Intermolecular Forces 4 7 Intermolecular Forces Intramolecular and


















- Slides: 26

Intermolecular Forces 4. 7 Intermolecular Forces

Intramolecular and Intermolecular Forces covalent bond and ionic bond: the forces that holds atom together making molecules. These are intramolecular forces. There also forces that cause molecules to attract each other. These are called intermolecular forces.

Intermolecular Forces Salt (Na. Cl) is a solid because of the strong electrostatic attraction of the Na+ and the Cl– ions: the ionic bond Q: Why is Cl 2 a gas, Br 2 a liquid and I 2 a solid? A: Intermolecular forces Q: Why is molasses “thick” while water has low viscosity? Same answer. Q: Why is it possible to float a needle on water?

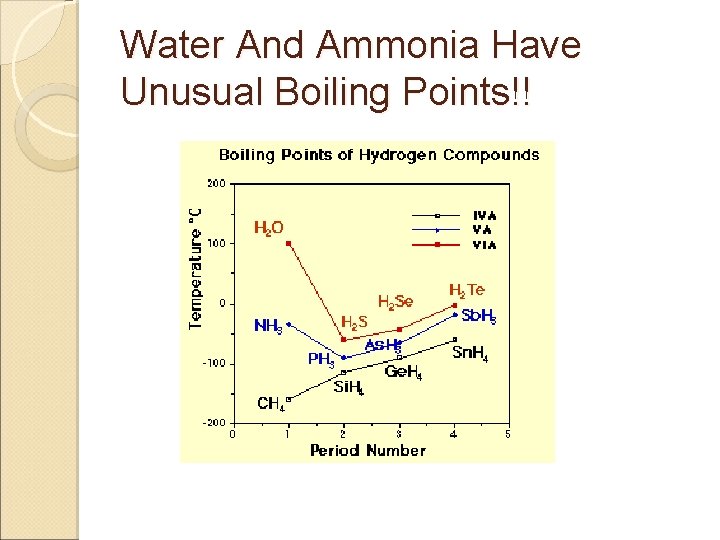
Intermolecular Forces (cont’d) Q: Why is water a liquid but H 2 S is a gas at 25ºC? Q: Why are real gases not ideal? A: van der Waals forces


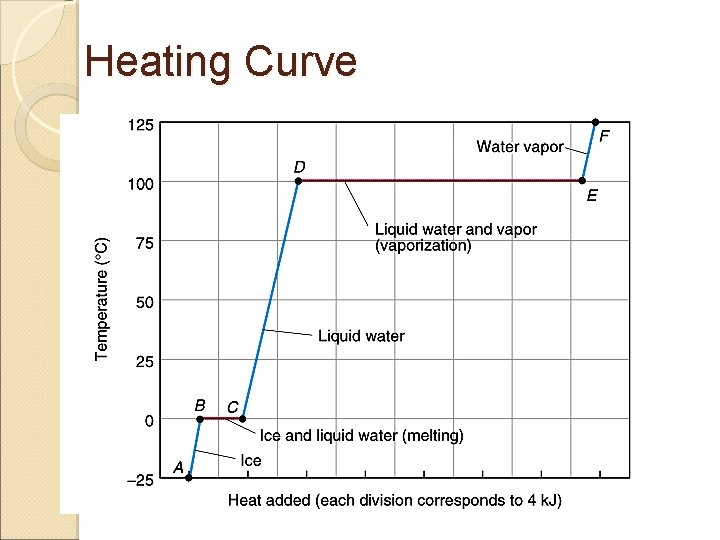
Heating Curve

Intermolecular Forces Much weaker than chemical bonds. Covalent bonds 200 k. J/mol and more. Intermolecular forces less than 50 k. J/mol When a liquid vaporizes, the intermolecular forces must be overcome. But no covalent bonds are broken.

