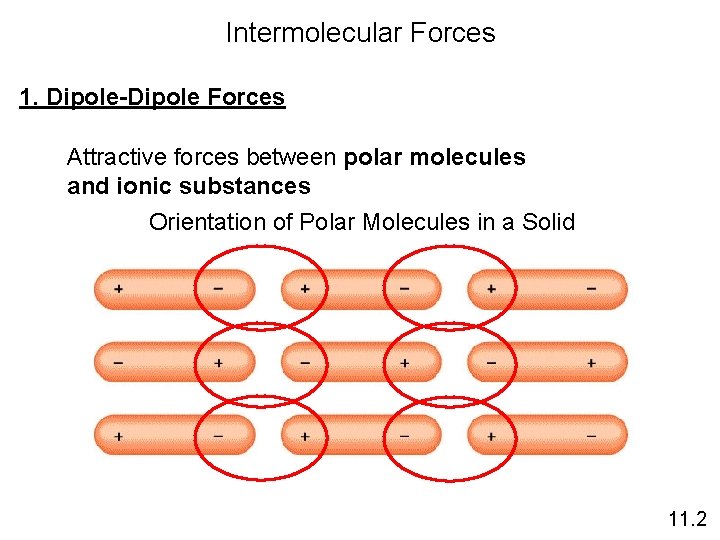
Intermolecular Forces 1. Dipole-Dipole Forces Attractive forces between polar molecules and ionic substances Orientation of Polar Molecules in a Solid 11. 2

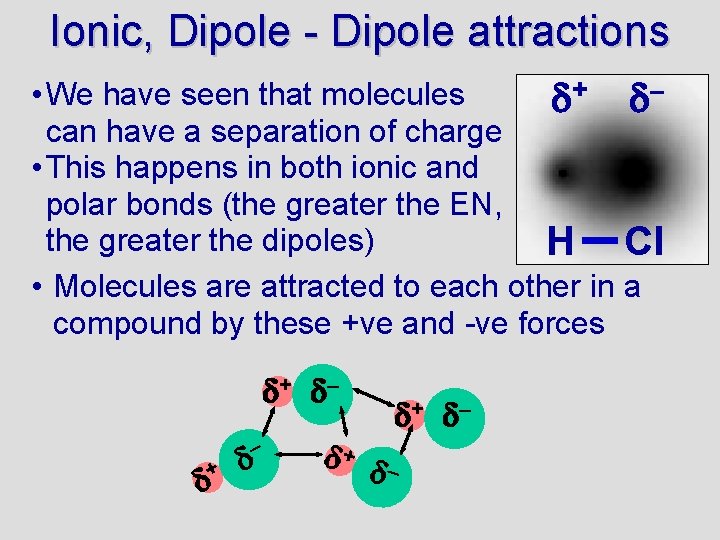
Ionic, Dipole - Dipole attractions • We have seen that molecules + – can have a separation of charge • This happens in both ionic and polar bonds (the greater the EN, the greater the dipoles) H Cl • Molecules are attracted to each other in a compound by these +ve and -ve forces + –

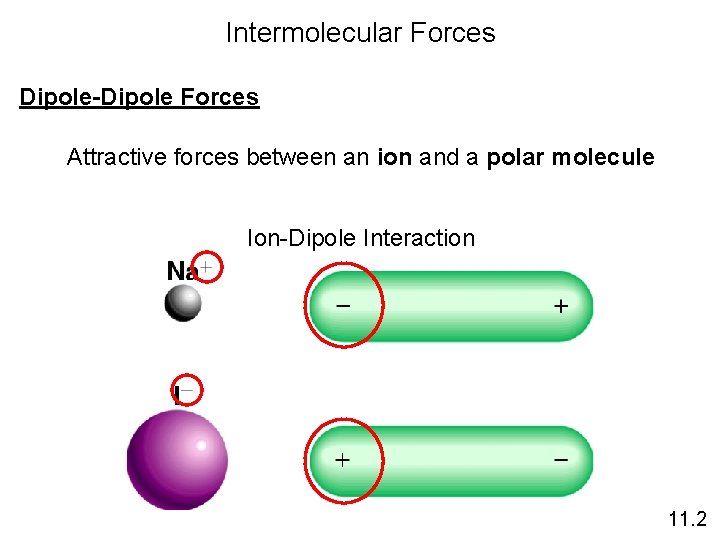
Intermolecular Forces Dipole-Dipole Forces Attractive forces between an ion and a polar molecule Ion-Dipole Interaction 11. 2

