Chapter 6 The Shape of Molecules Why Care









- Slides: 24

Chapter 6 –The Shape of Molecules

Why Care about Shapes of Molecules • Dot diagrams show atoms are connected in a molecule and how valence electrons are arranged – Problem: not 3 D

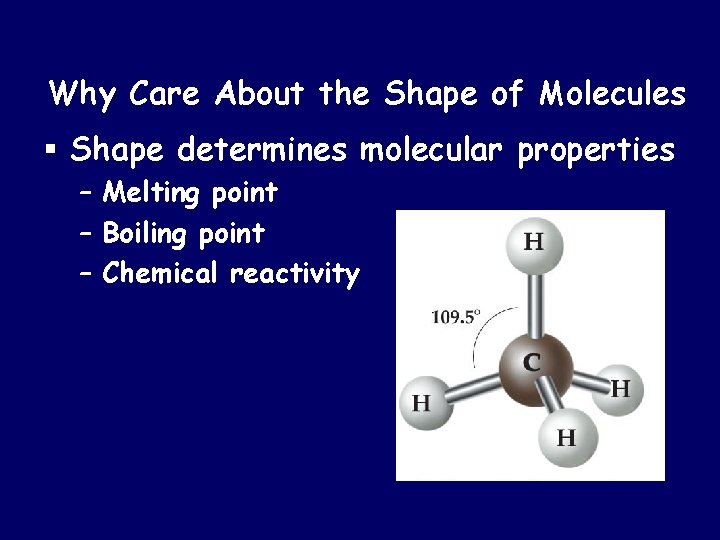
Why Care About the Shape of Molecules § Shape determines molecular properties – Melting point – Boiling point – Chemical reactivity

VSEPR Model (Valence Shell Electron Pair Repulsion) Theory that determines how molecules arrive at a three dimensional shape § Repulsion between the sets of valence-level electrons surrounding an atom causes these sets to be oriented as far apart as possible § All electrons are negatively charged. § Like charges repel. §

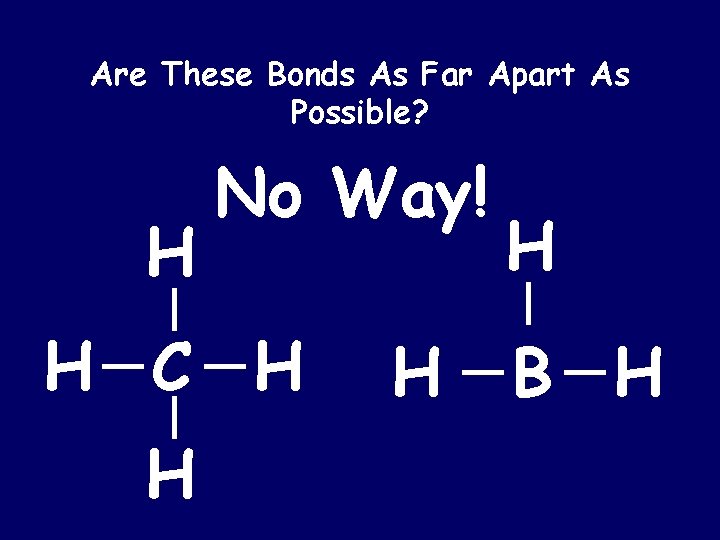
Are These Bonds As Far Apart As Possible? H No Way! H C H H B H

