Intermolecular Forces 1 Mr Tsigaridis Intermolecular Forces H



- Slides: 10

Intermolecular Forces 1 Mr. Tsigaridis

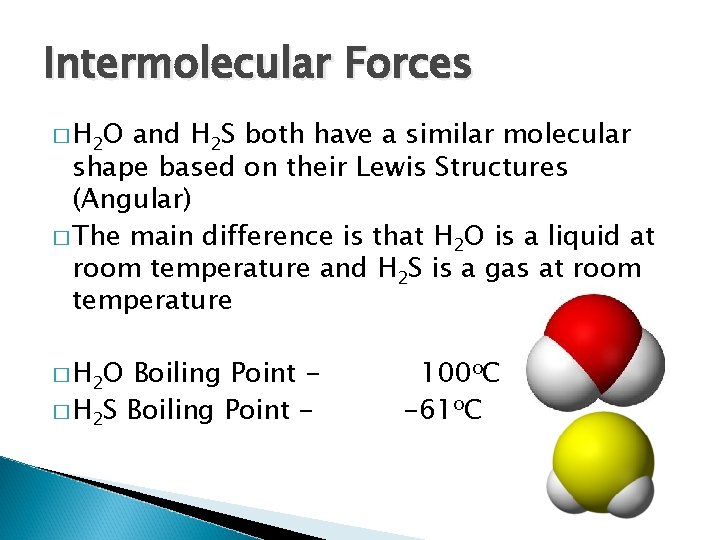
Intermolecular Forces � H 2 O and H 2 S both have a similar molecular shape based on their Lewis Structures (Angular) � The main difference is that H 2 O is a liquid at room temperature and H 2 S is a gas at room temperature � H 2 O Boiling Point � H 2 S Boiling Point - 100 o. C -61 o. C

Why the Difference? � Everything that you have learned about bonding has been about intramolecular forces (within molecules) � These are the bonds that build molecules and are responsible for the chemical properties of that compound � The physical properties are influenced by the intermolecular forces (between molecules)

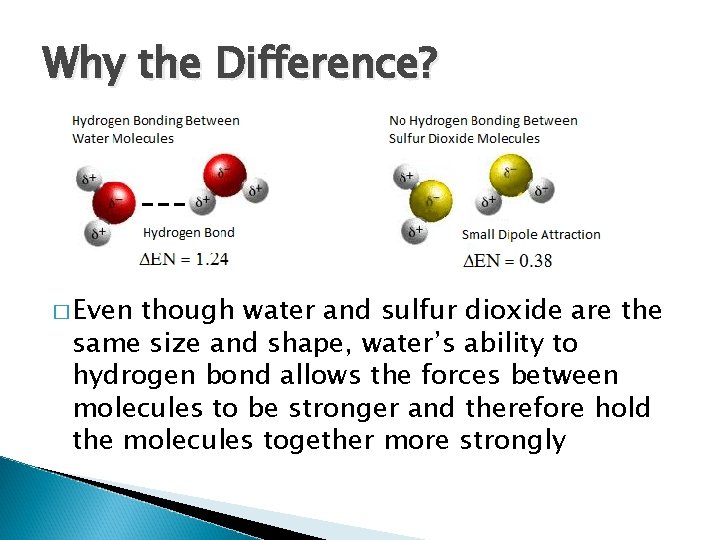
Why the Difference? � Even though water and sulfur dioxide are the same size and shape, water’s ability to hydrogen bond allows the forces between molecules to be stronger and therefore hold the molecules together more strongly

Johannes Van Der Walls � These types of interactions were extensively studied the Dutch theoretical physicist Johannes Van Der Walls � Intermolecular forces in fact are usually called Van Der Walls forces � These forces are responsible for the physical properties of compounds and molecules

Van Der Walls Forces � They are classified into 5 characteristic groups each one with its own unique characteristic and effect �Dipole - Dipole Forces �Hydrogen Bonding �Ion – Dipole Forces �Induced Dipole Forces �Dipole Induced Dipole Forces �Ion Induced Dipole Forces �London Dispersion Forces

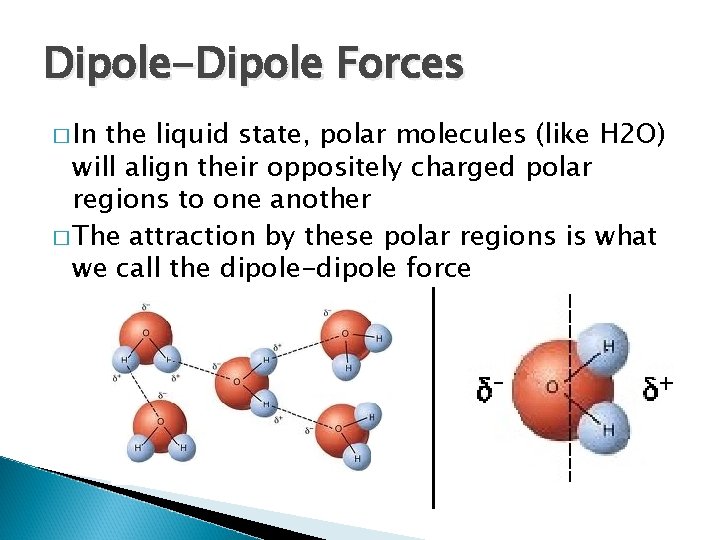
Dipole-Dipole Forces � In the liquid state, polar molecules (like H 2 O) will align their oppositely charged polar regions to one another � The attraction by these polar regions is what we call the dipole-dipole force

Hydrogen Bonding � Hydrogen bonding is a special type of dipole force that involves a hydrogen being sandwiched in between two very electronegative elements � Since there is a large difference in electronegativity between the element and the hydrogen, a very strong dipole interaction will exist

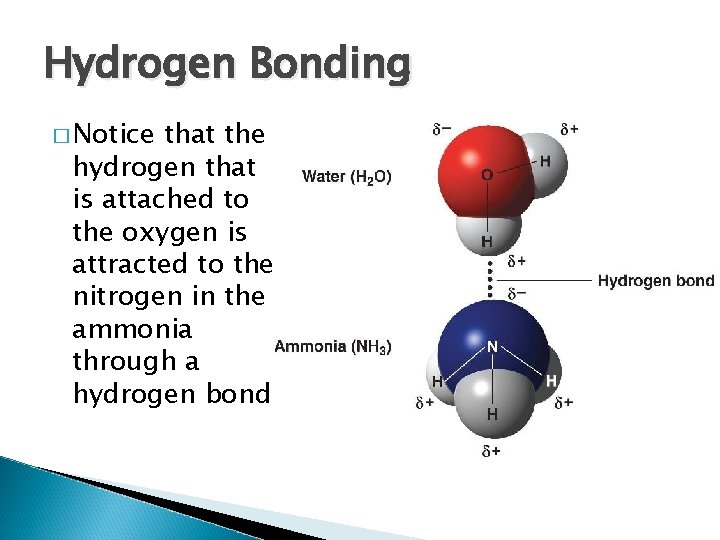
Hydrogen Bonding � Notice that the hydrogen that is attached to the oxygen is attracted to the nitrogen in the ammonia through a hydrogen bond

FONCl. Br � FONCl. Br is a Mnemonic device that can be used to help determine �F – Fluorine �O Oxygen �N Nitrogen � Cl Chlorine � Br Bromine � If the hydrogen is sandwiched between any of these two elements, it has the ability to form hydrogen bonds