Intermolecular Forces 2 Mr Tsigaridis Intermolecular Forces If




- Slides: 14

Intermolecular Forces 2 Mr. Tsigaridis

Intermolecular Forces If you recall from the last lesson, we discussed how intermolecular forces play a very important role in determining the physical properties of molecules When there is an uneven distribution of electrons in the electron clouds of molecules they create a type of polarization where one end of the molecule becomes partially positive and the other becomes partially negative

Intermolecular Forces This type of electrostatic difference is responsible for the dipole forces that were discussed These forces however can be extended to include ions as well

Ion – Dipole Forces When a dipole exists within a molecule, the forces of attraction and repulsion do not only exist between another polar molecule, but they can exist between ions as well Another physical property becomes elucidated with this interaction, the dissolution of solutes in solvents

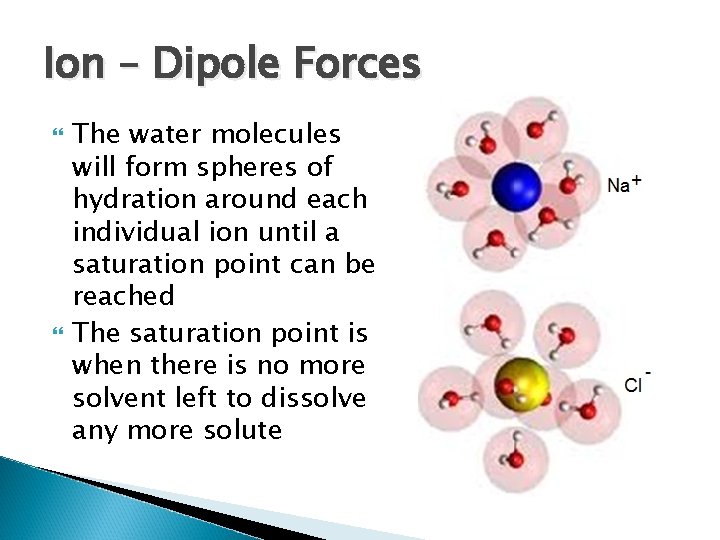
Ion – Dipole Forces The reason salt and sugar, which are ionic solids, can dissolve in solvents like water is because of this ion – dipole force An Ion, like Na+ or Cl-, will be attracted to the - or + ends of the water molecules respectively This occurs because the forces that are exerted by the ions on the polar molecules and vice versa are enough to overcome the ion-ion attraction of the Na+ and Cl- ions

Ion – Dipole Forces The water molecules will form spheres of hydration around each individual ion until a saturation point can be reached The saturation point is when there is no more solvent left to dissolve any more solute

Induced Dipole Forces The basis of these forces is charging by induction It is a forced dipole moment that is caused by the distortion of the electron cloud when a charged particle passes by the molecule The induced charged can happen in one of two ways

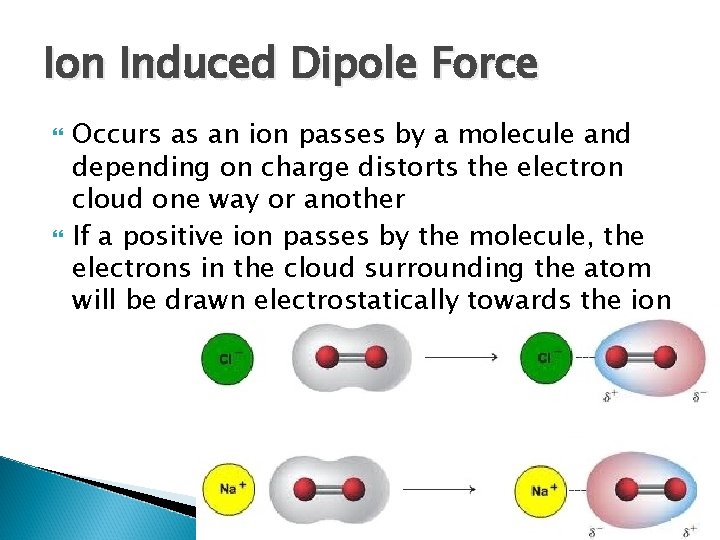
Ion Induced Dipole Force Occurs as an ion passes by a molecule and depending on charge distorts the electron cloud one way or another If a positive ion passes by the molecule, the electrons in the cloud surrounding the atom will be drawn electrostatically towards the ion