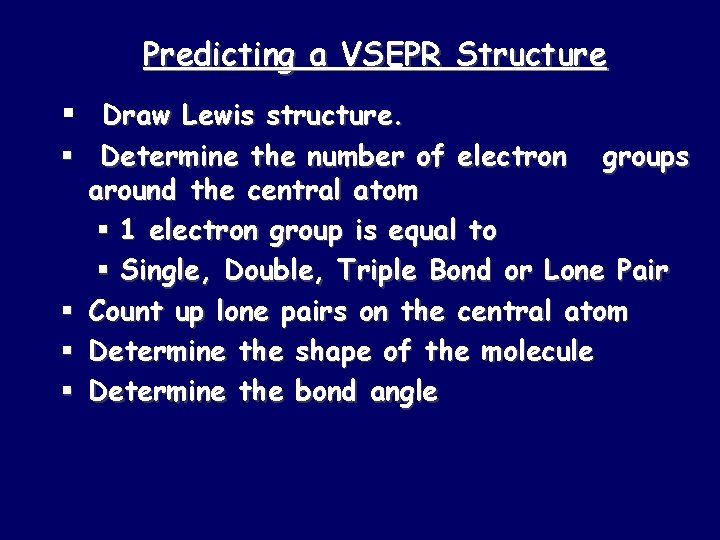
Predicting a VSEPR Structure § Draw Lewis structure. § Determine the number of electron groups around the central atom § 1 electron group is equal to § Single, Double, Triple Bond or Lone Pair § Count up lone pairs on the central atom § Determine the shape of the molecule § Determine the bond angle

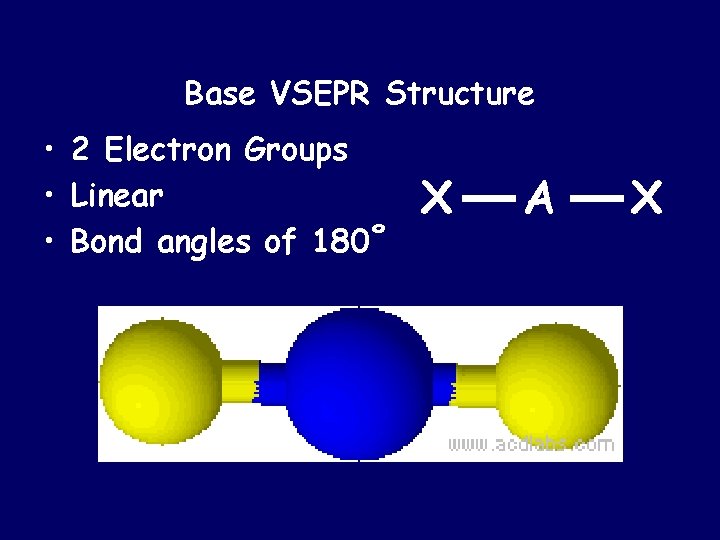
Base VSEPR Structure • 2 Electron Groups • Linear • Bond angles of 180˚ X A X

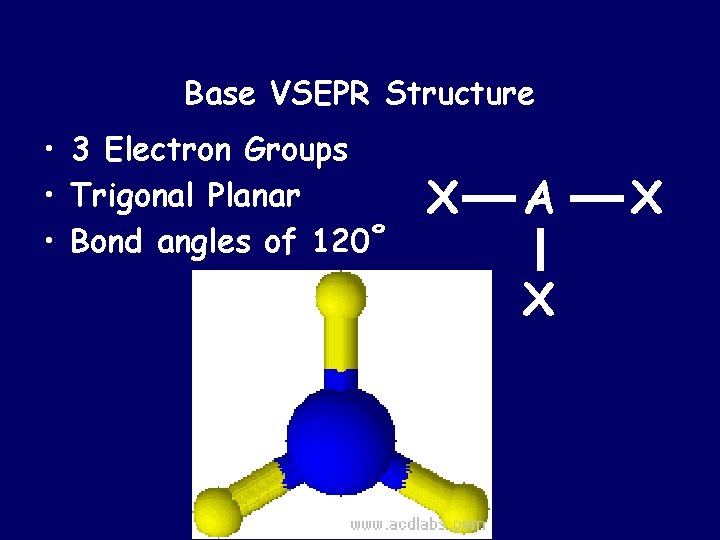
Base VSEPR Structure • 3 Electron Groups • Trigonal Planar • Bond angles of 120˚ X A X X

