Intermolecular Forces Chapter 12 pg 147 168 Interatomic





















- Slides: 36

Intermolecular Forces Chapter 12, pg. 147 -168

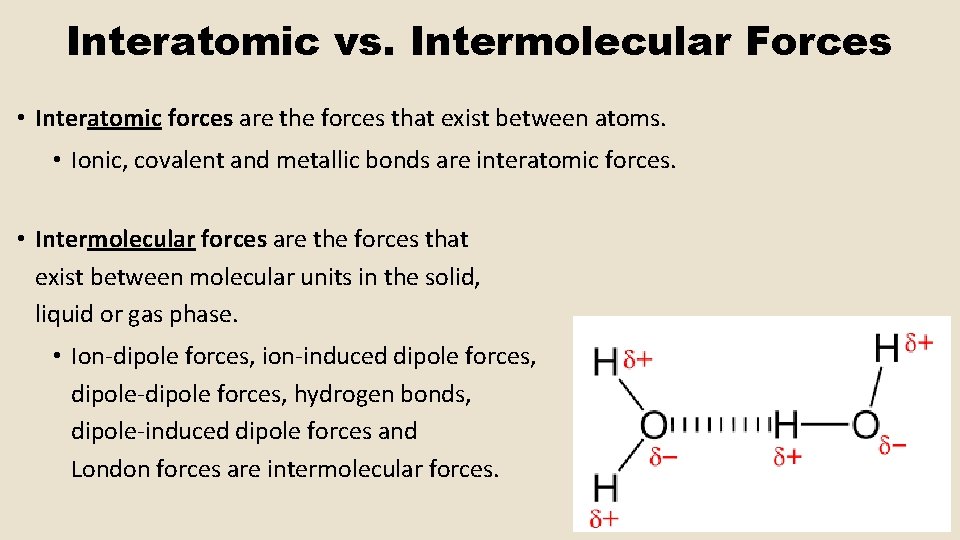
Interatomic vs. Intermolecular Forces • Interatomic forces are the forces that exist between atoms. • Ionic, covalent and metallic bonds are interatomic forces. • Intermolecular forces are the forces that exist between molecular units in the solid, liquid or gas phase. • Ion-dipole forces, ion-induced dipole forces, dipole-dipole forces, hydrogen bonds, dipole-induced dipole forces and London forces are intermolecular forces.

Ion-Dipole Forces • An ion-dipole force is the force of attraction between an ion and a polar molecule (i. e. a dipole). • The strength of the ion-dipole force is determined by: 1. Size of the charge on the ion. 2. Strength of the dipole. 3. Distance between the centre of the ion and the midpoint of the dipole.

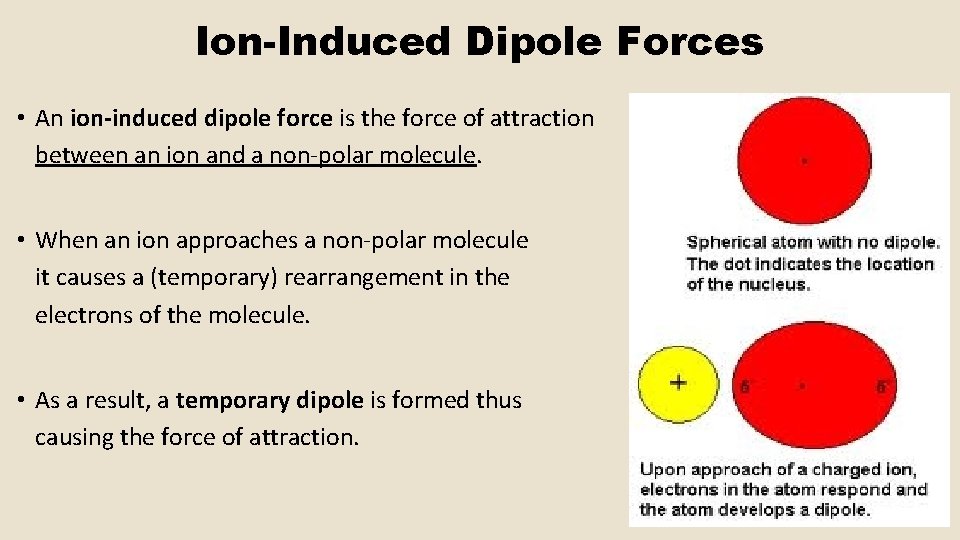
Ion-Induced Dipole Forces • An ion-induced dipole force is the force of attraction between an ion and a non-polar molecule. • When an ion approaches a non-polar molecule it causes a (temporary) rearrangement in the electrons of the molecule. • As a result, a temporary dipole is formed thus causing the force of attraction.

