Questions Why do some solids dissolve in water








- Slides: 13

Questions • Why do some solids dissolve in water but others do not? • Why are some substances gases at room temperature, but others are liquid or solid? • What gives metals the ability to conduct electricity, what makes non-metals brittle? • The answers have to do with … Intermolecular forces

Intermolecular forces Overview • There are 2 types of attraction in molecules: intramolecular bonds & intermolecular forces • We have already looked at intramolecular bonds (ionic, polar, non-polar) • Intermolecular forces (IMF) have to do with the attraction between molecules (vs. the attraction between atoms in a molecule) • IMFs come in six flavours: 1) ionic, 2) dipole - dipole, 3) H-bonding, 4) London forces, 5) covalent (network solids), 6) metallic

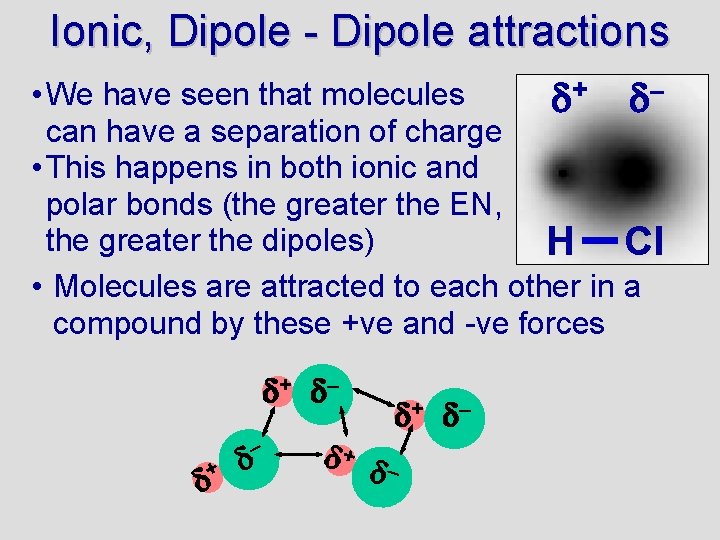
Ionic, Dipole - Dipole attractions • We have seen that molecules + – can have a separation of charge • This happens in both ionic and polar bonds (the greater the EN, the greater the dipoles) H Cl • Molecules are attracted to each other in a compound by these +ve and -ve forces + –

H - bonding • H-bonding is a special type of dipole - dipole attraction that is very strong • It occurs when N, O, or F are bonded to H Q- Calculate the EN for HCl and H 2 O A- HCl = 2. 9 -2. 1 = 0. 8, H 2 O = 3. 5 -2. 1 = 1. 4 • The high EN of NH, OH, and HF bonds cause these to be strong forces (about 5 x stronger than normal dipole-dipole forces) • They are given a special name (H-bonding) because compounds containing these bonds are important in biological systems

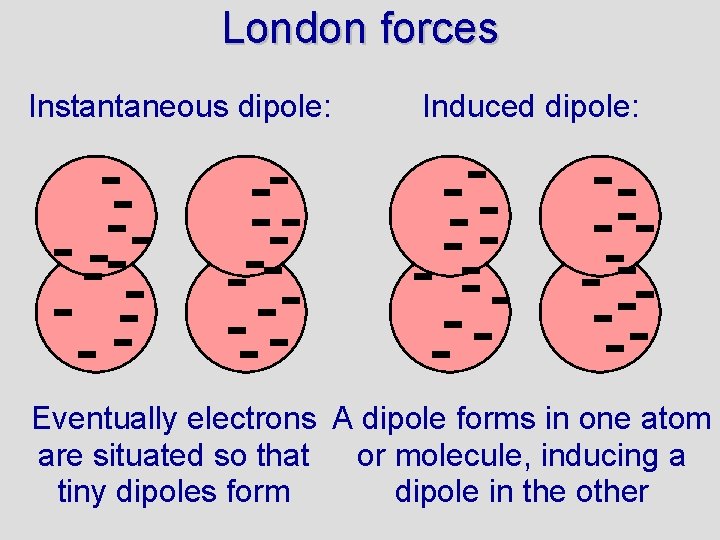
London forces • Non-polar molecules do not have dipoles like polar molecules. How, then, can non-polar compounds form solids or liquids? • London forces are named after Fritz London (also called van der Waal forces) • London forces are due to small dipoles that exist in non-polar molecules • Because electrons are moving around in atoms there will be instants when the charge around an atom is not symmetrical • The resulting tiny dipoles cause attractions between atoms/molecules • Read 10. 3 (pg. 351 - 355) and answer …

London forces Instantaneous dipole: Induced dipole: Eventually electrons A dipole forms in one atom are situated so that or molecule, inducing a tiny dipoles form dipole in the other

Testing concepts 1. Which attractions are stronger: intermolecular or intramolecular? 2. How many times stronger is a covalent bond compared to a dipole-dipole attraction? 3. What evidence is there that nonpolar molecules attract each other? 4. Which chemical in table 10. 1 has the weakest intermolecular forces? Which has the strongest? How can you tell? 5. Suggest some ways that the dipoles in London forces are different from the dipoles in dipole-dipole attractions. 6. A) Which would have a lower boiling point: O 2 or F 2? Explain. B) Which would have a lower boiling point: NO or O 2? Explain.

