Intramolecular Forces vs Intermolecular Forces Intramolecular Forces forces












- Slides: 23

Intramolecular Forces vs. Intermolecular Forces Intramolecular Forces --forces within a molecule. --tend to be very strong. --hold the atoms in molecules/formula units together. --include: ionic bonding, covalent bonding and metallic bonding. --been there…done that!

Intramolecular Forces vs. Intermolecular Forces --forces between molecules. --tend to be weaker than intramolecular forces. --matter has entropy (the tendency to be disordered) --therefore, a force must be present to keep the individual atoms, molecules, or ions of a solid or liquid, in place, organized.

Intermolecular Forces are also called Van der Waals Forces There are four types: 1. London/dispersion 2. Dipole-dipole forces 3. Hydrogen bonds 4. Molecule-ion attractions

London Dispersion Forces • Due to attractive forces between e-s of one atom and the nucleus of another • occurs btw molecules that are non-polar • stronger for atoms/molecules with more electrons

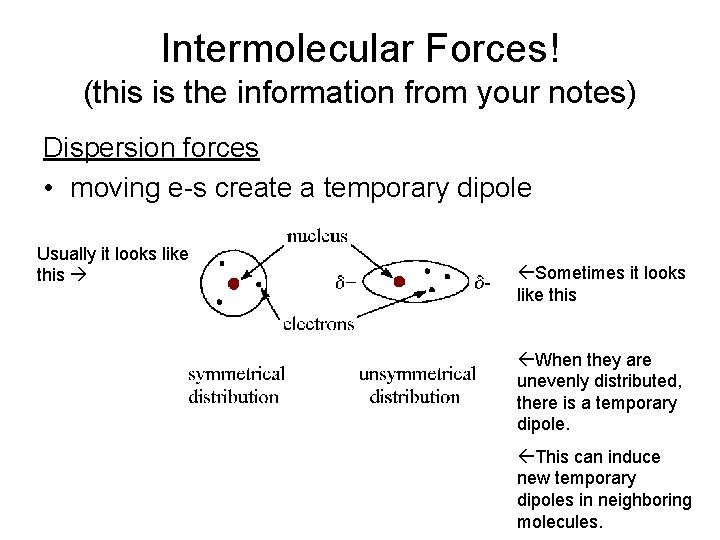
Intermolecular Forces! (this is the information from your notes) Dispersion forces • moving e-s create a temporary dipole Usually it looks like this ßSometimes it looks like this ßWhen they are unevenly distributed, there is a temporary dipole. ßThis can induce new temporary dipoles in neighboring molecules.

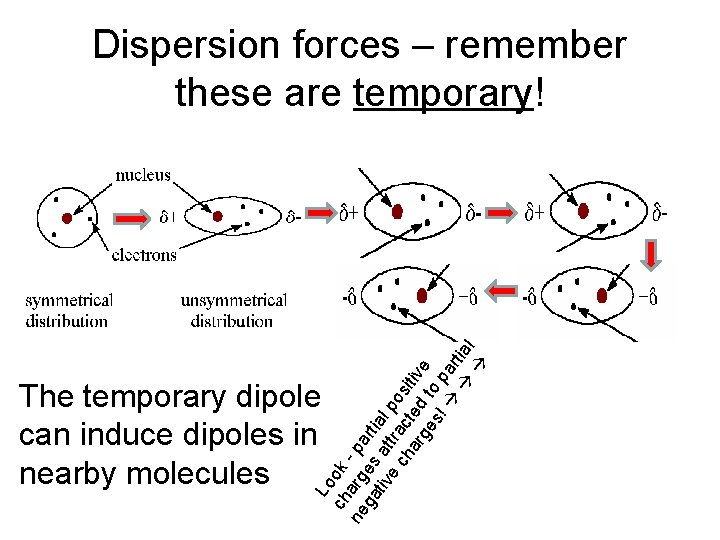
Lo ch ok ne arg - pa ga es rti tiv at al e tra po ch c si ar ted tiv ge to e s! par tial Dispersion forces – remember these are temporary! The temporary dipole can induce dipoles in nearby molecules