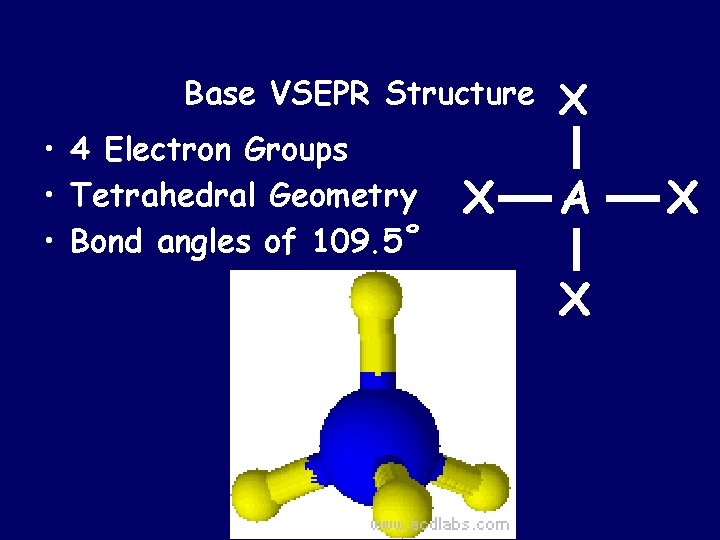
Base VSEPR Structure • 4 Electron Groups • Tetrahedral Geometry • Bond angles of 109. 5˚ X X A X X

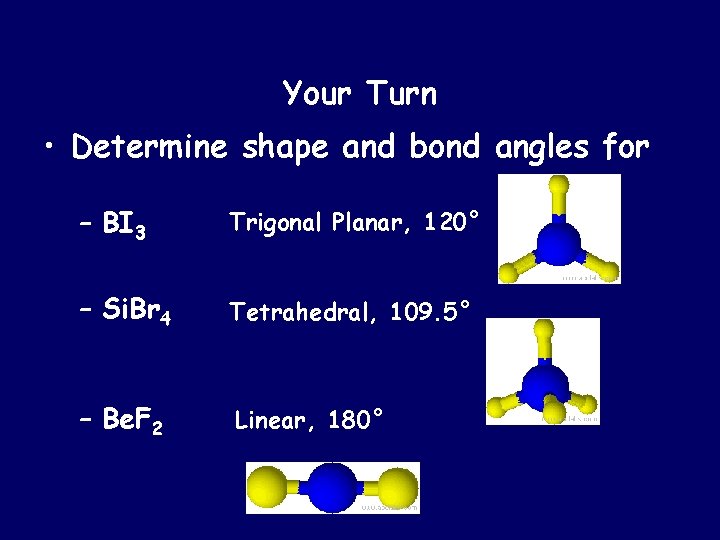
Your Turn • Determine shape and bond angles for – BI 3 Trigonal Planar, 120° – Si. Br 4 Tetrahedral, 109. 5° – Be. F 2 Linear, 180°

Lone Pairs of Electrons • Lone Pairs of Electrons make the bond angle a little bit smaller • More repulsion

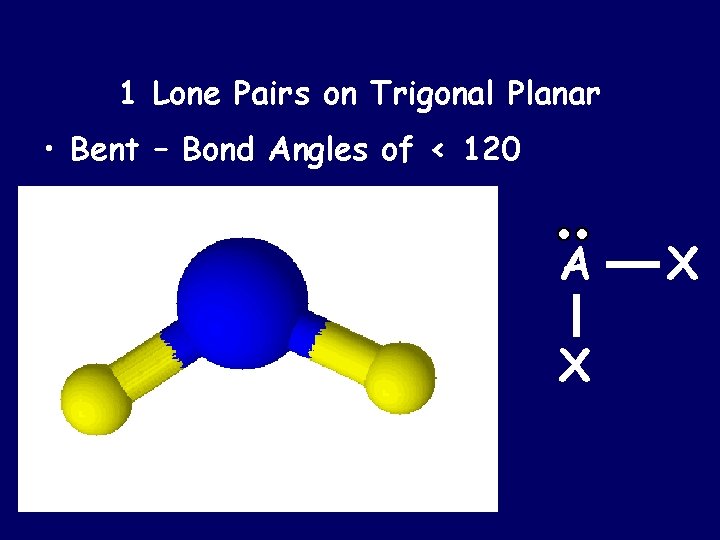
1 Lone Pairs on Trigonal Planar • Bent – Bond Angles of < 120 A X X

