Intermolecular Forces Intermolecular forces The attraction and repulsion







- Slides: 14

Intermolecular Forces

Intermolecular forces • The attraction and repulsion forces between molecules – (intramolecular forces are the forces within the molecule – bonding) • Cotton vs plastic raincoat • Broken down into three types

Van der Waals Forces • Intermolecular forces • Forces holding molecules in order • But very weak compared to the covalent bonding between elements • Energy to boil water vs the amount to decompose water

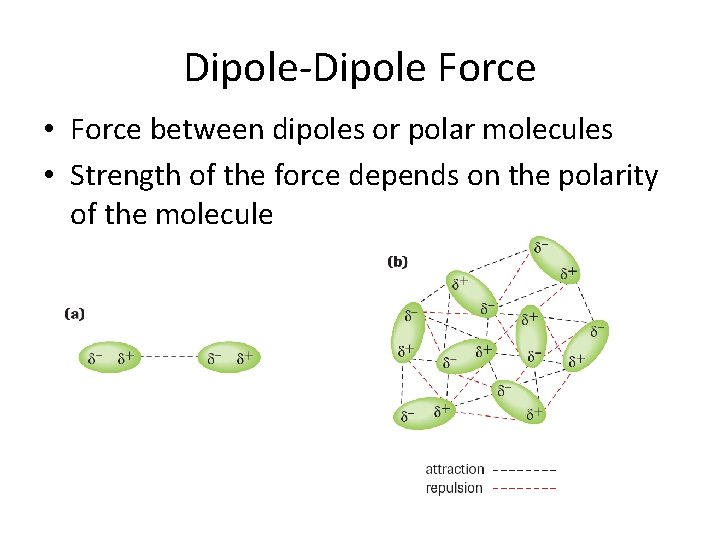
Dipole-Dipole Force • Force between dipoles or polar molecules • Strength of the force depends on the polarity of the molecule

London Force • Force between nonpolar molecules – While the overall molecule does not have a dipole the electrons moving within the molecule will create a momentary dipole – The more electrons the molecule has the higher the London Force

Predicting boiling points • Can use the number of electrons as a prediction of the boiling point. • This is because the intermolecular forces are related to how difficult the molecules are to separate

Isoelectronic Molecules • Iso – meaning the same • electronic – electrons • These molecules would have the same number of electrons and the London forces will be similar • However this does not mean that the boiling points would be the same. • Remember the boiling point depends on the London forces AND the dipole-dipole

Summary • More polar -> stronger dipole-dipole -> higher boiling point • More electrons -> larger London Forces -> higher boiling point • Using this knowledge to predict relative boiling points for certain cases

Lab Exercise 3. 4

Hydrogen bonding • Hydrogen (proton) is shared with a lone pair. • Hydrogen needs to be bonded with an atom with a high electronegativity • Need to have at least one lone pair on the atom bonded to Hydrogen to give hydrogen bonding to another molecule • Three possible structures: HF (g), anything with – OH and –NH. • Example is sugar C 12 H 22 O 11(s), or better written as C 12 H 14 O 3(OH)8, we can see there are 8 –OH groups to hydrogen bond

Examples • DNA and proteins • Water, ice – Reason for different densities

Physical Properties of Liquids • Intermolecular forces (IMF) contribute to various • Surface tension – in the liquid IMF are in all directions but at the surface it is only away from the surface. • Cohesion – attraction between like molecules • Adhesion – attraction between unlike molecules

Properties Cont’d • How easily the liquid evaporates – Weak forces – easy to evaporate – volatility

Summary • Intermolecular forces are the attraction and repulsion of positive and negative charges • All molecules have London forces – with momentary diploes • Polar molecules have dipole-dipole forces • Hydrogen bonding occurs when a hydrogen is attracted to the lone pair from an adjacent molecule • IMF affect many various physical properties