How much can dissolve How much can dissolve








- Slides: 12

How much can dissolve?

How much can dissolve? l If you continue adding sugar to lemonade, eventually the point is reached when no more sugar dissolves and the excess granules sink to the bottom of the glass. l Solubility (sol yuh BIH luh tee) is the maximum amount of a solute that can be dissolved in a given amount of solvent at a given temperature.

Comparing Solubilities l The amount of a substance that can dissolve in a solvent depends on the nature of these substances. l In one beaker, 1 g of solute A dissolves completely, but additional solute does not dissolve and falls to the bottom of the beaker

Comparing Solubilities l 1 g of solute B dissolves completely, and two more grams also dissolve before solute begins to fall to the bottom. l If the temperature of the water is the same in both beakers; you can conclude that substance B is more soluble than substance A.

Concentrations l. A concentrated solution is one in which a large amount of solute is dissolved in the solvent. l. A dilute solution is one that has a small amount of solute in the solvent.

Types of Solutions–Saturated l. A saturated solution is a solution that contains all the solute it can hold at a given temperature. l An unsaturated solution is any solution that can dissolve more solute at a given temperature. l Each time a saturated solution is heated to a higher temperature, it becomes unsaturated.

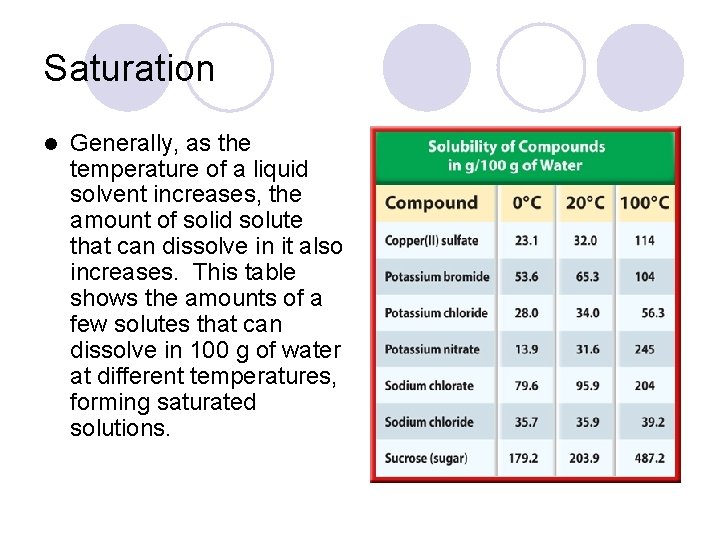
Saturation l Generally, as the temperature of a liquid solvent increases, the amount of solid solute that can dissolve in it also increases. This table shows the amounts of a few solutes that can dissolve in 100 g of water at different temperatures, forming saturated solutions.

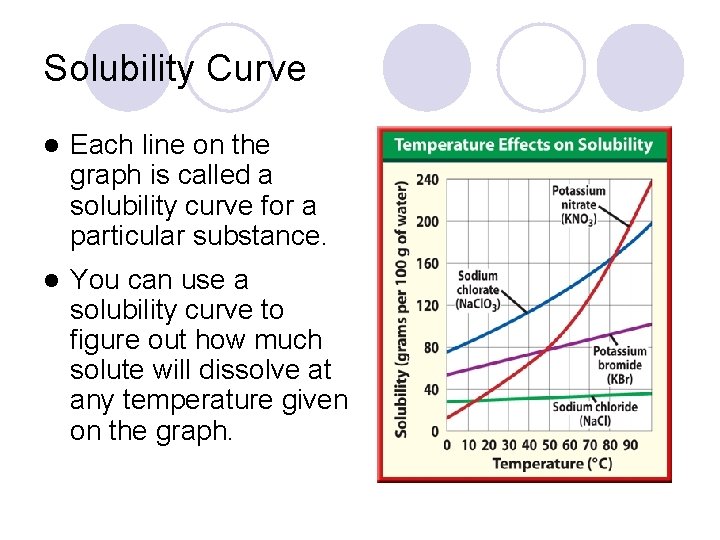
Solubility Curve l Each line on the graph is called a solubility curve for a particular substance. l You can use a solubility curve to figure out how much solute will dissolve at any temperature given on the graph.

Supersaturated Solutions l. A supersaturated solution is one that contains more solute than a saturated solution at the same temperature. l Supersaturated solutions are unstable.

Supersaturated Sodium acetate l For example, if a seed crystal of sodium acetate is dropped into the supersaturated solution, excess sodium acetate crystallizes out

Energy Flow l As the supersaturated solution of sodium acetate crystallizes, the solution becomes hot. l Energy is given off as new bonds form between the ions and the water molecules

Energy Flow l Another result of solution energy is to reduce the temperature of the solution. l Some substances, such as ammonium nitrate, must draw energy from the surroundings to dissolve. l This is what happens when a cold pack is activated to treat minor injuries or to reduce swelling.