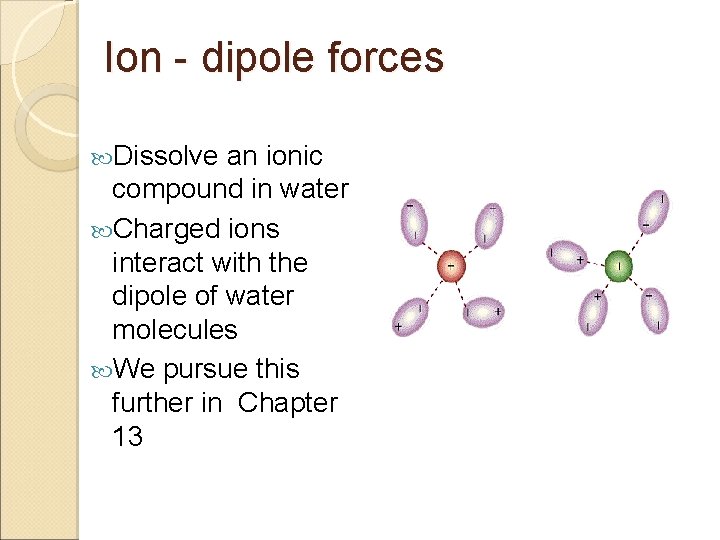
Ion - dipole forces Dissolve an ionic compound in water Charged ions interact with the dipole of water molecules We pursue this further in Chapter 13

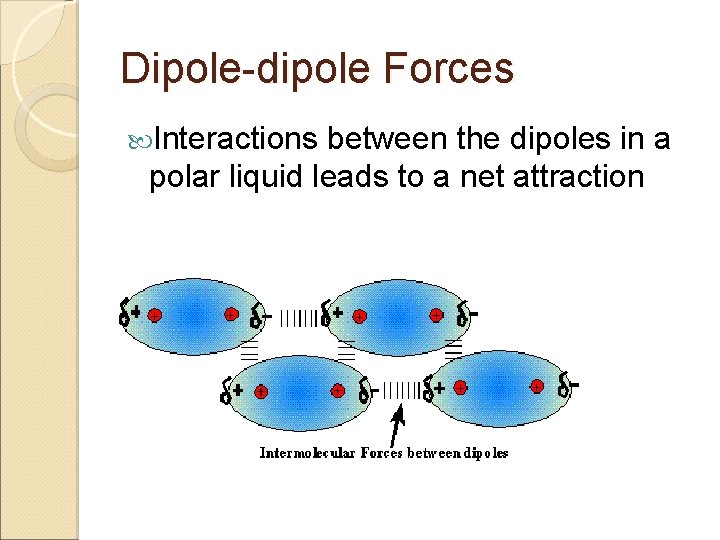
Dipole-dipole Forces Interactions between the dipoles in a polar liquid leads to a net attraction

Dipole - Induced dipole force In a mixture of two liquids were one is polar and the other is not, the dipole of the polar molecule can induce a dipole in the other. The energy of this force depends on the polarizabilty of the non-polar molecule. Larger molecules are more polarizable

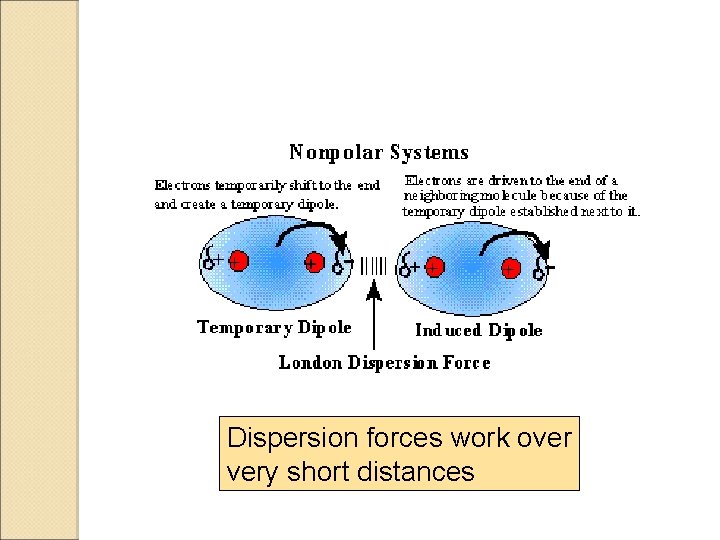
London Dispersion Forces How do we account for the fact that non-polar gases can be liquefied and solidified? Fritz London proposed instant dipoles. These are dipoles that result from the random movement of the electron cloud.

