4 7 Intermolecular Forces intermolecular force the force













- Slides: 20

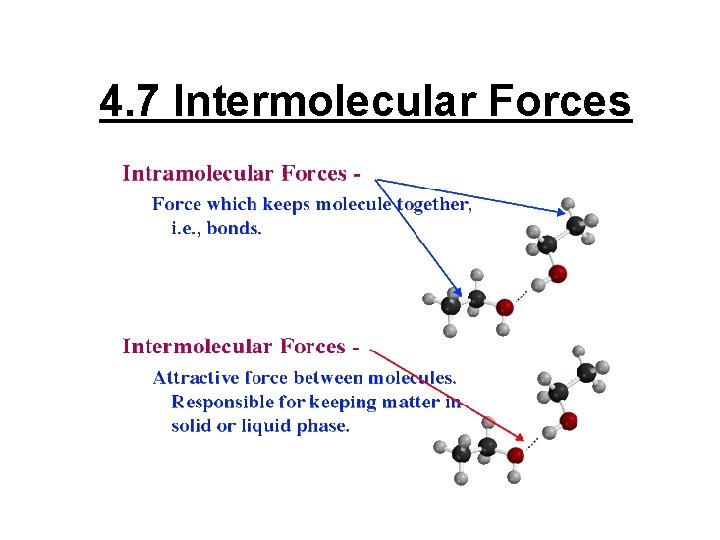
4. 7 Intermolecular Forces

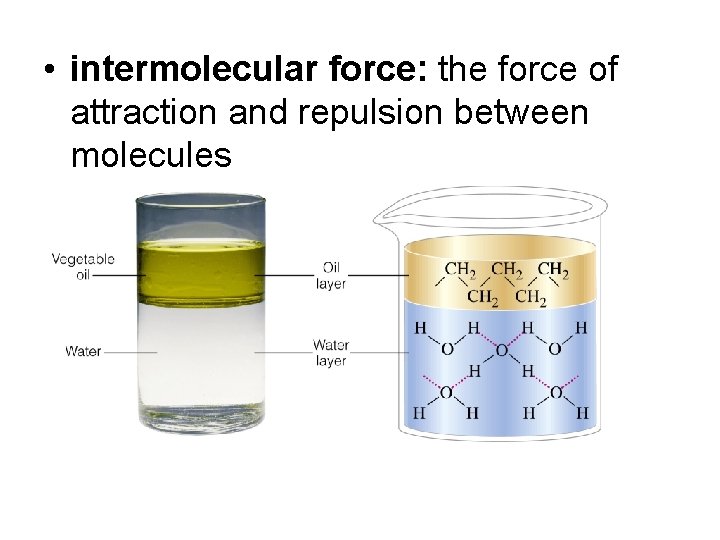
• intermolecular force: the force of attraction and repulsion between molecules

• Like all chemical bonding, intermolecular forces are explained as mutual electrostatic attractions. • These forces are much weaker than covalent bonds. • The forces have an effect on a chemicals melting point, boiling point, capillary action, surface tension, volatility and solubility.

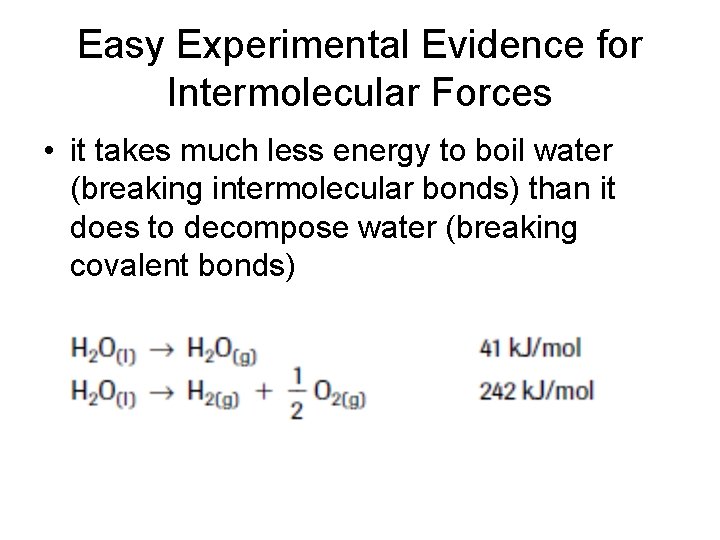
Easy Experimental Evidence for Intermolecular Forces • it takes much less energy to boil water (breaking intermolecular bonds) than it does to decompose water (breaking covalent bonds)

van der Waals and Hydrogen Bonding • Collectively some intermolecular forces are classified under the heading of van der Waals forces. – dipole-dipole – London dispersion forces – Hydrogen bonding was added later to explain the anomalies.