Van der Waals Forces • Van der Waals forces are a category of intermolecular forces which occur between polar and/or non-polar molecules. • There are 3 types of Van der Waals forces (to be discussed on future slides). • Van Der Waals forces are weaker than ion-dipole and ion-induced dipole forces*. • This is because the charge on any ion is larger than the partial charge on a polar molecule (or an induced partial charge on a non-polar molecule).

Dipole-Dipole Forces • A dipole-dipole force is the force of attraction between two polar molecules. • The strength of a dipole-dipole force is determined by the polarity of the bonds within the molecule, i. e. the more polar the bonds, the stronger the IMF. • All dipole-dipole forces are relatively weak forces of attraction with the exception of one type of dipole-dipole force known as hydrogen bonds.

Hydrogen Bonds • A hydrogen bond is a force of attraction between a hydrogen atom bonded to oxygen, nitrogen or fluorine in a molecule and the lone pair of electrons on the oxygen, nitrogen or fluorine atom bonded to hydrogen in an adjacent molecule. • Hydrogen bonds are one of the strongest intermolecular forces (IMF) known but they are still weaker than interatomic forces.

Dipole-Induced Dipole Forces • A dipole-induced dipole force is a force of attraction between a polar and a non-polar molecule. • When a dipole approaches a non-polar molecule it causes a (temporary) rearrangement in the electrons of the molecule. • As a result, a temporary dipole is formed thus causing the force of attraction.

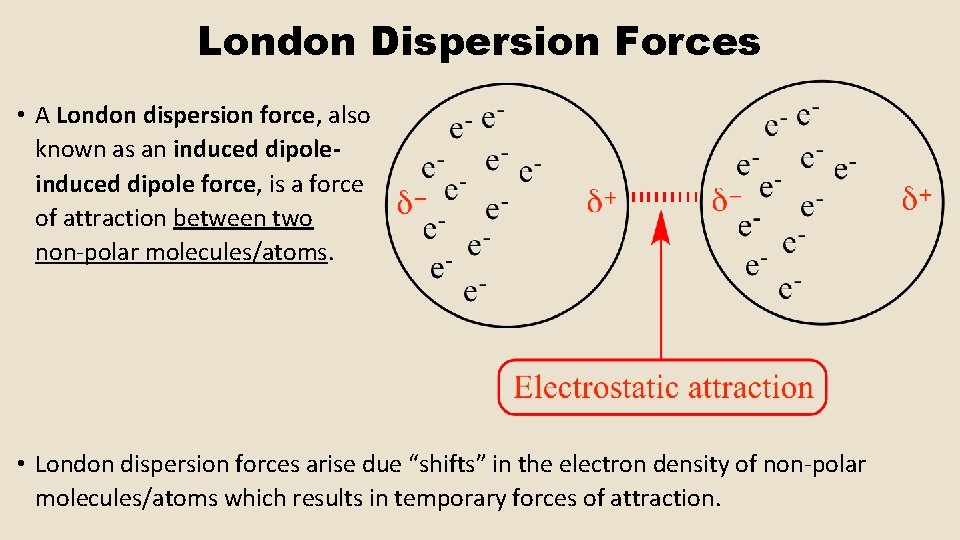
London Dispersion Forces • A London dispersion force, also known as an induced dipole force, is a force of attraction between two non-polar molecules/atoms. • London dispersion forces arise due “shifts” in the electron density of non-polar molecules/atoms which results in temporary forces of attraction.