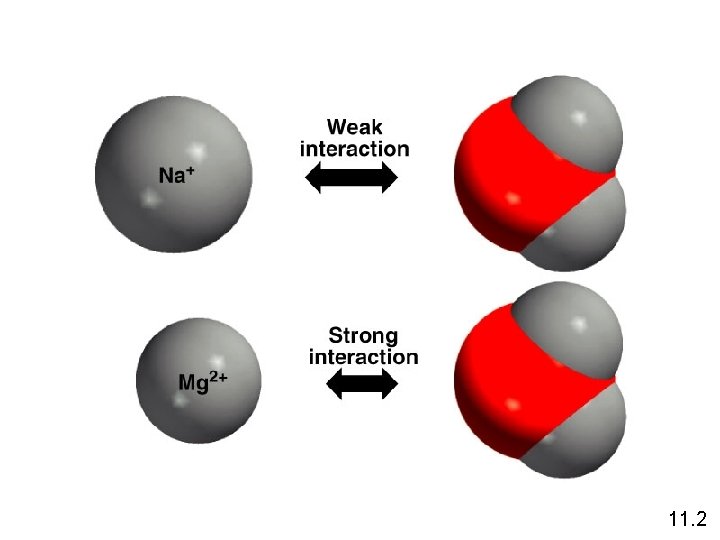
11. 2

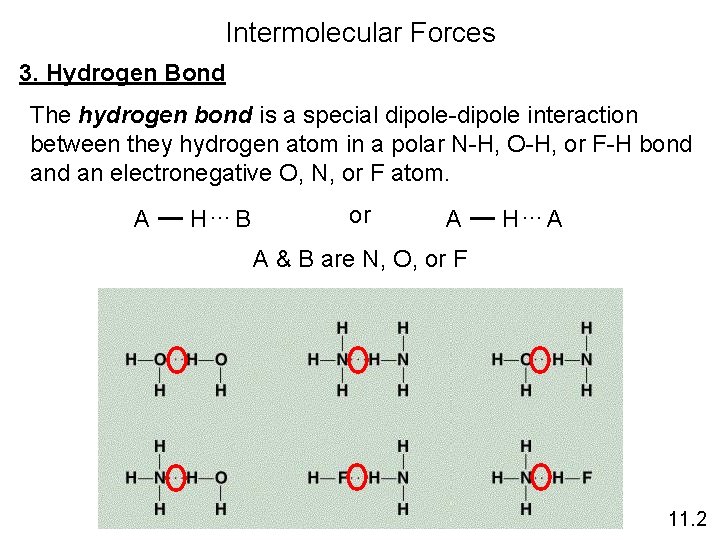
Intermolecular Forces 3. Hydrogen Bond The hydrogen bond is a special dipole-dipole interaction between they hydrogen atom in a polar N-H, O-H, or F-H bond an electronegative O, N, or F atom. A H…B or A H…A A & B are N, O, or F 11. 2

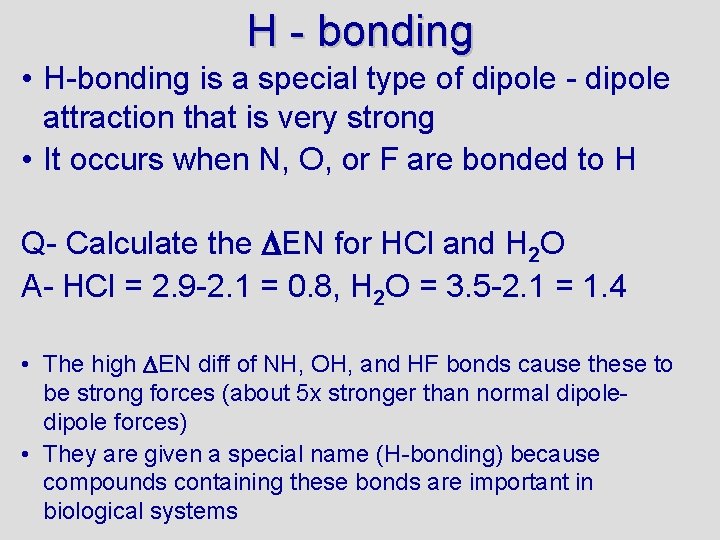
H - bonding • H-bonding is a special type of dipole - dipole attraction that is very strong • It occurs when N, O, or F are bonded to H Q- Calculate the EN for HCl and H 2 O A- HCl = 2. 9 -2. 1 = 0. 8, H 2 O = 3. 5 -2. 1 = 1. 4 • The high EN diff of NH, OH, and HF bonds cause these to be strong forces (about 5 x stronger than normal dipole forces) • They are given a special name (H-bonding) because compounds containing these bonds are important in biological systems