Dipole-dipole attractions • Occurs in POLAR MOLECULES • attractions between the negative end of one polar molecule and the positive end of another • it occurs as a result of the simultaneous attraction of a dipole by its surrounding dipoles • the strength of a dipole depends on the polarity of the molecule

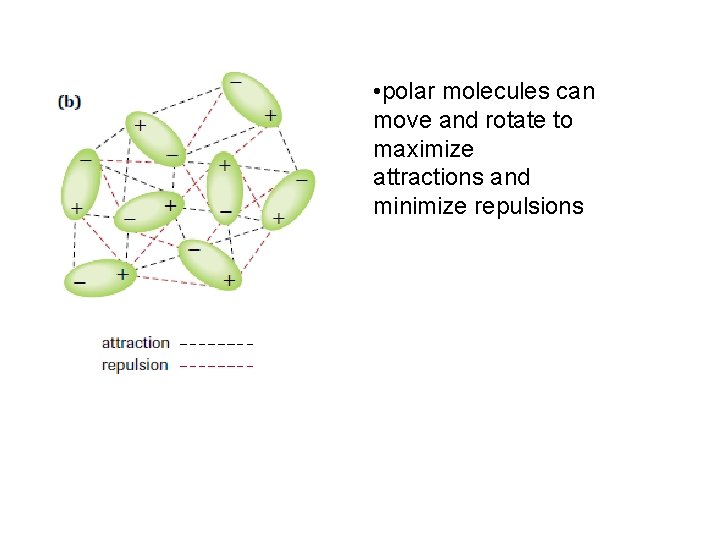
• polar molecules can move and rotate to maximize attractions and minimize repulsions