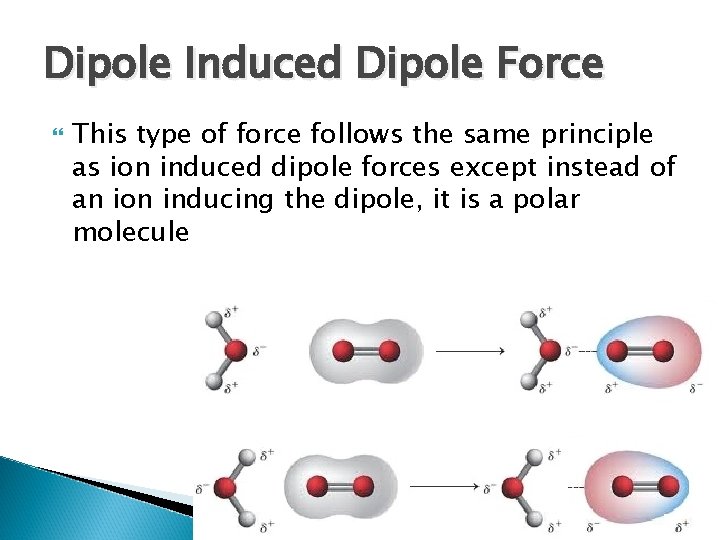
Dipole Induced Dipole Force This type of force follows the same principle as ion induced dipole forces except instead of an ion inducing the dipole, it is a polar molecule

Intermolecular Forces One observation that you may have made is the fact that the intermolecular forces we have dealt with thus far have all dealt with polar molecules or molecules becoming polar There is only a single intermolecular force that deals strictly with non-polar molecules It occurs in all molecules but because its force is the weakest, it is observed in molecules that are non polar

London Dispersion Forces The reason that this force is of particular interest to non-polar molecules is that it is the only force associated with non-polar molecules Bond pairs of electrons are in constant motion within the bond This motion cause momentary dipoles from the uneven distribution of electrons within the bond Conceptually this is very difficult to imagine

London Dispersion Forces It produces an instant where one bond in the molecule becomes polarized At the same instant, the neighbouring molecule may be going through an equal but opposite momentary dipole creating an electrostatic attraction between the two molecules This produces a dipole moment

London Dispersion Forces This affect can produce more than one dipole moment in a molecule at a time, effectively called a multipole The rule here is that the larger the molecule the greater the total number of bonds and therefore the greater the London Dispersion force that can exist at an instant between molecules

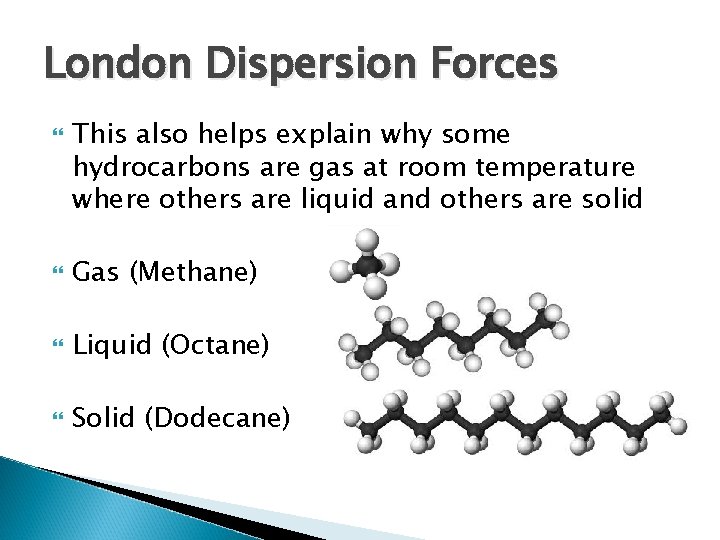
London Dispersion Forces This also helps explain why some hydrocarbons are gas at room temperature where others are liquid and others are solid Gas (Methane) Liquid (Octane) Solid (Dodecane)