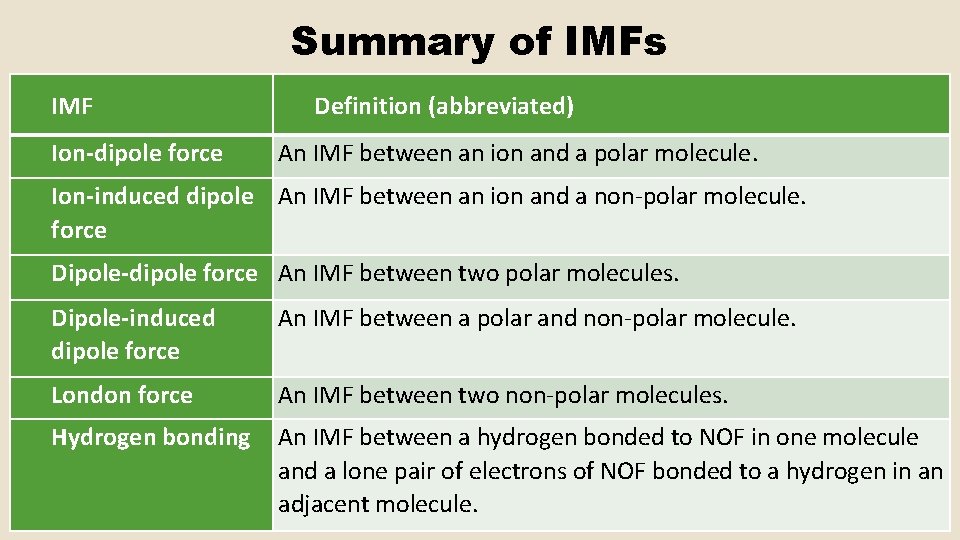
Summary of IMFs IMF Ion-dipole force Definition (abbreviated) An IMF between an ion and a polar molecule. Ion-induced dipole An IMF between an ion and a non-polar molecule. force Dipole-dipole force An IMF between two polar molecules. Dipole-induced dipole force An IMF between a polar and non-polar molecule. London force An IMF between two non-polar molecules. Hydrogen bonding An IMF between a hydrogen bonded to NOF in one molecule and a lone pair of electrons of NOF bonded to a hydrogen in an adjacent molecule.

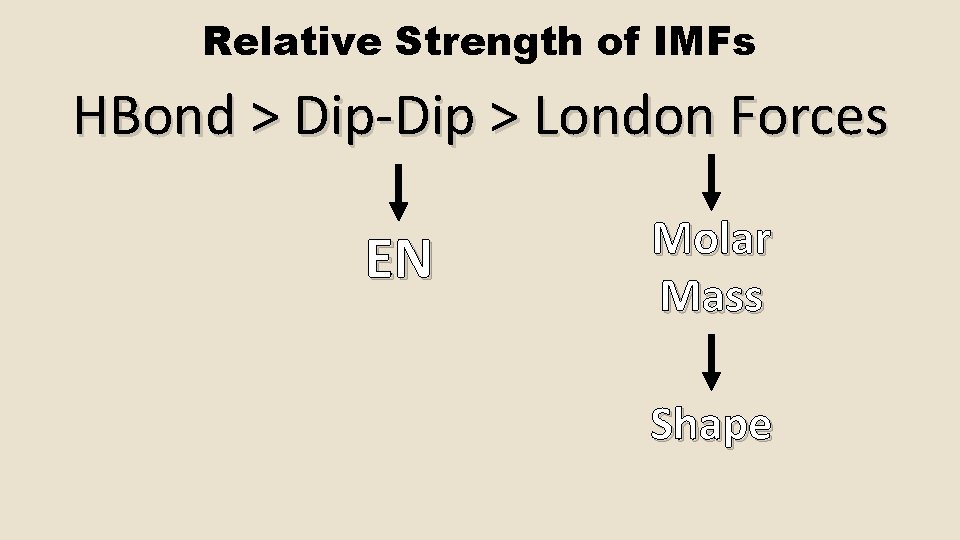
Relative Strength of IMFs HBond > Dip-Dip > London Forces EN Molar Mass Shape