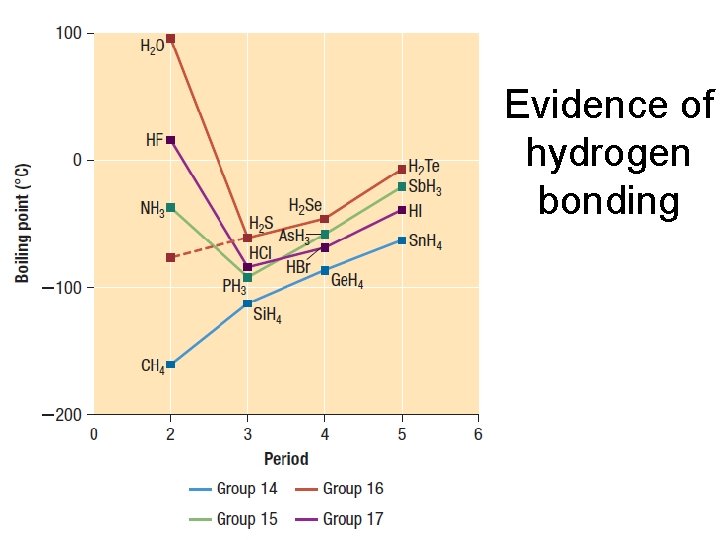
Evidence of hydrogen bonding

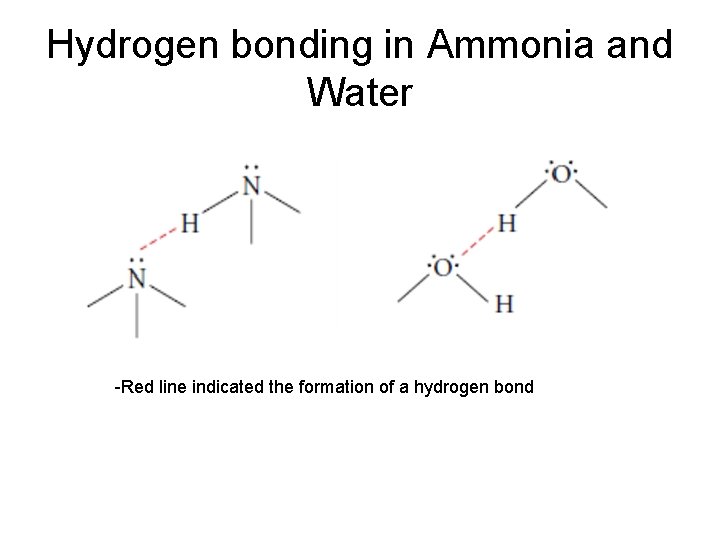
Hydrogen bonding • exists when hydrogen atoms are bonded to highly electronegative atoms like N, O, F • the hydrogen is simultaneously attracted to a pair of e- on the N, O, or F atom of an adjacent molecule • it’s like covalent bonding but instead of electrons being shared it is a proton • It is unique to these few atoms because of the strong electronegativity of N, O, F and hydrogen’s small size

Hydrogen bonding in Ammonia and Water -Red line indicated the formation of a hydrogen bond

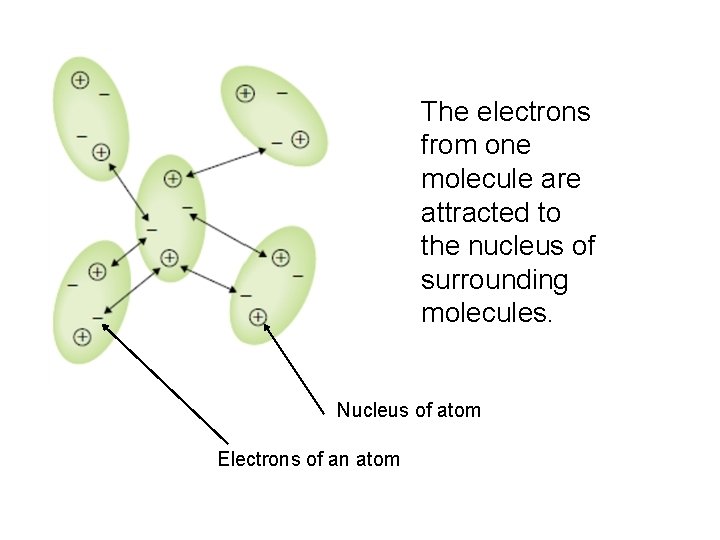
London Dispersion Forces • the simultaneous attraction of the electrons of one molecule by the positive nuclei in the surrounding molecules • it occurs in all molecules but is the dominant force in non-polar molecules • the strength is directly related to the number of electrons in a molecule

The electrons from one molecule are attracted to the nucleus of surrounding molecules. Nucleus of atom Electrons of an atom

Predicting Boiling Points with Dipole-Dipole & London Forces • isoelectric molecules (2 different molecules that have the same econfiguration) have approximately the same strength of London Forces • Example: bromine and iodine monochloride have 70 electrons per molecule they would experience the same level of London forces

• the more polar the molecule, the stronger the dipole-dipole force • Example: - bromine does not contain a polar bond because the electrons are shared equally; therefore it does not contain dipole-dipole forces - Iodine monochloride does have a slight polar bond 0. 5; therefore it does contain dipole-dipole forces

• The greater the number of e- per molecule, the stronger the London force and therefore, the higher the boiling point • Example: Which has the highest melting point: methane (CH 4), ethane (C 2 H 6), propane (C 3 H 8), or butane (C 4 H 10) – butane

• You cannot perform a comparison of London forces to dipole-dipole forces because they are not the same thing!

Surface Tension • Surface-tension is the resistance of liquid to increase its surface areas • It is caused by the attraction between the liquid's interior molecules to each other. If surface area is to increase molecules from the interior must migrate toward the surface which requires energy and is resisted by the liquid

Capillary Action at Work! Capillary action is the spontaneous rising of a liquid in a narrow tube. It is a result of cohesion and adhesion. The xylem of plants uses capillary action to transport water up to the leaves in tress. I

Viscosity • The measure of a liquid’s resistance to flow • If a liquid experiences a high level of intermolecular forces it will more slowly than one with a lower level of intermolecular forces.

Homework • Pg 247 #1 -8