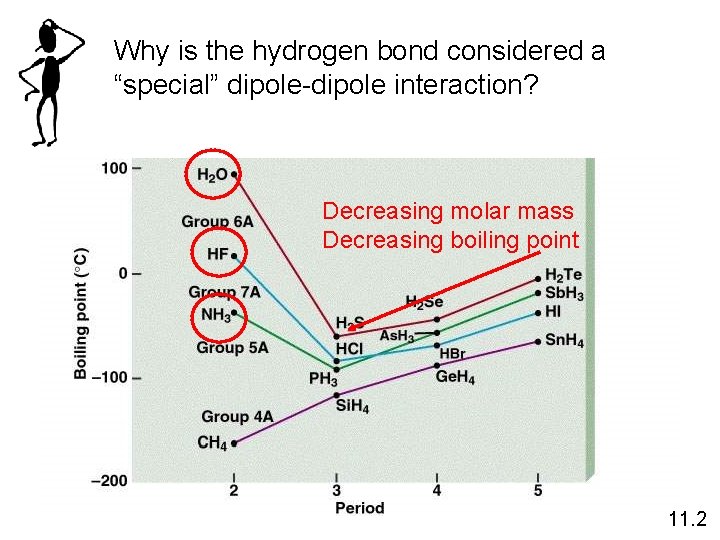
Why is the hydrogen bond considered a “special” dipole-dipole interaction? Decreasing molar mass Decreasing boiling point 11. 2

Van der waals forces • Non-polar molecules do not have dipoles like polar molecules. • How, then, can non-polar compounds form solids or liquids? • These forces are due to small dipoles that exist in non-polar molecules • Because electrons are moving around in atoms there will be instants when the charge around an atom is not symmetrical • The resulting tiny dipoles cause attractions between atoms/molecules

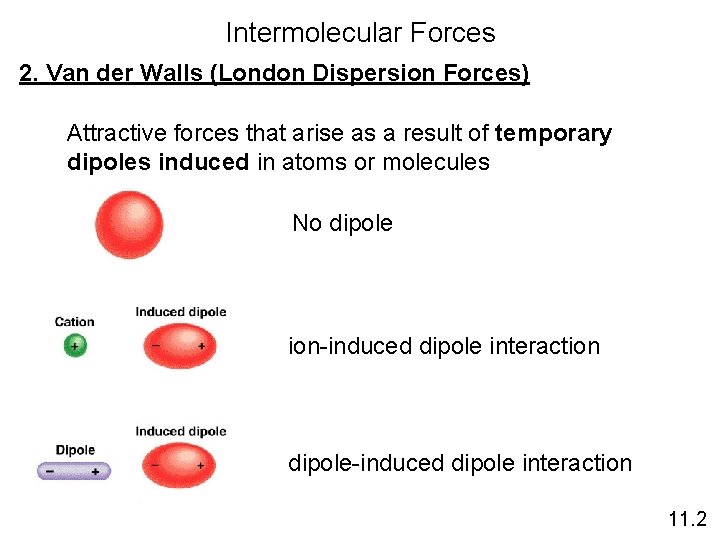
Intermolecular Forces 2. Van der Walls (London Dispersion Forces) Attractive forces that arise as a result of temporary dipoles induced in atoms or molecules No dipole ion-induced dipole interaction dipole-induced dipole interaction 11. 2