Class Exercise •

Physical Properties & IMFs 1. Phase of Matter and Melting & Boiling Points 2. Vapour Pressure and Volatility 3. Solubility 4. Surface Tension 5. Capillarity 6. Viscosity 7. Thermal Expansion 8. Thermal Conductivity 9. Density 10. Chemistry of Water

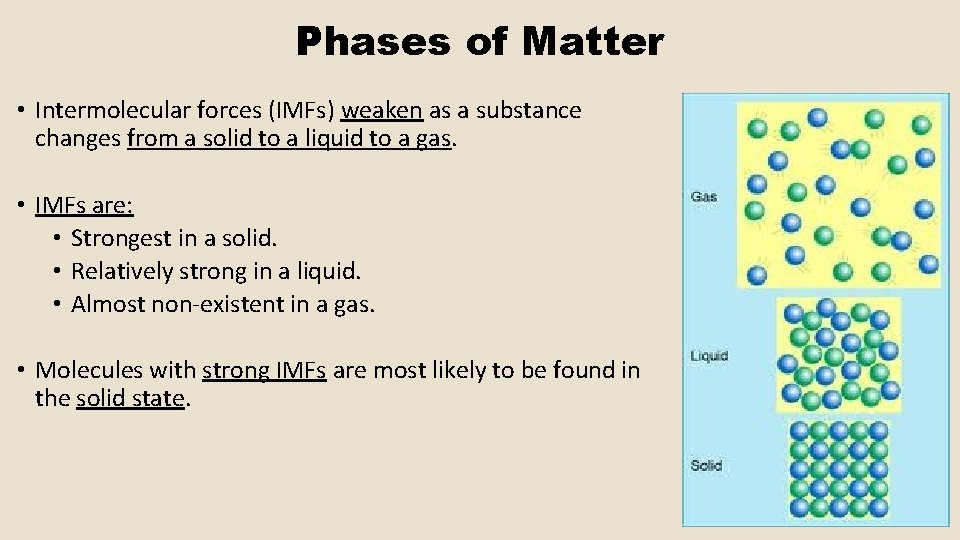
Phases of Matter • Intermolecular forces (IMFs) weaken as a substance changes from a solid to a liquid to a gas. • IMFs are: • Strongest in a solid. • Relatively strong in a liquid. • Almost non-existent in a gas. • Molecules with strong IMFs are most likely to be found in the solid state.

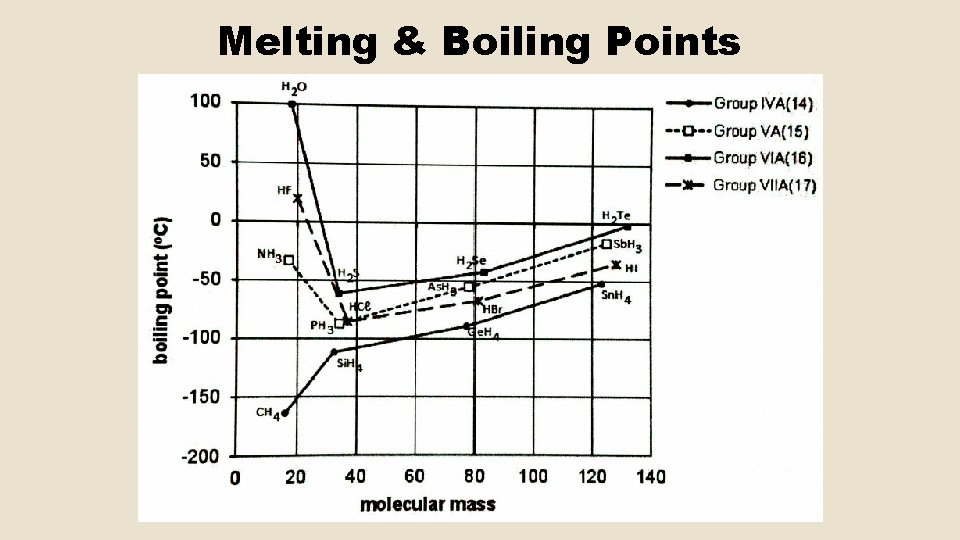
Melting & Boiling Points • As IMFs weaken, a substance will change phase (solid liquid, liquid gas) and the particles move further apart. • Definition: The melting point is the temperature at which a compound changes from solid to liquid. • As a substance boils, the particles completely separate from one another. There are (almost) no IMFs present between gas particles. • Definition: The boiling point is the temperature at which the vapour pressure of the liquid equals the external atmospheric pressure.