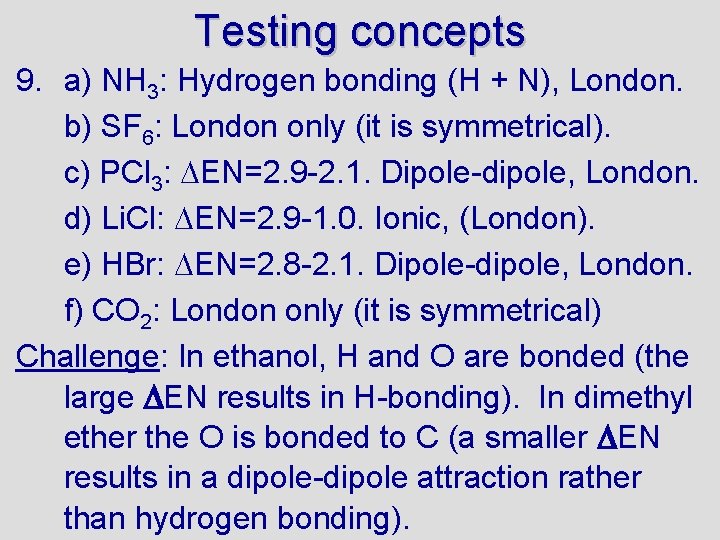
7. Which would you expect to have the higher melting point (or boiling point): C 8 H 18 or C 4 H 10? Explain. 8. What two factors causes hydrogen bonds to be so much stronger than typical dipole-dipole bonds? 9. So far we have discussed 4 kinds of intermolecular forces: ionic, dipole-dipole, hydrogen bonding, and London forces. What kind(s) of intermolecular forces are present in the following substances: a) NH 3, b) SF 6, c) PCl 3, d) Li. Cl, e) HBr, f) CO 2 (hint: consider EN and molecular shape/polarity) Challenge: Ethanol (CH 3 CH 2 OH) and dimethyl ether (CH 3 OCH 3) have the same formula (C 2 H 6 O). Ethanol boils at 78 C, whereas dimethyl ether boils at -24 C. Explain why the boiling point of the ether is so much lower than the boiling point of ethanol. Challenge: try answering the question on the next slide.

Testing concepts 1. Intramolecular are stronger. 2. A covalent bond is 100 x stronger. 3. The molecules gather together as liquids or solids at low temperatures. 4. Based on boiling points, F 2 (-188) has the weakest forces, H 2 S has the strongest (-61). 5. London forces – Are present in all compounds – Can occur between atoms or molecules – Are due to electron movement not to EN – Are transient in nature (dipole-dipole are more permanent). – London forces are weaker

Testing concepts 6. A) F 2 would be lower because it is smaller. Larger atoms/molecules can have their electron clouds more easily deformed and thus have stronger London attractions and higher melting/boiling points. B) O 2 because it has only London forces. NO has a small EN, giving it small dipoles. 7. C 8 H 18 would have the higher melting/boiling point. This is a result of the many more sites available for London forces to form. 8. 1) a large EN, 2) the small sizes of atoms.

Testing concepts 9. a) NH 3: Hydrogen bonding (H + N), London. b) SF 6: London only (it is symmetrical). c) PCl 3: EN=2. 9 -2. 1. Dipole-dipole, London. d) Li. Cl: EN=2. 9 -1. 0. Ionic, (London). e) HBr: EN=2. 8 -2. 1. Dipole-dipole, London. f) CO 2: London only (it is symmetrical) Challenge: In ethanol, H and O are bonded (the large EN results in H-bonding). In dimethyl ether the O is bonded to C (a smaller EN results in a dipole-dipole attraction rather than hydrogen bonding).

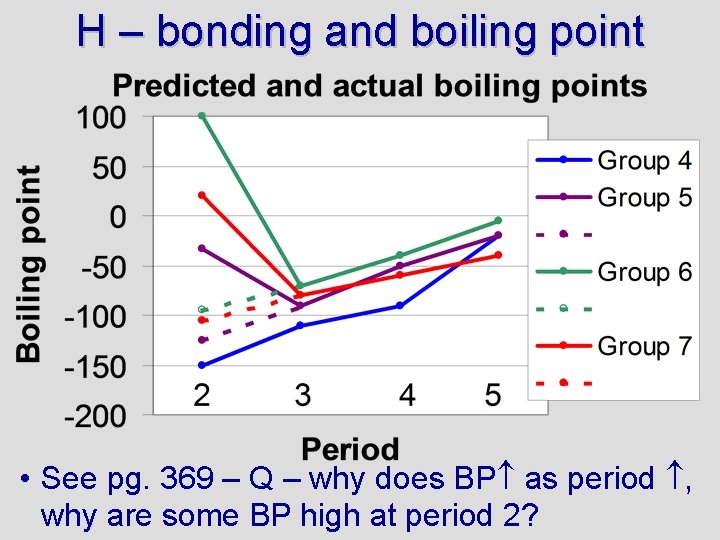
H – bonding and boiling point • See pg. 369 – Q – why does BP as period , why are some BP high at period 2?

Testing concepts Boiling points increase down a group (as period increases) for two reasons: 1) EN tends to increase and 2) size increases. A larger size means greater London forces. Boiling points are very high for H 2 O, HF, and NH 3 because these are hydrogen bonds (high EN), creating large intermolecular forces For more lessons, visit www. chalkbored. com