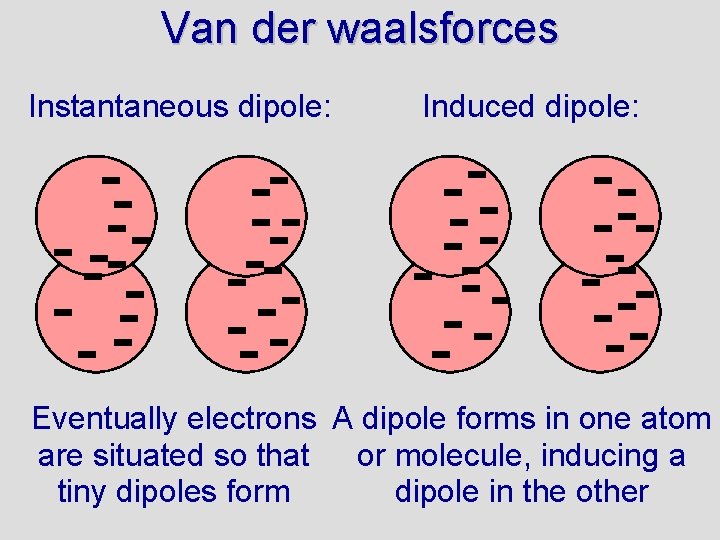
Van der waalsforces Instantaneous dipole: Induced dipole: Eventually electrons A dipole forms in one atom are situated so that or molecule, inducing a tiny dipoles form dipole in the other

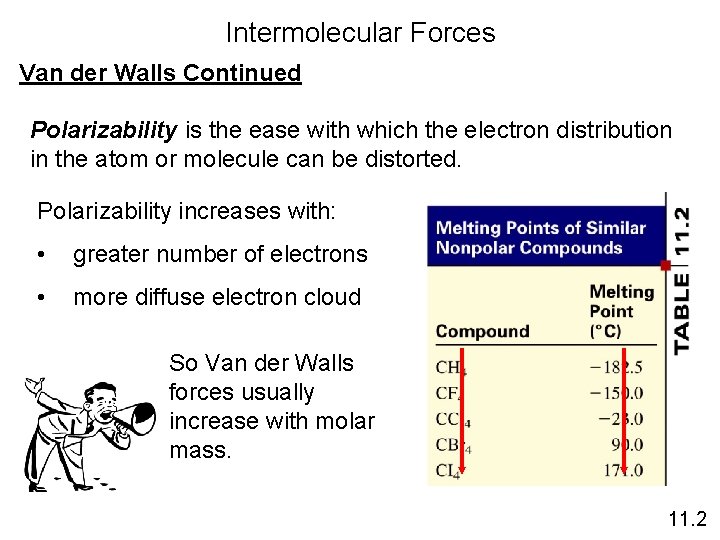
Intermolecular Forces Van der Walls Continued Polarizability is the ease with which the electron distribution in the atom or molecule can be distorted. Polarizability increases with: • greater number of electrons • more diffuse electron cloud So Van der Walls forces usually increase with molar mass. 11. 2

Van der Walls Forces • Are present in all compounds • Can occur between atoms or molecules • Are due to electron movement not to EN • Are transient in nature (dipole-dipole are more permanent). • are weaker than dipole-dipole and H-bonding

What type(s) of intermolecular forces exist between each of the following molecules? HBr is a polar molecule: dipole-dipole forces. There also dispersion forces between HBr molecules. CH 4 is nonpolar: Wan der Walls forces. SO 2 O SO 2 is a polar molecule: dipole-dipole forces. There also dispersion forces between SO 2 molecules. 11. 2

Testing concepts 1. Which attractions are stronger: intermolecular or intramolecular? Intramolecular are stronger. A covalent bond is 100 x stronger. 2. What evidence is there that nonpolar molecules attract each other? Nonpolar molecules gather together as liquids or solids at low temperatures. 3. Which would have a lower boiling point: O 2 or F 2? Explain. Both have Van der Walls forces but F 2 would be lower because it is smaller. Larger atoms/molecules can have their electron clouds more easily deformed and thus have stronger Van der Walls attractions and higher melting/boiling points. H 2 S has the strongest (-61). 4. Which would have a lower boiling point: NO or O 2? Explain. O 2 because it has only Van der Walls forces. NO has a small EN, giving it small dipoles.