Dispersion forces work over very short distances

Polarizability The ease with which a dipole can be induced depends on the polarizability of the molecule Large molecules are more polarizable - easier to distort the electron cloud

Polarizability and Boiling Points Boiling points (K) of halogens F 2 Cl 2 Br 2 I 2 85. 1 238. 6 332. 0 457. 6 Boiling points of Noble gases He Ne Ar Kr 4. 6 27. 3 87. 5 120. 9 166. 1 Xe

Water And Ammonia Have Unusual Boiling Points!!

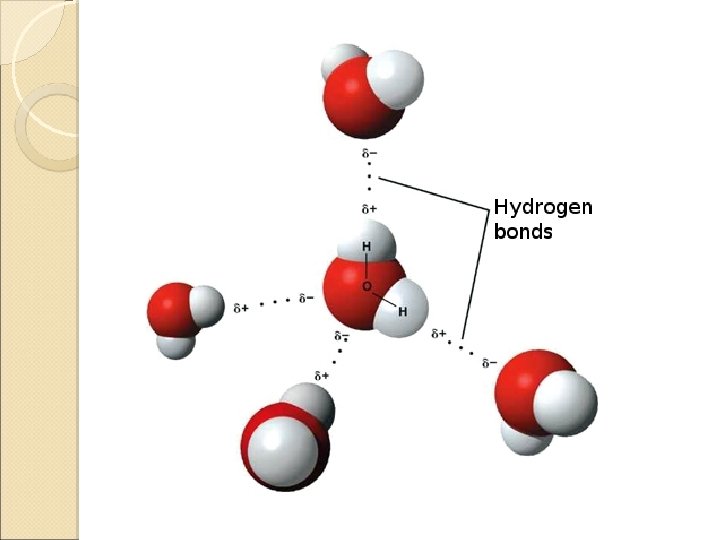
The Hydrogen Bond A special dipole-dipole intermolecular attraction ◦ H in a polar bond (H-F, H-O or H-N) ◦ an unshared electron pair on a nearby electronegative ion or atom (generally F, O or N) Hydrogen bonds (4 to 25 k. J/mol, or larger) are weaker than covalent bonds but stronger than most dipole-dipole or dispersion forces.


Molecules with H bonding HF (can behave as if it were H 2 F 2) NH 3 H 2 O (ice is less dense than liquid at 0ºC) alcohols (e. g. CH 3 OH) amines (e. g CH 3 NH 2) carboxylic acids (e. g. CH 3 COOH)

Summary

Liquids - viscosity Viscosity (resistance to flow) Molecules that have strong intermolecular forces cannot move very easily - more viscous Viscosity decreases at higher temperatures. The kinetic energy overcomes the intermolecular forces

Liquids - surface tension Surface tension: the energy that must be expended to increase the surface of a liquid

Surface tension Liquids with strong intermolecular bonds have high surface tensions Water: strong H-bonds, so high surface tension, 7. 29 10– 2 J/m 2 Mercury: atoms held by metallic bonds, so even higher surface tension, 4. 6 10 – 1 J/m 2

Wetting & capillary action Water wets clean glass (spreads out) but beads on a waxy surface Water climbs up a capillary tube Mercury does not wet glass Mercury level is depressed in capillary tube

To wet or not to wet? Competition between two tendencies Cohesive forces; intermolecular forces that bind similar molecules together. Keeps liquid as a bead Adhesive forces: Intermolecular forces between molecules of a liquid and those of a surface. Makes liquid spread out

Are we all wet? ? Water on clean glass adhesive forces > cohesive forces explains the upward meniscus of water Water on polished table cohesive forces win because H 2 O molecules are not attracted to wax Mercury on glass - cohesive forces win because Hg molecules are not attracted to glass Explains depression in capillary tube and downward meniscus

Volatile A liquid in an open container will evaporate As vapour moves away, the liquid releases more molecules into the vapour phase to try to build up to the correct vapour pressure Liquids with high vapour pressure evaporate more quickly - they are volatile