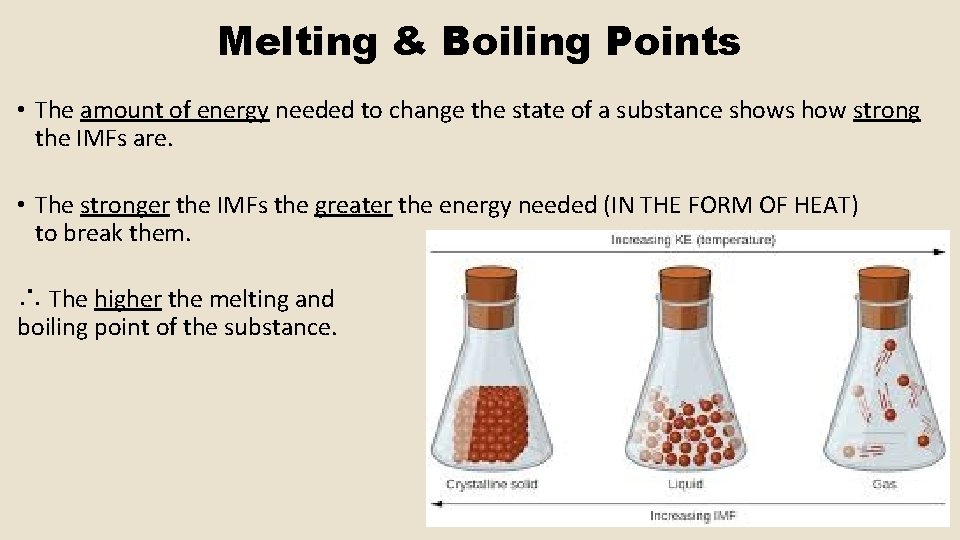
Melting & Boiling Points • The amount of energy needed to change the state of a substance shows how strong the IMFs are. • The stronger the IMFs the greater the energy needed (IN THE FORM OF HEAT) to break them. ∴ The higher the melting and boiling point of the substance.

Melting & Boiling Points

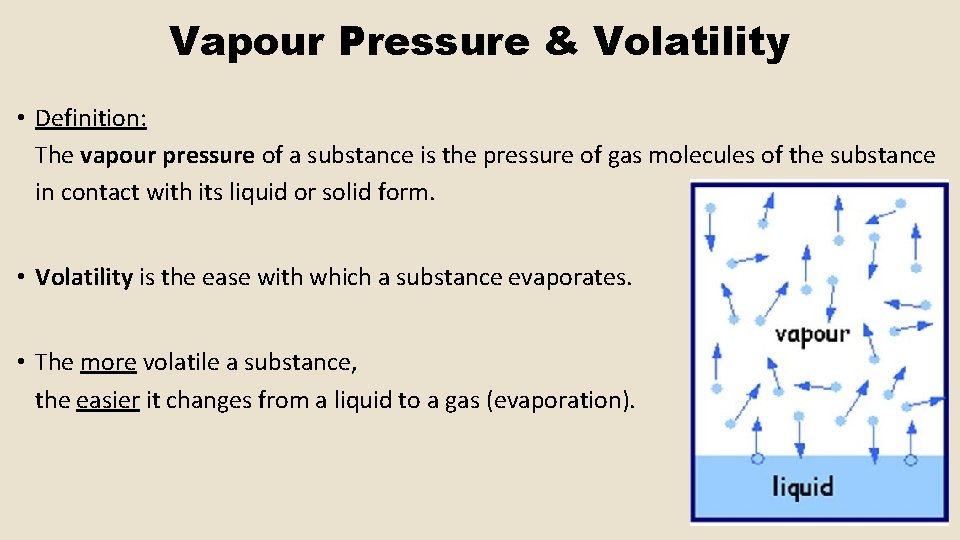
Vapour Pressure & Volatility • Definition: The vapour pressure of a substance is the pressure of gas molecules of the substance in contact with its liquid or solid form. • Volatility is the ease with which a substance evaporates. • The more volatile a substance, the easier it changes from a liquid to a gas (evaporation).