5. Which would you expect to have the higher melting point (or boiling point): C 8 H 18 or C 4 H 10? Explain. C 8 H 18 would have the higher melting/boiling point. This is a result of the many more sites available for Van der Walls forces to form. 6. What two factors causes hydrogen bonds to be so much stronger than typical dipole-dipole bonds? 1) a large EN 2) the small sizes of atoms.

9. What kind(s) of intermolecular forces are present in the following substances: a) NH 3, b) SF 6, c) PCl 3, d) Li. Cl, e) HBr, f) CO 2 (hint: consider EN and molecular shape/polarity) a) NH 3: Hydrogen bonding (H + N), Van der Walls b) SF 6: Van der Walls only (it is symmetrical). c) PCl 3: EN=2. 9 -2. 1. Dipole-dipole, Van der Walls. d) Li. Cl: EN=2. 9 -1. 0. Ionic, (Van der Walls). e) HBr: EN=2. 8 -2. 1. Dipole-dipole, Van der Walls. f) CO 2: Van der Walls only (it is symmetrical)

Challenge: Ethanol (CH 3 CH 2 OH) and dimethyl ether (CH 3 OCH 3) have the same formula (C 2 H 6 O). Ethanol boils at 78 C, whereas dimethyl ether boils at -24 C. Explain why the boiling point of the ether is so much lower than the boiling point of ethanol. • Challenge: In ethanol, H and O are bonded (the large EN results in H-bonding). In dimethyl ether the O is bonded to C (a smaller EN results in a dipole-dipole attraction rather than hydrogen bonding).

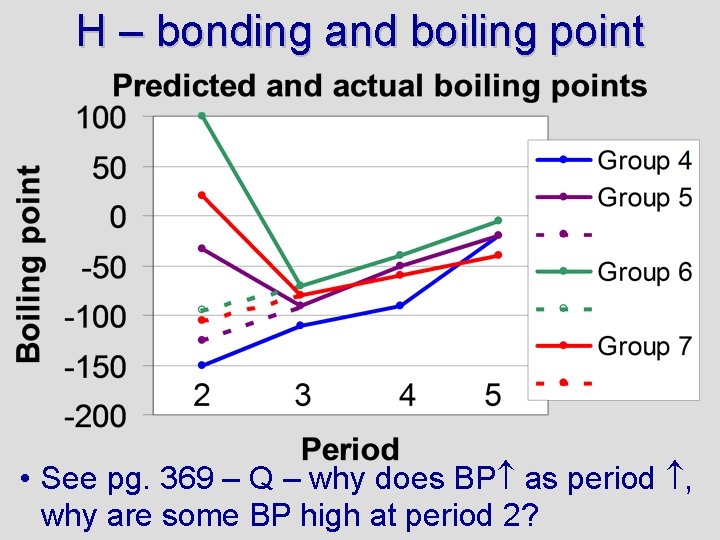
H – bonding and boiling point • See pg. 369 – Q – why does BP as period , why are some BP high at period 2?

Graph Q Boiling points increase down a group (as period increases) for two reasons: 1) EN tends to increase and 2) size increases. A larger size means greater London forces. Boiling points are very high for H 2 O, HF, and NH 3 because these are hydrogen bonds (high EN), creating large intermolecular forces For more lessons, visit www. chalkbored. com

Testing concepts 6. A) F 2 would be lower because it is smaller. Larger atoms/molecules can have their electron clouds more easily deformed and thus have stronger London attractions and higher melting/boiling points. B) O 2 because it has only London forces. NO has a small EN, giving it small dipoles.

Testing concepts 9. a) NH 3: Hydrogen bonding (H + N), London. b) SF 6: London only (it is symmetrical). c) PCl 3: EN=2. 9 -2. 1. Dipole-dipole, London. d) Li. Cl: EN=2. 9 -1. 0. Ionic, (London). e) HBr: EN=2. 8 -2. 1. Dipole-dipole, London. f) CO 2: London only (it is symmetrical)