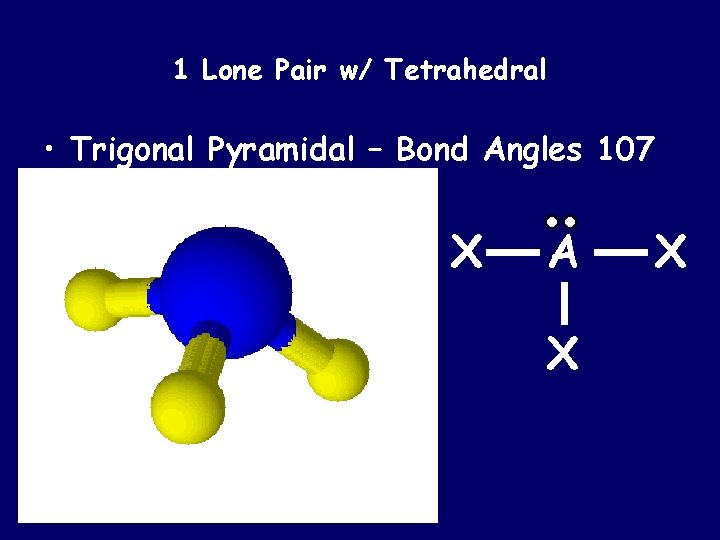
1 Lone Pair w/ Tetrahedral • Trigonal Pyramidal – Bond Angles 107 X A X X

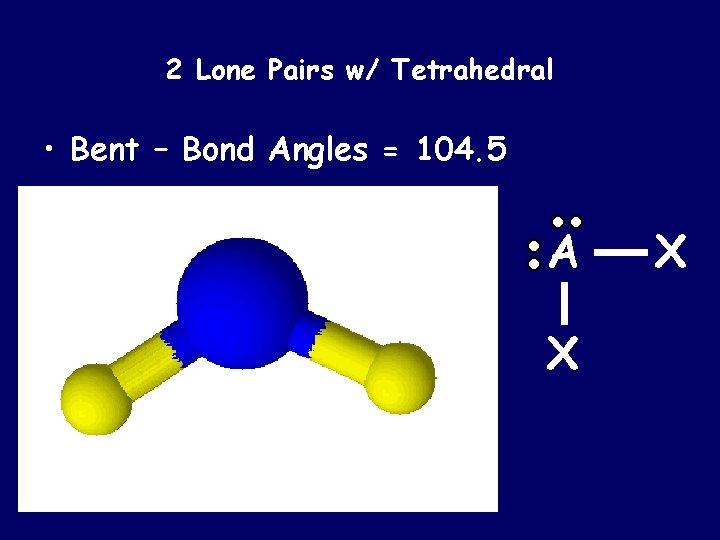
2 Lone Pairs w/ Tetrahedral • Bent – Bond Angles = 104. 5 A X X

Your Turn • Determine shape and bond angles for – PBr 3 Trigonal Pyrimidal, 107° – O 3 Bent, <120° – Se. O 2 Bent, <120°

Electronegativity • The ability of an atom in a molecule to attract shared electrons to itself. • • – Developed by Linus Pauling Values range 0. 7 to 4. 0 Fluorine = 4. 0 Francium and Cesium = 0. 7 What is the periodic trend? – Top to Bottom – Decrease – Left to Right Increase

Molecular Polarity • A polar molecule is one that has a partially positive and partially negative side • Molecules are Always nonpolar if they are one of the 3 base shapes w/ the same atom at the ends • Molecules are Always polar if their bond dipoles do not cancel out • Molecules are polar if they do not have the same atoms at the end, or bent, or pyramidal

Intermolecular Forces • Forces of attraction between molecules • Intermolecular Forces are responsible for melting and boiling points • Strong IMF raise BP and MP • Three different types of IMF • London Forces, Dipole-Dipole, and Hydrogen Bonding