Vapour Pressure & Volatility • The volatility of a substance, and therefore the size of its vapour pressure, depends on the strength of the IMFs present within the substance. • The stronger the IMFs in a liquid, the more energy required to break the IMFs, the slower the rate of evaporation, the lower the volatility and the smaller the vapour pressure. • The weaker the IMFs in a liquid, the less energy required to break the IMFs, the faster the rate of evaporation, the higher the volatility and the larger the vapour pressure.

Class Exercise •

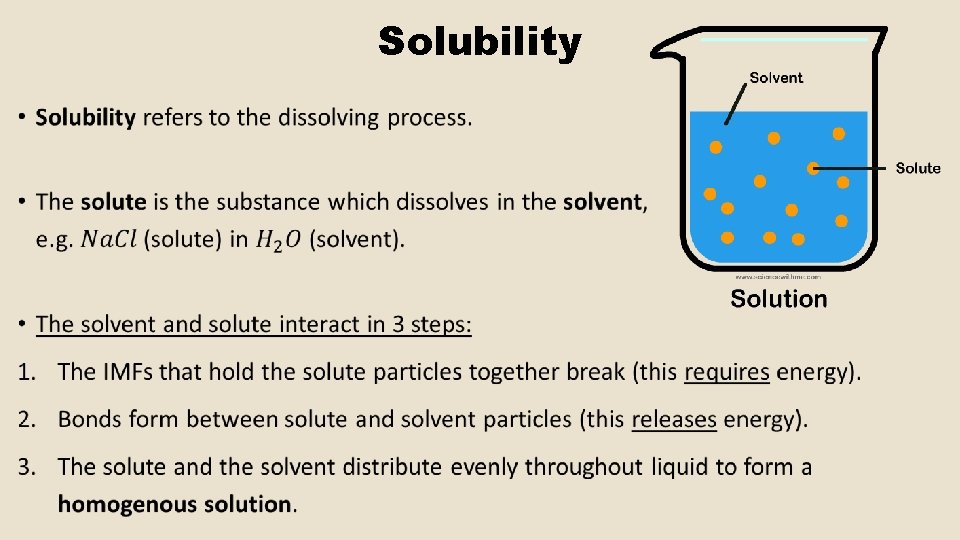
Solubility •

Solubility •

Surface Tension • Surface tension is the energy required to increase the surface area of a liquid. • The unbalanced force at the surface causes molecules at the surface to: • Rearrange so the least amount of molecules are at the surface. • Pack closer together than the rest of the molecules in the liquid. • Be more ordered and resistant to changing the arrangement of the molecules at the surface. ∴ The surface will have a “skin”.

Surface Tension • The stronger the IMFs, the greater the surface tension. • Due to (strong) hydrogen bonds, water has a high surface tension which explains why: • Water forms spherical droplets (spheres have the smallest surface area per unit volume). • Some insects can walk on the surface of water.

Capillarity • Capillarity is the ability of a liquid to flow against gravity. • Capillary action causes liquid to rise spontaneously in a narrow space such as a thin tube. It occurs due to forces of adhesion and cohesion. • The IMFs between liquid molecules are called forces of cohesion. • The IMFs between the liquid molecules and the solid surrounding surface are called the forces of adhesion.

Capillarity • CONCAVE CONVEX

Viscosity • Viscosity is the resistance of a substance to flow. • The greater the viscosity, the slower the flow. • Viscosity is determined by how long it takes for a liquid to flow out of a narrow tube under gravity. • Viscosity depends on the strength of the IMFs present in the substance. • The stronger the IMFs, the higher the viscosity.