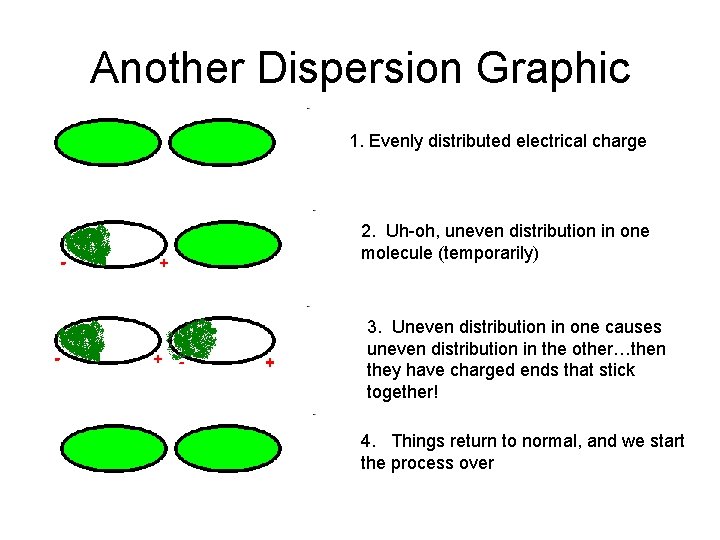
Another Dispersion Graphic 1. Evenly distributed electrical charge 2. Uh-oh, uneven distribution in one molecule (temporarily) 3. Uneven distribution in one causes uneven distribution in the other…then they have charged ends that stick together! 4. Things return to normal, and we start the process over

Check in! • What kind of molecules experience London/Dispersion forces? • What can make London forces stronger?

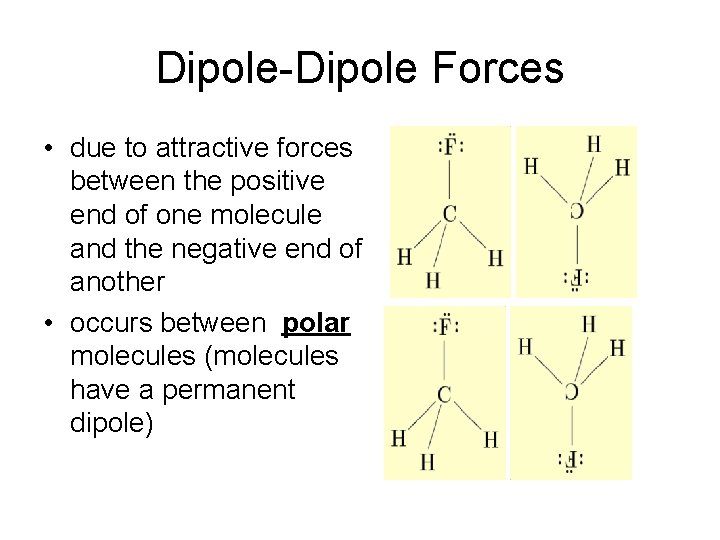
Dipole-Dipole Forces • due to attractive forces between the positive end of one molecule and the negative end of another • occurs between polar molecules (molecules have a permanent dipole)

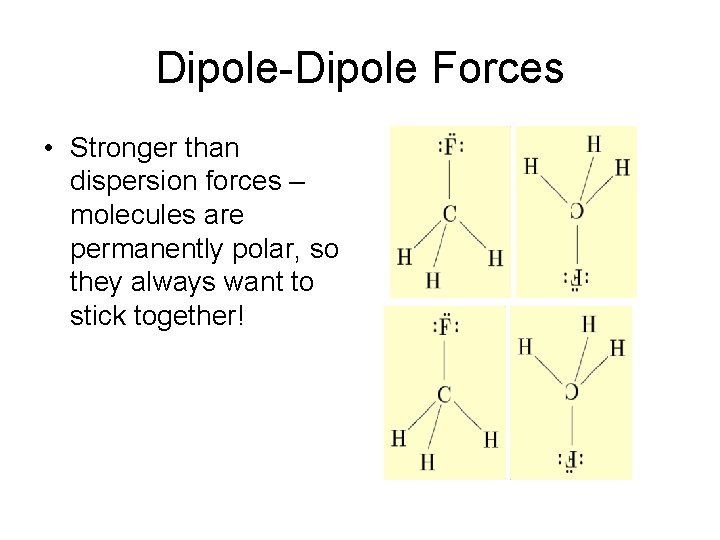
Dipole-Dipole Forces • Stronger than dispersion forces – molecules are permanently polar, so they always want to stick together!