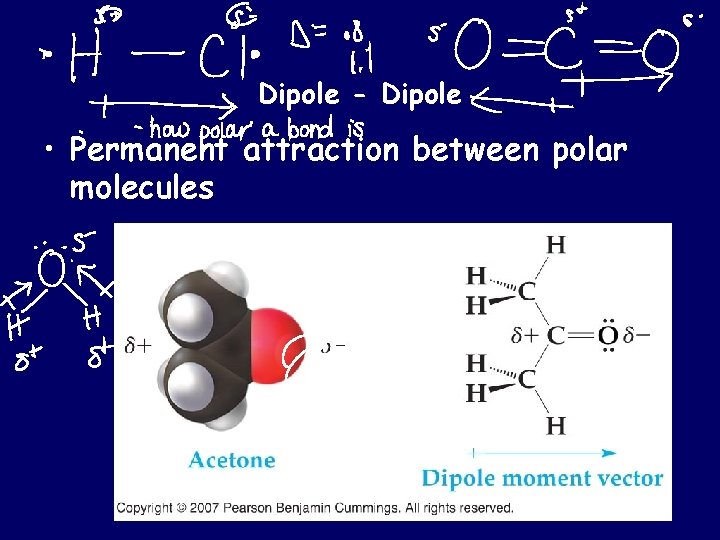
Dipole - Dipole • Permanent attraction between polar molecules

Hydrogen Bonding • Extra Strong attraction between polar molecules where H is bonded to F, N, O


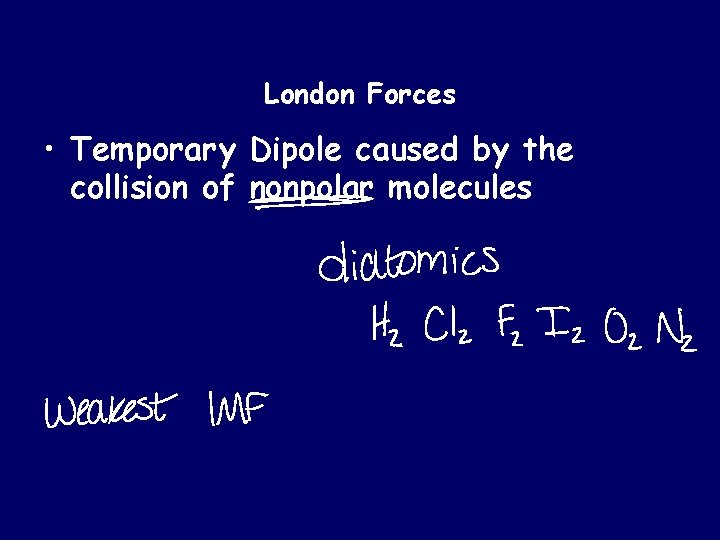
London Forces • Temporary Dipole caused by the collision of nonpolar molecules


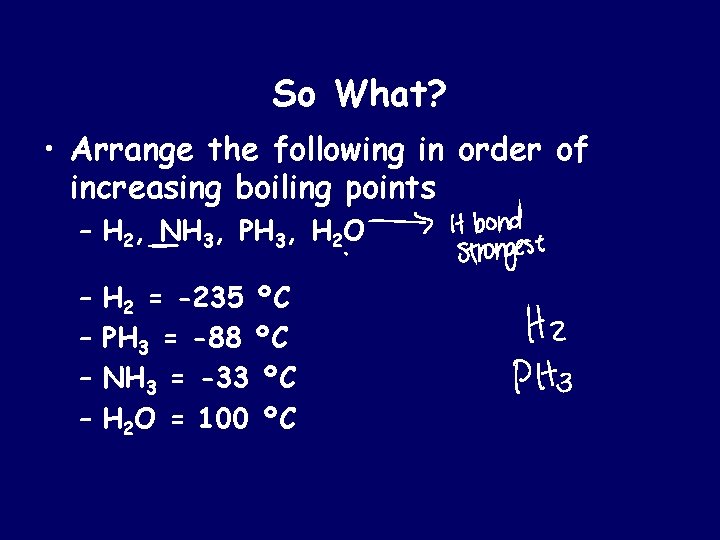
So What? • Arrange the following in order of increasing boiling points – H 2, NH 3, PH 3, H 2 O – – H 2 = -235 ºC PH 3 = -88 ºC NH 3 = -33 ºC H 2 O = 100 ºC