Thermal Expansion • When the temperature of a substance is increased, heat energy is transferred to the particles. • The particles begin to move faster and thus further apart. • As a result, the substance expands. • A mercury thermometer works using the principle of thermal expansion.

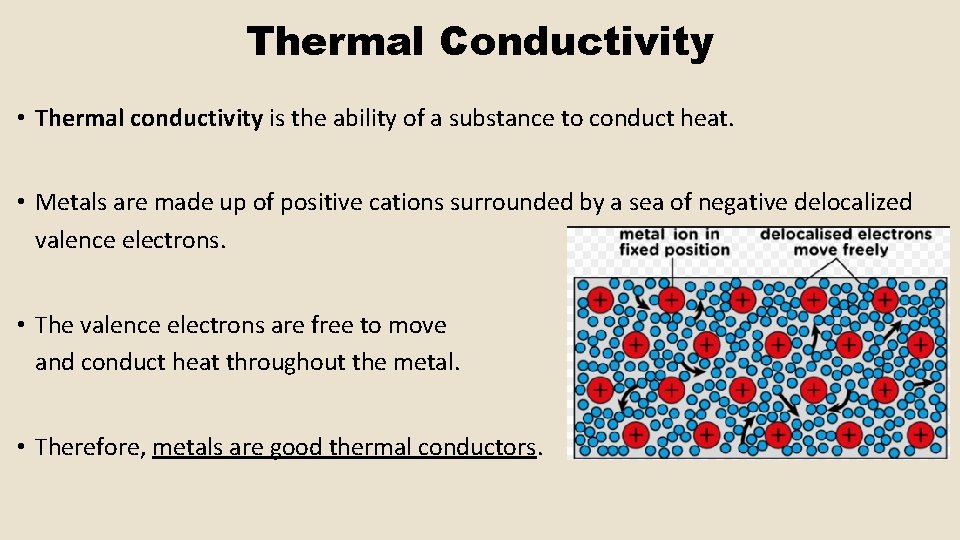
Thermal Conductivity • Thermal conductivity is the ability of a substance to conduct heat. • Metals are made up of positive cations surrounded by a sea of negative delocalized valence electrons. • The valence electrons are free to move and conduct heat throughout the metal. • Therefore, metals are good thermal conductors.

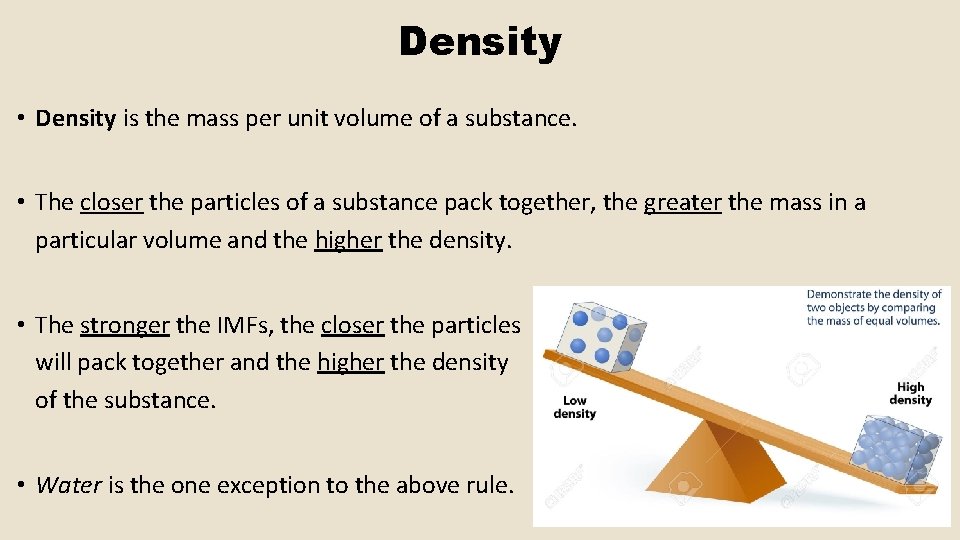
Density • Density is the mass per unit volume of a substance. • The closer the particles of a substance pack together, the greater the mass in a particular volume and the higher the density. • The stronger the IMFs, the closer the particles will pack together and the higher the density of the substance. • Water is the one exception to the above rule.