Check in! • What kinds of molecules experience Dipole-Dipole Forces?

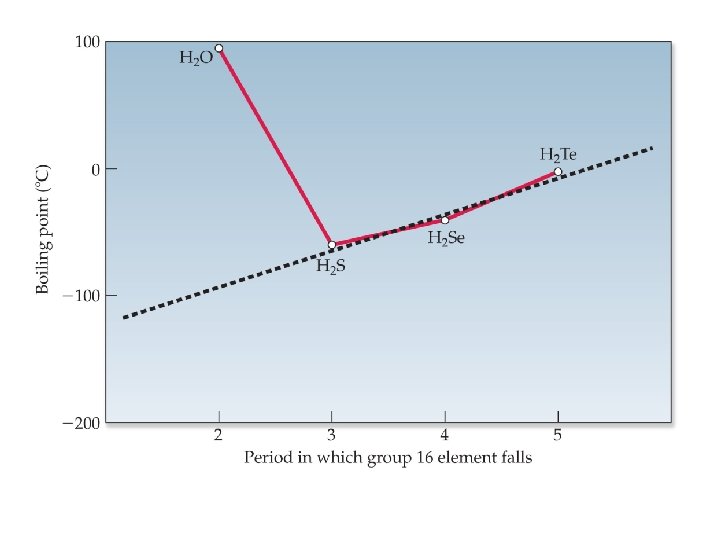
Hydrogen Bonding • a special dipole-dipole attraction between Hydrogen and three other elements with very high electronegativities and small radii (Oxygen, Nitrogen and Fluorine) • explains high boiling point of water

Hydrogen Bonding • A specific and EXTRA STRONG dipole interaction… • H must be bonded to O, N, or F these atoms are electronegative enough, to make a BIG enough dipole, to count as a different kind of force

H-bonding animation • http: //bcs. whfreeman. com/thelifewire/conte nt/chp 02/02020. html

Check in! • What is one (important) molecule that experiences Hydrogen Bonding?

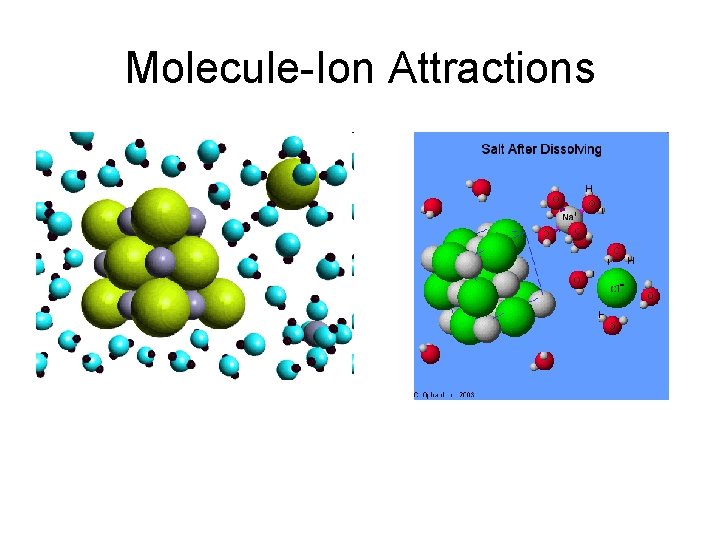
Molecule-ion Attractions • ionic compounds dissolve in water and other polar liquids because of attraction between the dipoles and the ions

Molecule-Ion Attractions

Check in! • If Al. Cl 3 dissolves in water, what pieces come off? What is the name of the force that accomplishes this?

And just for fun

Please take out a piece of paper • Divide it into 4 sections, label each with one kind of intermolecular force (look at your notes) • A’s on your own explain and draw pictures for Dispersion forces and dipole-dipole forces • B’s on your own explain and draw pictures for Hydrogen bonding and Molecule-ion interactions • In 5 minutes turn and share with your neighbor • You should have pictures for all 4 by the end

Now please take out… • Intermolecular bonding and boiling points • Use the data to complete each graph, then start working on the questions • When you have finished the questions decide which type of intermolecular force is present for EACH compound listed


Polar molecules • What has to be true for a molecule to be polar? (2 things) • What is one example of a polar molecule from your lab? • What is one example of a nonpolar molecule?