Summary of Physical Properties & IMFs • Melting and Boiling Points: • The STRONGER the IMFs, the more energy (in the form of heat) required to break the IMFs, and the HIGHER the MP and BP. • Vapour Pressure: • The STRONGER the IMFs, the LONGER it takes to evaporate, and the SMALLER the vapour pressure. • Solubility: • Like dissolves like. • Surface Tension: • The STRONGER the IMFs, the HIGHER the surface tension.

Summary of Physical Properties & IMFs • Capillarity: • Adhesive forces > Cohesive forces then the meniscus will be CONCAVE. • Cohesive forces > Adhesive forces then the meniscus will be CONVEX. • Viscosity: • The STRONGER the IMFs, the GREATER the viscosity. • Thermal Expansion: • The HIGHER the temperature, the MORE a substance expands. • Thermal Conductivity: • Metals are BETTER conductors of heat than non-metals. • Density: • The STRONGER the IMFs, the GREATER the density (except with water).

Chemistry of Water • Water is an amazing molecule without which life would not exist. • The strong hydrogen bonds present between water molecules cause it to have a number of unique physical properties such as: • Water has a high boiling point. • Water has a high heat of vaporisation. • Solid water (ice) is less dense than liquid water.

Water’s High Boiling Point • Water has a high boiling point due to strong hydrogen bonds present between water molecules. • Therefore, water requires a lot of heat energy to evaporate. • Water draws this heat from the surrounding environment. • As a result, evaporation causes cooling. • People have been able to use this fact to create fridges that don’t need electricity:

Water’s High Heat of Vaporisation • Since the hydrogen bonds in water are so strong, it requires a lot of energy to break them. • Water will absorb a large amount of energy before changing phase from liquid to gas. • In chemical terms: water has a high heat of vaporisation. • As a result, water is able to absorb a lot of heat from the sun and retain the heat allowing it to be warm enough to sustain life. • This also helps to moderate the climate in coastal areas.

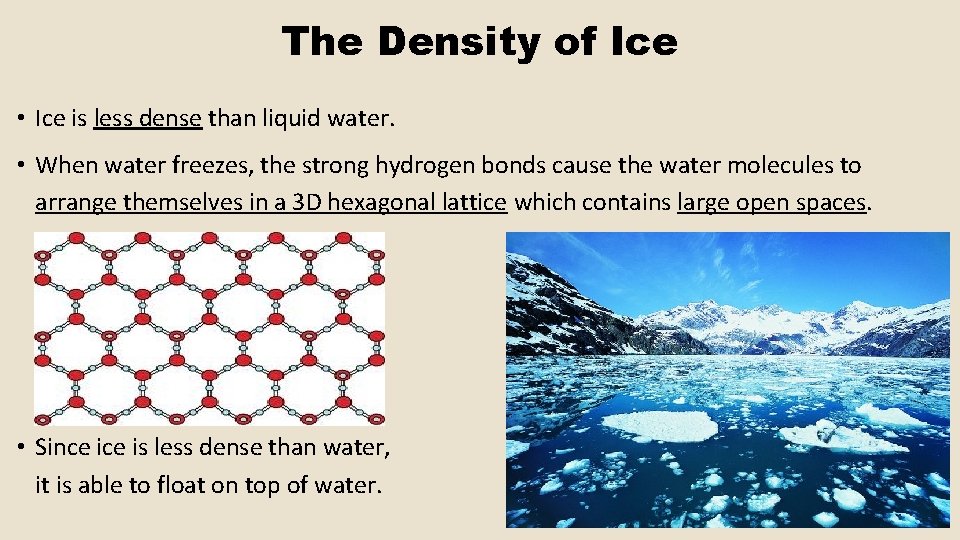
The Density of Ice • Ice is less dense than liquid water. • When water freezes, the strong hydrogen bonds cause the water molecules to arrange themselves in a 3 D hexagonal lattice which contains large open spaces. • Since is less dense than water, it is able to float on top of water.